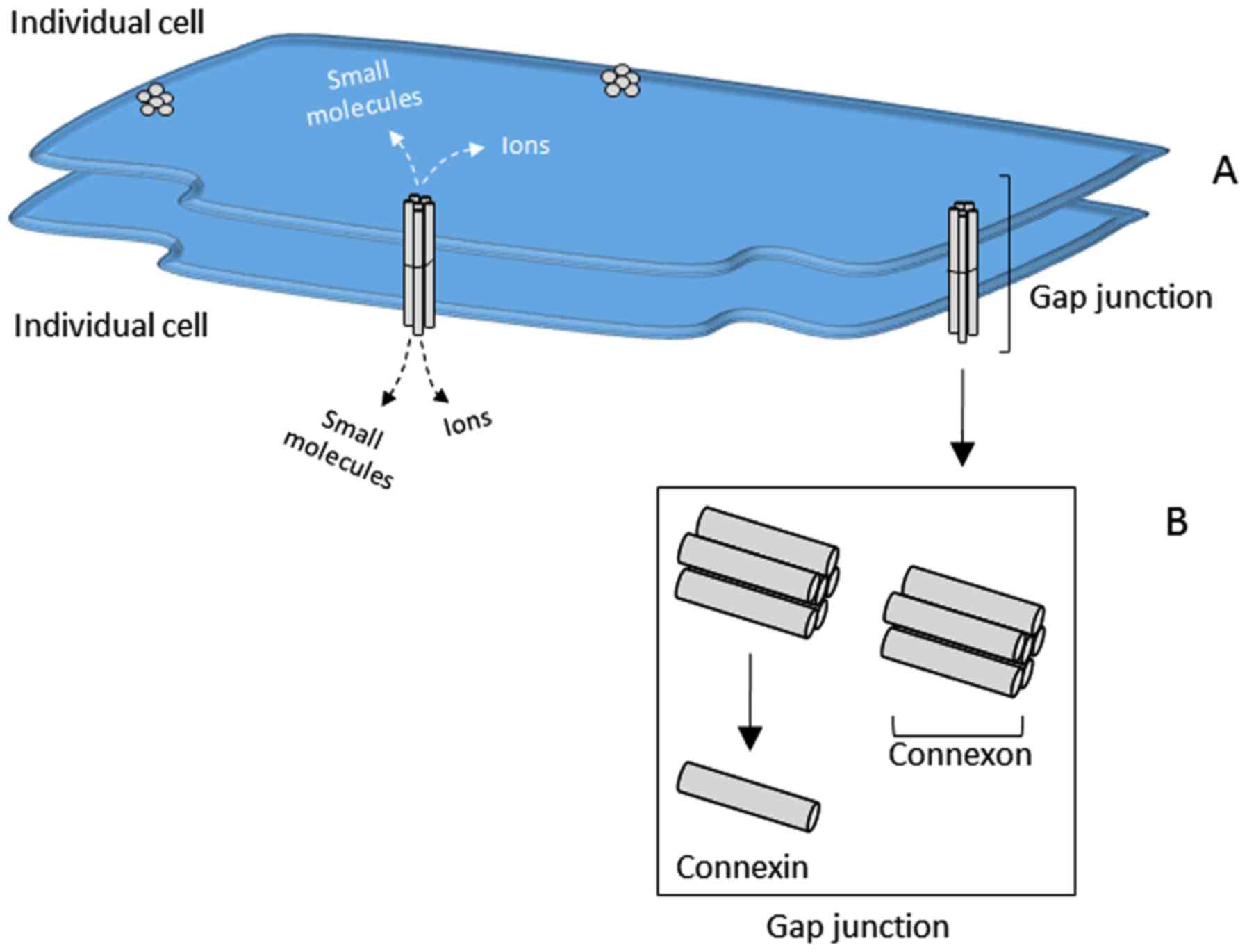
1. Introduction
Gap junctions are intercellular channels that
connect the cytoplasm of neighboring cells. These channels allow
for crosstalk between cells by regulating the direct intercellular
exchange of secondary messengers, small metabolites or inorganic
ions, through a process known as gap junction intercellular
communication (GJIC). Located at the plasma membrane of the cell,
gap junctions are composed of two hemi-channels called connexons,
which are assembled from six oligomerized protein subunits called
connexins (Fig. 1). The connexin
proteins that make up the hemi-channel subunits can be homomeric,
consisting of a single protein type of connexin family member, or
heteromeric/hetero-multimeric, consisting of two or more connexin
family members. When the hemi-channels from two opposing cells come
together to form a gap junction pore, the channels can likewise be
homotypic, where both hemi-channels are composed of a group of a
single type of connexin protein, or heterotypic, containing
hemi-channels composed of more than one family member.
In general, connexins and gap junctions are found in
numerous cell types throughout the body. To date, 21 different
connexins have been identified in humans (1). Expression of these connexin proteins
varies among cell types, and cells often express more than one
connexin (2,3). Since the initial observation that
GJIC is lost in cancer cells, the function of connexins and the
role of GJIC in human cancer has been researched and debated
(4–6). Since the 1960s, much of this research
has centered around the hypothesis that loss of direct
intercellular communication is characteristic of cancer cells and
is an important driver of tumorigenesis (1).
Connexin 43 (Cx43) is a predominant connexin protein
that has been studied in a variety of human cancers including
prostate, lung, liver, brain, and breast. Though scientific
evidence indicates that connexins, including Cx43 have a tumor
suppressive role in primary tumors, their role in cancer
progression and metastasis is quite controversial. Studies that
examine human breast cancer tissue suggest that levels of Cx43
either increase or decrease with cancer stage (7–10).
Studies also suggest that Cx43 localization to gap junctions could
play a part in determining disease severity, suggesting that
preserving Cx43 gap junctions could be an important distinction
between normal and malignant breast epithelial cells (7,10,11,23,25–28).
However, evidence supports both pro- and anti-metastatic roles for
Cx43 and many reports indicate that levels of Cx43 are both
elevated and reduced in breast cancer (7,9,11–24).
Consequently, there has been prominent debate in breast cancer
regarding whether Cx43 promotes or inhibits breast cancer
progression and metastasis because experimental findings have been
somewhat contradictory (11–21).
To date, it appears that in primary breast tumors,
overall Cx43 expression is either decreased or increased, with a
shift from the protein's normal localization in the plasma membrane
within gap junctions to a primarily cytoplasmic localization
(7,10,22,23,25–28).
In the majority of studies, the consensus is that GJIC is lost
within primary tumors. However, in the event of breast cancer
metastases, it has been suggested that Cx43 may become upregulated,
which may or may not coincide with a restoration of GJIC (9). Although some consensus, particularly
with primary tumors, has been reached, the exact role of Cx43 in
breast cancer, particularly with metastasis, remains elusive. This
report seeks to review the role of Cx43 in human breast and to
present the current literature on clinical observations of Cx43
from studies that focus solely on human breast and breast cancer
tissue samples.
