Introduction
Pancreatic cancer (PC) is a devastating disease with
a 5-year survival rate <7% (1,2). In
most cases, the initial diagnosis of PC is made when the disease
has progressed to a late stage, thus a poor prognosis is
inevitable. Although surgery has made great progress to improve the
overall survival of PC patients, the majority still survive less
than 5 years (2–5). According to National Comprehensive
Cancer Network (NCCN) criteria, combination therapy of gemcitabine
with paclitaxel is currently the best choice for PC patients.
Gemcitabine is a nucleoside analogue widely used as an important
drug against carcinoma (6).
Docetaxel, a semisynthetic analogue of paclitaxel, is a widely used
anti-mitotic drug for various cancers, including PC (7). However, the combination regimen of
gemcitabine plus paclitaxel is still unsatisfactory in the case of
overall survival (OS), and severe side effects are common (8). Therefore, an alternative approach
with minimal drug-related side effects to increase the OS of PC
patients is urgently needed.
Baicalein, a kind of traditional Chinese herbal
medicine, has been reported to be a potent chemotherapeutic
adjuvant for its properties of selectively inducing apoptosis in
various human cancer cells with minimal influence on normal cells
(9–15). It has been demonstrated that
baicalein has the capacity to induce apoptosis and inhibits the
proliferation of PC cells in pre-clinical studies (10,16,17).
Furthermore, baicalein is able to activate caspase-3 by forming
hydrogen bonds with residues Ser251 and Asp253 at its active site
and results in cell apoptosis in colon cancer (14). However, whether baicalein has
synergistic effects with gemcitabine or docetaxel in the treatment
of pancreatic cancer is still unclear. In the current study, we
examined the effects of combination therapy of baicalein with
gemcitabine or docetaxel on proliferation, apoptosis, migration,
and cell cycle of pancreatic cells in vitro. Furthermore, we
gained insight into the underlying mechanism of combination
treatment containing baicalein. Our results suggest that
synergistic effects of baicalein with gemcitabine or docetaxel on
apoptosis of PANC-1 cells is dependent on caspase-3/PARP signaling
pathway.
Materials and methods
Cell lines and reagents
Human pancreatic cell line PANC-1, MIA PaCa-2 and
HPAF-II were purchased from Chinese Academy of Life Science
(Shanghai, China). DMEM medium was purchased from HyClone
Laboratories Inc. (Shrewsbury, NJ, USA). Fetal bovine serum (FBS)
was from Gibco Co. (Carlsbad, CA, USA). Baicalein was purchased
from Xinran Co. (Shanghai, China). Gemcitabine and docetaxel were
purchased from Sigma-Aldrich (St. Louis, MO, USA) and were
dissolved according to the manufacturer's instructions. DMSO and
DAPI were from Roche.
Cell culture
PANC-1, MIA PaCa-2 and HPAF-II cells were cultured
in DMEM medium containing 10% FBS and 1% penicillin and
streptomycin in incubator with 5% CO2 at 37°C. Drugs at
different dosage were given at the indicated time point.
3-(4, 5-Dimethylthiazol-2-yl)-2,
5-diphenyltetrazolium (MTT) assay
Drug sensitivity was detected using the MTT assay.
Briefly, cells were trypsinized and seeded in 96-well plates
(Corning Inc., Corning, NY, USA) at 5×103 cells per
well. The cells were cultured overnight and then replenished with
fresh medium containing drugs at indicated concentrations. The
cells were then incubated for 48 h. A total of 20 μl of MTT
(Sigma-Aldrich) dissolved in PBS at 5 mg/ml was added directly to
the wells at the indicated time points. The plates were then
incubated for an additional 4 h at 37°C for MTT reaction. The
supernatant was then removed. A total of 100 μl of DMSO was
added to dissolve the formed formazan crystals, and the optical
density was measured at 490 nm on a PerkinElmer 2030 VICTOR X
Multilabel Plate Reader (Perkin-Elmer, Waltham, MA, USA). The
results are represented as the average value of 3 independent
experiments. The percentage of viable cells was calculated as cell
viability (%) = (OD of treatment/OD of control) × 100.
Analysis of cytotoxic synergy
The viability of PANC-1, MIA PaCa-2 and HPAF-II
cells were examined by MTT assay as described above and the CI
values were analyzed using the Calcusyn software, which calculates
CI value by the following equation: CI = (D)1/(Dx)1 + (D)2/(Dx)2 +
(D)1(D)2/(Dx)1(Dx)2, where (Dx)1 and (Dx)2 are the doses for x%
inhibition by drug 1 and