2. Connexin 43 expression in the normal
human breast
The normal, non-malignant, human breast contains a
ductal epithelial tree that is composed of a contiguous layer of
luminal epithelial cells that is surrounded by an outer layer of
basal, myoepithelial cells. In the human breast, two predominant
connexins have been described, Cx26 and Cx43 (7,22,24,25,29).
Studies to decipher which connexins are expressed in human breast
have indicated that Cx26 and Cx43 are expressed in individual
compartments of the epithelial tree (1–5).
Evidence suggests that Cx26 appears to be predominantly expressed
in the luminal epithelium and Cx43 appears to be predominantly
expressed in the myoepithelium (7,22,24,29,30).
An initial study by Wilgenbus et al evaluated
Cx26, Cx32, and Cx43 in non-malignant and malignant human tissues,
including breast, by immunohistochemical (IHC) analysis and
immunoblotting with antibodies directed to each of the connexin
proteins (25). Although no
discernable expression of Cx26 and Cx32 was reported, Cx43 was
found to be expressed in normal breast and the connective tissue of
invasive ductal carcinomas of the breast. Following, a study by
Pozzi et al evaluated Cx43 by IHC and reverse transcriptase
PCR (RT-PCR) in human breast samples from archived tissues that
were obtained from non-pregnant women who had undergone reductive
mammoplasty (29). This group
identified Cx26, Cx32, and Cx43 in human breast using RT-PCR.
However, only Cx43, localized to myoepithelial cells, was detected
by IHC. It is of potential interest to note that Cx43 expression
appears to be predominant when the method of detection for each
connexin relies on protein detection using antibodies.
A follow-up study by Monaghan et al, also
using human breast obtained from reduction mammoplasties, confirmed
the expression of Cx43 in human breast (24). In this study, IHC, immunoblotting,
and RT-PCR were used to evaluate connexins including Cx26 and Cx43
in normal breast tissue. IHC staining indicated that Cx43 is
predominantly expressed in the basal myoepithelium of the ducts
whereas Cx26 was detected among the luminal epithelial cells of the
ducts. Additional studies by Jamieson et al (22) and Kanczuga-Koda et al
(7) confirmed the observation by
IHC analysis that Cx43 is expressed in myoepithelial cells in
normal, non-malignant human breast. Furthermore, consensus suggests
that Cx43 is localized primarily to the plasma membrane at the
lateral surface of myoepithelial cells, presumably at sites of gap
junction formation between the myoepithelial cells (10,22,24,26).
In the study by Monaghan et al, normal breast
samples were also evaluated for Cx43 by RT-PCR, which confirmed
that Cx26 and Cx43 mRNA are present (24). This observation confirms the
expression of Cx26 and Cx43, but it does not rule out the
possibility that the expression signal from either Cx26 or Cx43
could have originated in the epithelial cells and/or other stromal
tissue found in the human breast. However, while Cx43 expression
has been reported in the stromal compartment of different types of
breast malignancies, it has not been extensively reported in the
non-epithelial cell compartment of non-malignant, normal human
breast tissue (22,25).
In the study by Monoaghan et al, the authors
also purified populations of luminal and basal cells from the
normal human breast samples (24).
These cells were cultured in vitro and were further
evaluated by immunofluorescent (IF) staining and RT-PCR. Isolated
basal cell populations showed an increase in Cx43 staining by IF as
a function of time in culture. The isolated luminal cell cultures
were shown to primarily express Cx26, similar to the in vivo
tissue analysis done in this study. By western blotting, Cx43 but
not Cx26 was present in purified luminal and basal cells. By
RT-PCR, Cx43 was more abundant than Cx26 but still present.
Certainly, cell culturing techniques and antibody sensitivity
toward individual connexins are among the possibilities suggested
for the differences in observations between the studies described
here. Additionally, if Cx43 is localized to the plasma membrane of
basal myoepithelial cells that border luminal cells, it is
potentially logical to presume that both luminal and basal cell
types would be positive for Cx43 protein. Furthermore, multipotent
cells may have been present in isolated cell populations,
consistent with the observation that Cx43 expression increased with
time. None-the-less, these findings suggest a potential role for
Cx43 in normal human breast function and it follows that a
disruption of normal Cx43 expression and function, potentially
between luminal and basal cell populations or the surrounding
stroma, could play a role in the development of human breast
malignancy.
3. Connexin 43 expression and breast cancer
prognosis
Since Cx43 protein expression is relatively abundant
in normal human breast myoepithelial cells, studies to determine
whether Cx43 expression increases or decreases in breast cancer
relative to normal tissue and in relation to cancer stage is a
prominent area of investigation. A study by Jamieson et al,
sought to evaluate the pattern of expression of Cx26 and Cx43 in
human breast tumors (n=27, 12 grade 2 tumors and 15 grade 3 tumors)
relative to their expression in normal human breast (22). The study authors confirmed Cx43 in
normal human breast tissue localizes to myoepithelial cells and
further observed that the stroma of invasive breast carcinomas
contained Cx43-positive cells, unlike the stroma of normal breast
tissue. These findings were consistent with earlier studies from
Wilgenbus et al who also observed stromal staining of Cx43
in samples of invasive ductal carcinomas (25). Together, these studies indicate
that there is an increase in Cx43 protein expression in the stroma
of invasive breast carcinoma.
Interestingly, the majority of the carcinomas
examined at in the study by Jamieson et al showed
upregulation of Cx26 and/or Cx43 in carcinoma cells (22). The authors suggest that this marked
increase in Cx43 protein expression levels goes against the
hypothesis that GJIC suppresses tumorigenesis. However, if Cx43
protein is elevated but not localized or functional at the site of
gap junctions, then this would suggest either gap junction
independent functions for Cx43 exist or that cancer cells
upregulate Cx43 protein as a compensatory mechanism. It is
important to note that 4 out of 12 of the grade 2 tumors and 10 out
of 15 grade 3 tumors exhibited carcinoma cells that were
Cx43-positive and the observed staining was mainly diffuse and
cytoplasmic. The authors speculate that the ductal epithelial cells
of normal human breast express connexins other than Cx26 or Cx43
and, that the upregulation of Cx26 and/or Cx43 by the carcinoma
cells could alter GJIC between hyperplastic or malignant cells and
normal non-cancerous cells (22).
A study by Laird et al reported observations
that Cx43 protein expression is reduced or lost in breast tumors
compared to normal breast tissue (23). However, the authors did not observe
increased Cx43 staining in the stromal compartment. In the study by
Laird et al, 32 surgical specimens from breast cancer
patients who did not receive pre-operative neoadjuvant chemotherapy
or radiation therapy were evaluated and compared to adjacent normal
tissue from corresponding patient samples (23). Similar to the observations made by
Jamieson et al, the non-malignant breast tissue specimens
showed moderate to high levels of Cx43 staining (22). The 32 specimens evaluated by Laird
et al included malignant samples from ductal carcinoma in
situ (DCIS), invasive lobular carcinoma (ILC), and invasive
ductal carcinoma (IDC) but no difference in Cx43 expression was
observed between the different histological types and almost all
the samples were negative for Cx43 staining (23). The study authors also used IHC to
evaluate estrogen receptor (ER), progesterone receptor (PR), and
human epidermal growth factor receptor 2 (HER2) in relation to Cx43
expression but no correlation to any of the three receptors was
observed. Taken together, these observations suggest that loss of
Cx43, and presumably GJIC regulated by Cx43, is a common feature of
breast malignancies regardless of histological or receptor defined
subtype.
A later study by Kanczuga-Koda et al
evaluated a cohort of samples from normal breast (n=25), samples
with evidence of dysplasia (n=40), and breast cancer samples
including intraductal and invasive carcinomas (n=29) (7). Consistent with earlier observations,
the majority of intraductal carcinomas showed little to no
immunoreactivity with Cx43 antibodies suggesting that Cx43
expression is lost or reduced during malignancy (22,23).
However, in many of the cases of invasive breast carcinomas that
were evaluated in the study by Kanczuga-Koda et al, diffuse
cytoplasmic staining was observed suggesting a noticeable amount of
Cx43 protein is present (7). The
authors suggest that their findings are consistent with the study
by Jamieson et al, who also observed increased Cx43 staining
in invasive carcinomas (22).
However, it should be noted that in the study by Jamieson et
al, the increased Cx43 staining was also associated with
increased Cx43 in the tumor stroma (22). Whether or not this distinction is
significant, remains to be determined.
A follow-up study by Kanczuga-Koda et al
evaluated 71 breast cancer patient samples that only included
invasive ductal carcinomas of histological grade 2 or grade 3
(10). While the authors found no
association between Cx43 expression with age, tumor size, or lymph
node status, there was a significant positive correlation with Cx43
expression and histological grade 3 tumors. The authors also
evaluated Bak and Bcl-2 expression to follow-up on findings from a
study that implicated Cx43 in apoptosis and found a significant
association between Cx43 expression and the pro-apoptotic protein,
Bak (10,31). No association with Cx43 and the
anti-apoptotic protein, Bcl-2 was found (10).
A more recent study by Teleki et al sought to
evaluate the expression of Cx26, Cx32, Cx43, and Cx46 in breast
cancer patient samples prior to and after neoadjuvant chemotherapy
(27). In this study, the authors
used IHC to evaluate each connexin in samples taken from patients
before and after treatment with a variety of chemotherapeutic
agents including docetaxel, epirubicin, cyclophosphamide,
doxorubicin, viorelbine, fuorouracil, and trastuzumab. These
samples were also correlated with Ki67 expression staining and
information regarding ER, PR, and HER2 status was available for
most of the patient samples. The pre-chemotherapy levels of Cx43
correlated with ER status where high Cx43 expression was more
likely to be found in ER-positive samples both before and after
chemotherapy treatment. The observation that Cx43 is associated
with ER status was also made in an earlier study by Shipitsin et
al (30). Conversely, Cx43
expression negatively correlated with HER2 expression
pre-chemotherapy (27). Since
previous reports indicate that expression of Cx43 is hormone
responsive, the finding that Cx43 expression positively correlates
with ER status seems reasonable (32–35).
However, this finding is contradictory to the study by Laird et
al which reported no association between Cx43 and ER or HER2
(23). No relationship between
Cx43 and Ki67 was reported as Ki67 staining was not provided for
the Cx43 pre-chemotherapy treatment samples for comparison
(27). Together, the observations
made in this study by Teleki et al indicate that elevated
Cx43 expression corresponds with ER expression but not HER2.
Furthermore, Cx43 expression is not changed due to chemotherapy
treatment.
An additional observation that can be deduced from
the Teleki et al study is that the presence of Cx43-positive
cells in both pre-chemotherapy and post-chemotherapy samples
suggest that breast cancer cells are positive for Cx43 (27). This observation is consistent with
the study by Kanczuga-Koda et al which reported a
significant level of Cx43 staining in invasive breast carcinoma
samples (7). However, no
additional measures were taken to distinguish between epithelial
and stromal components in breast cancer samples in the former
(27). As this study also
evaluated Cx26, Cx32, and Cx46, it seems pertinent to discuss the
overall conclusions that the authors made was that a reduced level
of Cx26 post-chemotherapy suggested a better prognosis. Likewise,
high levels of Cx46, regardless of whether expression was observed
before or after chemotherapy, suggested a better prognosis. These
additional observations open up the opportunity for discussion that
changing the mixture of connexins expressed in the breast could
have significant impact on malignancy and bring into question the
potential roles of heterotypic channels as well as hemi-channels in
breast cancer.
Almost all of the studies that evaluated Cx43
expression have focused on protein expression detection by IHC.
Therefore, Teleki et al performed a study to evaluate
connexin gene expression using mRNA platforms (26). The authors also performed an
additional protein based analysis on a cohort of tumor microarray
(TMA) samples which represented all histological grades of breast
cancer. The mRNA analysis was performed separately on data mined
from Affymetrix and Illumina platforms but both analyses yielded
similar results.
From the Affymetrix analysis, high Cx43
(GJA1) mRNA expression was associated with a reduced
relapse-free survival (RFS) and overall survival (OS) in
ER-negative patients (26). A
study from Stoletov et al confirmed this observation using
analysis from the Oncomine database (www.oncomine.org) (28). The analysis by Stoletov et
al indicates that higher Cx43 expression correlated with
increased patient death and recurrence. However, in the study by
Teleki et al the opposite was true when evaluating distant
metastasis-free survival (DMFS) (26). In this case, elevated Cx43 was
associated with longer DMFS in the whole patient cohort, in lymph
node-negative patients, and ER-positive patients. Similar to these
findings, the Illumina-based analysis showed a significant positive
association between elevated Cx43 and OS in the whole patient
cohort, the ER-positive group, the luminal A group, and the
ER-positive cohort that received endocrine therapy. A negative
association was reported for OS between elevated Cx43 and
ER-negative as well as triple-negative patients.
Interestingly, the Cx43 protein analysis using TMAs
also indicated that elevated Cx43 expression (scored as 1–3+ vs. 0
for no expression) associated with increased RFS in the whole
patient cohort and in histological grade 2 tumors (26). Further analysis of Ki67 staining,
as a marker of proliferation, showed low Cx43 stain in high
proliferating tumor cells but prominent cytoplasmic Cx43 staining
in low proliferating tumor cells. Though not discussed, it would be
interesting to determine if the same Cx43 protein expression
patterns were present for the stromal compartment or if there was
any association between Cx43 expression in the stroma and survival
or histological grade. Similar to the previous study by Teleki
et al, other connexins also held significant prognostic
value (27). Whether or not there
is a relationship between each connexin, GJIC, and prognosis
remains to be determined.
4. The relationship between connexin 43 and
tumor initiating cells
Breast tumors are composed of a heterogeneous
mixture of cells. Many avenues of investigation have argued for the
existence of a defined subgroup of cancer cells called tumor
initiating cells, which are characterized by stem cell-like
properties (36–39). These tumor initiating cells, often
referred to as cancer stem cells, are defined by their capacity to
maintain stem cell phenotypes including self renewal, asymmetrical
division, and an ability to promote migration and invasion. Several
groups have isolated tumor initiating cells using cell surface
markers specific for the normal stem cells of the same organ, and
for breast, these cells can be defined by a CD44+,
CD24− population (36–39).
A study by Shipitsin et al sought to identify
genes that are expressed in CD44+ (stem-like cells) and
CD24+ (differentiated) cells (30). The study authors isolated different
cell populations, representing CD44+ and
CD24+ cells, from human breast tissue obtained from
reduction mammoplasty as well as breast tumor samples and then
performed gene expression profiling to determine if specific genes
were enriched in each population. Interestingly, they found that
Cx43 was significantly expressed in the CD44+ subset.
Consequently, the study authors defined Cx43 as a CD44+
specific gene.
A follow-up study from the same group by Park et
al expanded these findings by looking at breast cancer samples
from 4 histological subgroups defined as IDC, IDC with DCIS, DCIS
with microinvasion, and DCIS only (40). Consistent with previous findings,
Cx43 expression correlated with CD44 expression. The findings from
this study indicated that Cx43 is low in DCIS with microinvasion
but significantly higher in IDC with DCIS. This observation was
also true for CD44+CD24−-positive cells.
Therefore, the correlation of Cx43 with these two distinct
histological subtypes corresponds with findings from the
CD44+CD24− cell populations within these same
histological subgroups. Altogether, these findings imply that Cx43
gene expression is enriched in CD44+ cell populations,
which further corresponds with specific histological IDC subtypes,
implicating Cx43 in breast cancer progression.
5. Cytoplasmic localization of connexin 43
points to decreased GJIC in human breast cancer
Many studies have questioned if the loss of GJIC
between a potential cancer cell and normal cells is an alteration
that leads to a cancer cell transformation (1). There are two methods that have been
used as a surrogate for GJIC in static, clinical samples:
localization and expression. As discussed, studies evaluating
expression have led to findings suggesting both increased and
decreased expression levels of Cx43 protein or mRNA (7,10,22,23,25–27).
However, expression levels are not necessarily reflective of
protein function, particularly if protein expression is increased.
Therefore, Cx43 localization can also be informative when trying to
determine if GJIC is compromised.
In the previously referenced study by Jamieson et
al, the localization of Cx26 and Cx43 in human breast tumors
was evaluated in addition to total expression levels (22). The study confirmed that Cx43 in
normal human breast tissue localizes to the myoepithelial cells
with the localization of Cx43 predominantly on the lateral aspect
of the plasma membrane (10,22,24,26).
Contrary to this observation, Cx43 localization in 4 of the 12
grade 2 tumors showed predominantly cytoplasmic staining,
suggesting that Cx43 was localized away from sites of gap junctions
(22). A similar observation was
made in the grade 3 tumors where 10 out of 15 samples showed
predominantly cytoplasmic staining of Cx43. Somewhat consistent
with this observation, the study by Laird et al determined
that Cx43 expression was predominantly lost, reporting no Cx43
expression in breast tumors, and consequently no Cx43-positive gap
junctions (23).
In the earlier study by Kanczuga-Koda et al,
IHC analysis showed primarily cytoplasmic expression of Cx43 but
only in 3 out of 11 intraductal carcinomas (7). The remaining 8 samples were negative
for Cx43, consistent with the Laird et al study (23). Samples of invasive carcinoma (n=29)
showed mainly diffuse cytoplasmic staining of Cx43 in the majority
of the samples (26/29) (7). This
finding could suggest an upregulation of Cx43 as tumors progress to
an invasive phenotype or that a dominant cell type within the tumor
that expressed cytoplasmic Cx43 made up the bulk of invasive
tumors. The cytoplasmic localization of Cx43 suggests that gap
junctions are lost in invasive breast carcinomas.
A later study by Kanczuga-Koda et al, also
confirmed cytoplasmic expression of Cx43 but in a larger subset of
samples, consistent with their assertion that Cx43 expression is
increased in invasive carcinomas (10). The study authors observed
cytoplasmic Cx43 staining in 55 out of 71 (~80%) invasive ductal
breast carcinoma samples evaluated. In the remaining 16 samples, 6
samples showed punctate staining of Cx43 in the plasma membrane of
the carcinoma cells, presumably representing sites of gap
junctions. A punctate plasma membrane staining for Cx43 was found
in a small number of samples (1–2 tumors) and in a small fraction
of cells within a total IHC section; a phenomenon that has been
reported in other studies as well (7,22).
The remaining 10 samples were negative for Cx43 (10). A more recent study by Teleki et
al similarly alluded to the idea that Cx43 becomes more
cytoplasmic in tumors with higher levels of proliferation,
suggesting a more invasive phenotype (26). All together, these studies confirm
the observation that Cx43 is predominantly cytoplasmic in breast
carcinomas, which likely correlates with invasiveness as well as a
loss of Cx43 GJIC.
6. Phosphorylated connexin 43 in the
progression of breast cancer
A major regulatory feature of Cx43 is that the
protein contains at least 16 reported phosphorylation sites
(41). Studies indicate that the
phosphorylation of Cx43 regulates the Cx43 lifecycle including
assembly of Cx43 into gap junctions and formation of gap junction
plaques at the plasma membrane as well as trafficking of Cx43 via
the endolysosomal and autophagosomal systems. The phospho-mediated
regulation of Cx43 can result in increased or decreased GJIC
(1). A study by Gould et
al, sought to determine whether Cx43 phosphorylation correlated
with breast cancer stage (42).
The researchers developed an antiserum (SA226P) that strongly and
selectively reacts with Cx43 phosphorylated on Serine (S) residues
at amino acid positions 279 and 282 (S279/S282). Previous studies
showed that S279 and S282 are phosphorylated by MAPK signaling
through ERK1/2 (43,44). Additional studies indicated that
phosphorylation of these residues can either enhance or inhibit
GJIC, depending on the particular experimental cell type (45–53).
In the study by Gould et al, a total of 98
breast samples were examined, which included tissue from normal
adult breast tissue, fibrocystic disease (FCD), fibroadenomas (FA),
ductal and lobular carcinomas in situ of all grades,
infiltrating ductal carcinomas, infiltrating lobular carcinomas,
and infiltrating carcinomas with mixed ductal and lobular features
(42). The study authors evaluated
phosphorylated Cx43 using the SA226P antiserum directed at Cx43
phosphorylated at S279/S282 by IHC and compared these findings with
IHC staining of total pan-Cx43. In the normal breast, FCD and FA
samples, the SA226P antiserum stained myoepithelial cells in all
samples, which was predominantly cytoplasmic and diffuse. Very few
cells showed membrane related staining with SA226P. No pan-Cx43
staining was observed in normal breast, which is largely
inconsistent with prior studies (7,10,22,24,26,29).
Interestingly, staining for pan-Cx43 was also not reported in
malignant samples. However, the phospho-Cx43 signal varied
depending on the grade of malignancy. While it is difficult to form
any conclusions regarding the pan-Cx43 IHC results, the SA226P IHC
results suggest that phosphorylation of Cx43 could vary between
normal and malignant breast.
The authors went on to confirm their findings by
immunoblotting for pan-Cx43 and phosphorylated Cx43 using the
SA226P antiserum (42). These
results showed that the intensity of the bands that reacted with
the SA226P antiserum were high in normal breast but were somewhat
decreased in papilloma and DCIS samples. A clear increase in
phosphorylated Cx43 levels was observed in infiltrating ductal
carcinomas, infiltrating lobular carcinomas, and infiltrating
carcinomas when compared to papilloma and DCIS samples, suggesting
that the amount of phosphorylated Cx43 increases with increasing
disease severity. A similar pattern was observed using the pan-Cx43
antibody. It seems worthwhile to note that because no pan-Cx43 was
observed in any of the IHC samples, it may be interesting to
revisit these studies using alternative pan-Cx43 antibodies to
independently confirm the IHC findings.
7. Increased connexin 43 expression and
membrane localization in breast cancer metastasis
Studies that evaluated Cx43 expression and
localization uniformly suggest that GJIC is lost during breast
malignancy whether it be due to loss of protein expression or a
predominant localization of Cx43 in the cytoplasm of tumor cells,
away from plasma membrane sites of gap junction formation (7,10,22,23,25).
Additional studies have also argued that Cx43 expression at the
mRNA and protein level correlates in a statistically significant
manner with poor prognosis (26–28).
As a result, many have questioned whether Cx43 plays a role in
metastasis and furthermore, whether Cx43-regulated GJIC is required
for the metastatic process. Studies using breast cancer and mammary
tumor cell lines have presented conflicting findings indicating
both pro-metastatic and anti-metastatic roles for Cx43 (11–21).
Therefore, evaluation of clinically relevant samples would be
informative.
A later study by Kanczuga-Koda et al
evaluated 51 samples from primary breast cancers that were matched
with metastatic lymph node samples taken from the same patient
(9). Cytoplasmic Cx43 staining by
IHC was observed in ~50% of the primary tumors and 42 of the 51
(82.4%) tumors exhibited some membrane associated Cx43 staining.
Interestingly, all the lymph node metastases were positive for
Cx43, even if the primary tumors were negative for Cx43 staining.
The localization of Cx43 in the lymph node metastases was
heterogeneous with cytoplasmic and membrane associated staining.
Moreover, the study authors observed a statistically significant
increase in Cx43 levels in 18 of the 51 (35.3%) metastases. Cx26
was also evaluated in this study and while Cx26 was mainly
cytoplasmic in both the primary tumor and their metastases, Cx26
levels were higher in the metastases compared with the primary
tumor.
As a whole, these findings suggest that Cx43 likely
plays a role in breast cancer metastasis to lymph node and that
Cx43-positive tumor cells in lymph node metastases are likely able
to localize to sights of gap junctions. These observations are
consistent with prior experimental studies that suggest
Cx43-mediated GJIC promotes metastasis (12,14,19).
It is not known whether the plasma membrane localized Cx43 in the
lymph node metastasis samples evaluated in the Kanczuga-Koda et
al study are in functional gap junctions, whether the proposed
channels are homotypic or heterotypic, or whether hemi-channels
could be implicated (9). It is
also worth mentioning that it is possible that the tumor cells in
the lymph node metastases could be a specific tumor cell subtype
that selects for Cx43 expression, which is a potential explanation
for why 100% of the lymph node metastasis samples were positive for
Cx43 despite some primary tumor samples staining negative for
Cx43.
Additional observations support a role for Cx43 in
mediating interaction with the vasculature during breast cancer
metastasis (28,42). The previously described study by
Gould et al, whose goal was to evaluate Cx43 phosphorylation
in human breast malignancies, showed a strong staining pattern for
phosphorylated Cx43, using their SA226P anti-serum, in capillary
endothelial cells within tumors (42). Along the same lines, Stoletov et
al observed small populations of Cx43-positive tumor cells that
are in direct contact with blood vessel surfaces (28). While the exact nature of Cx43 in
the vasculature is largely unclear, these studies point to a
potential interaction between tumor cells and the vasculature that
may be Cx43-dependent. It is reasonable to speculate that Cx43
could play a role in intravasation or extravasation during
metastasis, but based on these minimal observations, that
conclusion seems largely premature.
8. Conclusions
Since the expression of Cx43 was observed in the
myoepithelial cells of the human breast over two decades ago,
researchers continue to investigate the role of Cx43 in the
development and progression of breast cancer. Though not completely
understood, Cx43 gap junctions have a potential mechanistic
connection to the development of malignancy in human breast. Some
debate still exists regarding the levels of expression of Cx43 in
the malignant progression of breast cancer, however, it seems
evident from the literature that loss of GJIC is integral to breast
tumor formation. As reviewed here, a general trend seems to be that
loss of Cx43 GJIC is an early event in malignancy. Based on at
least one study, it may be reasonable to speculate that Cx43 also
plays a role during intravasation or extravasation during
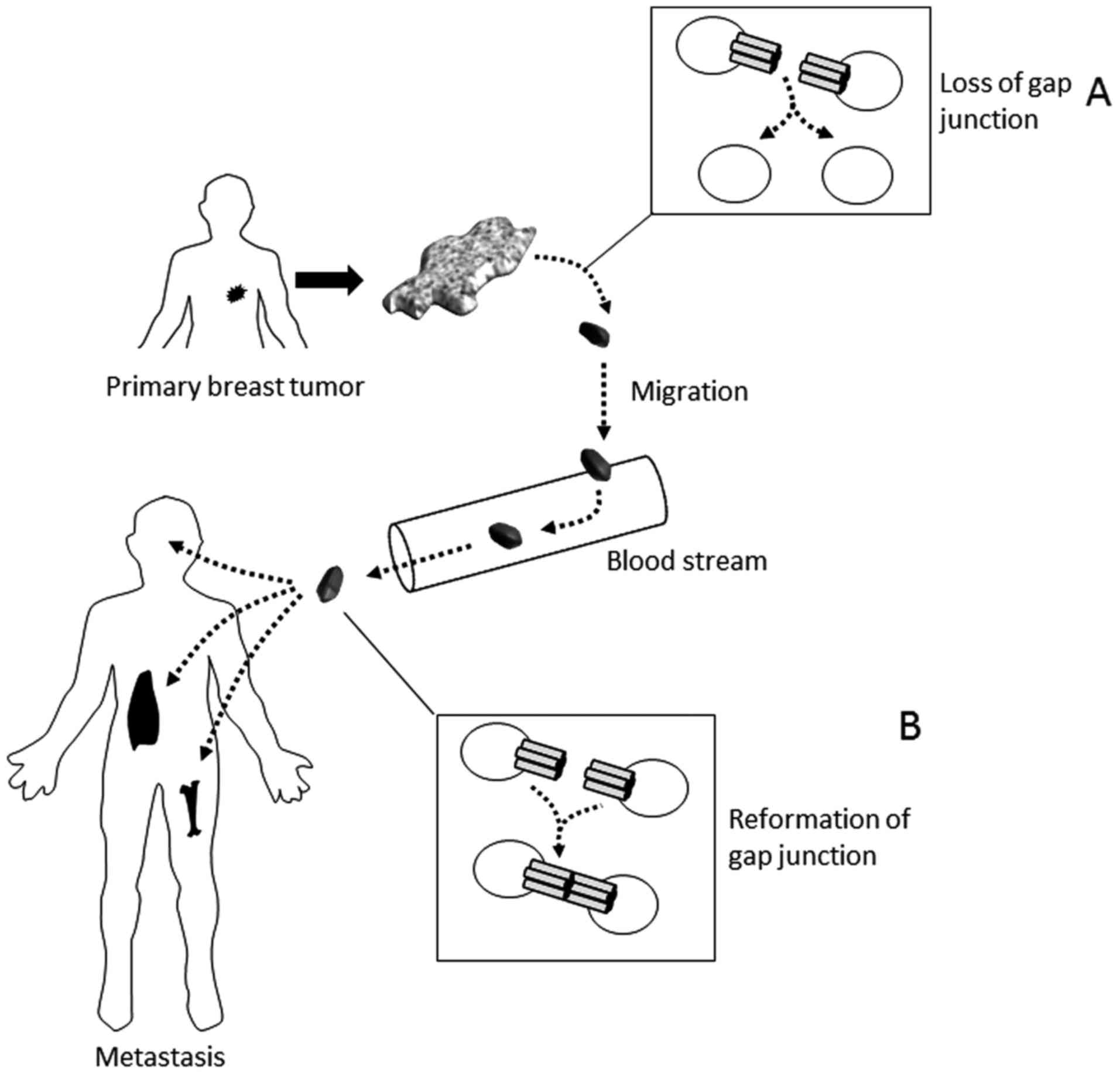
metastasis (28). Consequently, we
put forth a possible model that is thus far consistent with
published reports (Fig. 2),
however at this point, the conclusions, in particular with
metastasis, may be premature. Though some connection seems
apparent, the true role of Cx43 in breast cancer development and
progression remains unclear.
Efforts to therapeutically modulate Cx43 have
largely centered around chemicals that indirectly target Cx43
through changing membrane dynamics or through direct inhibition of
Cx43 gap junction activity (54,55).
Since restoring Cx43 gap junction intercellular communication is
preferable, at least at early stages of breast cancer development,
agents to restore rather than inhibit Cx43 gap junction
communication are needed. At least one such agent is available; a
therapeutic peptide called α-connexin carboxyl-terminal peptide,
which has been tested in several breast cancer cell lines (56). This 25-amino acid length peptide
mimics the c-terminal cytoplasmic domain of Cx43. However, clinical
application of peptide agents is somewhat limited due to certain
caveats including instability and poor oral availability (57). Furthermore, a highly studied area
of Cx43 research is the non-canonical (i.e., non-gap junction
related) functions of Cx43. Numerous reports demonstrate that the
carboxy-terminus (c-terminus) regulatory domain of Cx43, that is
located in the cytoplasm, is a primary site for protein-protein
interaction and phosphorylation (58). The c-terminal domain has been
reported to contribute to intracellular functions including
proliferation, apoptosis, migration, and transcription (58–62).
Consequently, two significant issues remain: deciding exactly how
to modulate Cx43 activity and identifying agents that can directly
target Cx43 to restore, rather than inhibit, gap junction
intercellular communication. As detection techniques and reagents
are refined, the analyses and information gained will continue to
improve. Targeted studies to evaluate discreet stages of the
metastatic process are needed to further our understanding of
Cx43's role in breast cancer progression. Ideally, evaluating
individual breast cancer subtypes (e.g., ER+,
HER2+, and triple-negative) is required for gaining
clear insight. Further insight may also be gained as the function
of heterotypic gap junctions and hemichannels in breast cancer is
revealed, or if non-gap junction functions for Cx43 and other
connexins are identified. Additional studies are needed to
elucidate the fundamental workings of this protein, which could
lead to an enhanced understanding of malignant progression and
reveal better potential therapeutic strategies for breast cancer
patients.
Acknowledgments
We would like to thank Dr Jennifer Jaroscak, for her
support and encouragement of our research endeavors these past two
years. This study was supported, in part, by a grant awarded to
E.S.Y. from the METAvivor Research and Support Inc. This study was
also supported, in part, by the South Carolina Clinical and
Translational Research Insitute, with an academic home at the
Medical University of South Carolina, through NIH grant nos. TL1
TR001451 and ULT TR001450, in the form of a training award to
C.B.W.
References
|
1
|
Aasen T, Mesnil M, Naus CC, Lampe PD and
Laird DW: Gap junctions and cancer: Communicating for 50 years. Nat
Rev Cancer. 16:775–788. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Falk MM: Connexin-specific distribution
within gap junctions revealed in living cells. J Cell Sci.
113:4109–4120. 2000.PubMed/NCBI
|
|
3
|
Goodenough DA and Paul DL: Gap junctions.
Cold Spring Harb Perspect Biol. 1:a0025762009. View Article : Google Scholar :
|
|
4
|
Loewenstein WR and Kanno Y: Intercellular
communication and the control of tissue growth: Lack of
communication between cancer cells. Nature. 209:1248–1249. 1966.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Loewenstein WR and Kanno Y: Intercellular
communication and tissue growth. I. Cancerous growth. J Cell Biol.
33:225–234. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fentiman IS and Taylor-Papadimitriou J:
Cultured human breast cancer cells lose selectivity in direct
intercellular communication. Nature. 269:156–158. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kańczuga-Koda L, Sulkowska M, Koda M,
Reszeć J, Famulski W, Baltaziak M and Sulkowski S: Expression of
connexin 43 in breast cancer in comparison with mammary dysplasia
and the normal mammary gland. Folia Morphol (Warsz). 62:439–442.
2003.
|
|
8
|
Kanczuga-Koda L, Sulkowska M, Koda M,
Rutkowski R and Sulkowski S: Increased expression of gap junction
protein-connexin 32 in lymph node metastases of human ductal breast
cancer. Folia Histochem Cytobiol. 45(Suppl 1): S175–S180. 2007.
|
|
9
|
Kanczuga-Koda L, Sulkowski S, Lenczewski
A, Koda M, Wincewicz A, Baltaziak M and Sulkowska M: Increased
expression of connexins 26 and 43 in lymph node metastases of
breast cancer. J Clin Pathol. 59:429–433. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kanczuga-Koda L, Sulkowski S, Tomaszewski
J, Koda M, Sulkowska M, Przystupa W, Golaszewska J and Baltaziak M:
Connexins 26 and 43 correlate with Bak, but not with Bcl-2 protein
in breast cancer. Oncol Rep. 14:325–329. 2005.PubMed/NCBI
|
|
11
|
Carystinos GD, Bier A and Batist G: The
role of connexin-mediated cell-cell communication in breast cancer
metastasis. J Mammary Gland Biol Neoplasia. 6:431–440. 2001.
View Article : Google Scholar
|
|
12
|
Elzarrad MK, Haroon A, Willecke K,
Dobrowolski R, Gillespie MN and Al-Mehdi AB: Connexin-43
upregulation in micrometastases and tumor vasculature and its role
in tumor cell attachment to pulmonary endothelium. BMC Med.
6:202008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kandouz M and Batist G: Gap junctions and
connexins as therapeutic targets in cancer. Expert Opin Ther
Targets. 14:681–692. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kapoor P, Saunders MM, Li Z, Zhou Z,
Sheaffer N, Kunze EL, Samant RS, Welch DR and Donahue HJ: Breast
cancer metastatic potential: correlation with increased heterotypic
gap junctional intercellular communication between breast cancer
cells and osteoblastic cells. Int J Cancer. 111:693–697. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li Z, Zhou Z and Donahue HJ: Alterations
in Cx43 and OB-cadherin affect breast cancer cell metastatic
potential. Clin Exp Metastasis. 25:265–272. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li Z, Zhou Z, Welch DR and Donahue HJ:
Expressing connexin 43 in breast cancer cells reduces their
metastasis to lungs. Clin Exp Metastasis. 25:893–901. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
McLachlan E, Shao Q and Laird DW:
Connexins and gap junctions in mammary gland development and breast
cancer progression. J Membr Biol. 218:107–121. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Plante I, Stewart MK, Barr K, Allan AL and
Laird DW: Cx43 suppresses mammary tumor metastasis to the lung in a
Cx43 mutant mouse model of human disease. Oncogene. 30:1681–1692.
2011. View Article : Google Scholar
|
|
19
|
Pollmann MA, Shao Q, Laird DW and Sandig
M: Connexin 43 mediated gap junctional communication enhances
breast tumor cell diapedesis in culture. Breast Cancer Res.
7:R522–R534. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Saunders MM, Seraj MJ, Li Z, Zhou Z,
Winter CR, Welch DR and Donahue HJ: Breast cancer metastatic
potential correlates with a breakdown in homospecific and
heterospecific gap junctional intercellular communication. Cancer
Res. 61:1765–1767. 2001.PubMed/NCBI
|
|
21
|
Shishido SN, Delahaye A, Beck A and Nguyen
TA: The anticancer effect of PQ1 in the MMTV-PyVT mouse model. Int
J Cancer. 134:1474–1483. 2014. View Article : Google Scholar :
|
|
22
|
Jamieson S, Going JJ, D'Arcy R and George
WD: Expression of gap junction proteins connexin 26 and connexin 43
in normal human breast and in breast tumours. J Pathol. 184:37–43.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Laird DW, Fistouris P, Batist G, Alpert L,
Huynh HT, Carystinos GD and Alaoui-Jamali MA: Deficiency of
connexin43 gap junctions is an independent marker for breast
tumors. Cancer Res. 59:4104–4110. 1999.PubMed/NCBI
|
|
24
|
Monaghan P, Clarke C, Perusinghe NP, Moss
DW, Chen XY and Evans WH: Gap junction distribution and connexin
expression in human breast. Exp Cell Res. 223:29–38. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wilgenbus KK, Kirkpatrick CJ, Knuechel R,
Willecke K and Traub O: Expression of Cx26, Cx32 and Cx43 gap
junction proteins in normal and neoplastic human tissues. Int J
Cancer. 51:522–529. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Teleki I, Szasz AM, Maros ME, Gyorffy B,
Kulka J, Meggyeshazi N, Kiszner G, Balla P, Samu A and Krenacs T:
Correlations of differentially expressed gap junction connexins
Cx26, Cx30, Cx32, Cx43 and Cx46 with breast cancer progression and
prognosis. PLoS One. 9:e1125412014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Teleki I, Krenacs T, Szasz MA, Kulka J,
Wichmann B, Leo C, Papassotiropoulos B, Riemenschnitter C, Moch H
and Varga Z: The potential prognostic value of connexin 26 and 46
expression in neoadjuvant-treated breast cancer. BMC Cancer.
13:502013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Stoletov K, Strnadel J, Zardouzian E,
Momiyama M, Park FD, Kelber JA, Pizzo DP, Hoffman R, VandenBerg SR
and Klemke RL: Role of connexins in metastatic breast cancer and
melanoma brain colonization. J Cell Sci. 126:904–913. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pozzi A, Risek B, Kiang DT, Gilula NB and
Kumar NM: Analysis of multiple gap junction gene products in the
rodent and human mammary gland. Exp Cell Res. 220:212–219. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shipitsin M, Campbell LL, Argani P,
Weremowicz S, Bloushtain-Qimron N, Yao J, Nikolskaya T,
Serebryiskaya T, Beroukhim R, Hu M, et al: Molecular definition of
breast tumor heterogeneity. Cancer Cell. 11:259–273. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Krutovskikh VA, Piccoli C and Yamasaki H:
Gap junction intercellular communication propagates cell death in
cancerous cells. Oncogene. 21:1989–1999. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Garfield RE, Puri CP and Csapo AI:
Endocrine, structural, and functional changes in the uterus during
premature labor. Am J Obstet Gynecol. 142:21–27. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Garfield RE, Sims S and Daniel EE: Gap
junctions: Their presence and necessity in myometrium during
parturition. Science. 198:958–960. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hendrix EM, Mao SJ, Everson W and Larsen
WJ: Myometrial connexin 43 trafficking and gap junction assembly at
term and in preterm labor. Mol Reprod Dev. 33:27–38. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Risek B, Guthrie S, Kumar N and Gilula NB:
Modulation of gap junction transcript and protein expression during
pregnancy in the rat. J Cell Biol. 110:269–282. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Clarke MF and Fuller M: Stem cells and
cancer: Two faces of eve. Cell. 124:1111–1115. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lynch MD, Cariati M and Purushotham AD:
Breast cancer, stem cells and prospects for therapy. Breast Cancer
Res. 8:2112006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Polyak K and Hahn WC: Roots and stems:
Stem cells in cancer. Nat Med. 12:296–300. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wicha MS, Liu S and Dontu G: Cancer stem
cells: An old idea - a paradigm shift. Cancer Res. 66:1883–1890;
discussion 1895–6. 2006. View Article : Google Scholar
|
|
40
|
Park SY, Lee HE, Li H, Shipitsin M, Gelman
R and Polyak K: Heterogeneity for stem cell-related markers
according to tumor subtype and histologic stage in breast cancer.
Clin Cancer Res. 16:876–887. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Solan JL and Lampe PD: Connexin43
phosphorylation: Structural changes and biological effects. Biochem
J. 419:261–272. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gould VE, Mosquera JM, Leykauf K, Gattuso
P, Dürst M and Alonso A: The phosphorylated form of connexin43 is
up-regulated in breast hyperplasias and carcinomas and in their
neoformed capillaries. Hum Pathol. 36:536–545. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Warn-Cramer BJ, Lampe PD, Kurata WE,
Kanemitsu MY, Loo LW, Eckhart W and Lau AF: Characterization of the
mitogen-activated protein kinase phosphorylation sites on the
connexin-43 gap junction protein. J Biol Chem. 271:3779–3786. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Warn-Cramer BJ, Cottrell GT, Burt JM and
Lau AF: Regulation of connexin-43 gap junctional intercellular
communication by mitogen-activated protein kinase. J Biol Chem.
273:9188–9196. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Laird DW: The life cycle of a connexin:
Gap junction formation, removal, and degradation. J Bioenerg
Biomembr. 28:311–318. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Leykauf K, Dürst M and Alonso A:
Phosphorylation and subcellular distribution of connexin43 in
normal and stressed cells. Cell Tissue Res. 311:23–30. 2003.
View Article : Google Scholar
|
|
47
|
Melchheier I, von Montfort C, Stuhlmann D,
Sies H and Klotz LO: Quinone-induced Cdc25A inhibition causes
ERK-dependent connexin phosphorylation. Biochem Biophys Res Commun.
327:1016–1023. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lee KM, Kwon JY, Lee KW and Lee HJ:
Ascorbic acid 6-palmitate suppresses gap-junctional intercellular
communication through phosphorylation of connexin 43 via activation
of the MEK-ERK pathway. Mutat Res. 660:51–56. 2009. View Article : Google Scholar
|
|
49
|
Riquelme MA, Burra S, Kar R, Lampe PD and
Jiang JX: Mitogen-activated protein kinase (MAPK) activated by
prostaglandin E2 phosphorylates connexin 43 and closes osteocytic
hemichannels in response to continuous flow shear stress. J Biol
Chem. 290:28321–28328. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kang NJ, Lee KM, Kim JH, Lee BK, Kwon JY,
Lee KW and Lee HJ: Inhibition of gap junctional intercellular
communication by the green tea polyphenol (-)-epigallocatechin
gallate in normal rat liver epithelial cells. J Agric Food Chem.
56:10422–10427. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Abdelmohsen K, Sauerbier E, Ale-Agha N,
Beier J, Walter P, Galban S, Stuhlmann D, Sies H and Klotz LO:
Epidermal growth factor- and stress-induced loss of gap junctional
communication is mediated by ERK-1/ERK-2 but not ERK-5 in rat liver
epithelial cells. Biochem Biophys Res Commun. 364:313–317. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Munoz JL, Rodriguez-Cruz V, Greco SJ,
Ramkissoon SH, Ligon KL and Rameshwar P: Temozolomide resistance in
glioblastoma cells occurs partly through epidermal growth factor
receptor-mediated induction of connexin 43. Cell Death Dis.
5:e11452014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Dubé E, Dufresne J, Chan PT and Cyr DG:
Epidermal growth factor regulates connexin 43 in the human
epididymis: Role of gap junctions in azoospermia. Hum Reprod.
27:2285–2296. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Evans WH, Bultynck G and Leybaert L:
Manipulating connexin communication channels: Use of
peptidomimetics and the translational outputs. J Membr Biol.
245:437–449. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
De Vuyst E, Boengler K, Antoons G, Sipido
KR, Schulz R and Leybaert L: Pharmacological modulation of
connexin-formed channels in cardiac pathophysiology. Br J
Pharmacol. 163:469–483. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Grek CL, Rhett JM, Bruce JS, Abt MA,
Ghatnekar GS and Yeh ES: Targeting connexin 43 with α-connexin
carboxyl-terminal (ACT1) peptide enhances the activity of the
targeted inhibitors, tamoxifen and lapatinib, in breast cancer:
Clinical implication for ACT1. BMC Cancer. 15:2962015. View Article : Google Scholar
|
|
57
|
Fosgerau K and Hoffmann T: Peptide
therapeutics: Current status and future directions. Drug Discov
Today. 20:122–128. 2015. View Article : Google Scholar
|
|
58
|
Leithe E, Mesnil M and Aasen T: The
connexin 43 C-terminus: A tail of many tales. Biochim Biophys Acta.
2017 May;16:2017.Epub ahead of print. View Article : Google Scholar
|
|
59
|
Moorby C and Patel M: Dual functions for
connexins: Cx43 regulates growth independently of gap junction
formation. Exp Cell Res. 271:238–248. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zhang YW, Nakayama K, Nakayama K and
Morita I: A novel route for connexin 43 to inhibit cell
proliferation: Negative regulation of S-phase kinase-associated
protein (Skp 2). Cancer Res. 63:1623–1630. 2003.PubMed/NCBI
|
|
61
|
Behrens J, Kameritsch P, Wallner S, Pohl U
and Pogoda K: The carboxyl tail of Cx43 augments p38 mediated cell
migration in a gap junction-independent manner. Eur J Cell Biol.
89:828–838. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Hebert C and Stains JP: An intact
connexin43 is required to enhance signaling and gene expression in
osteoblast-like cells. J Cell Biochem. 114:2542–2550. 2013.
View Article : Google Scholar : PubMed/NCBI
|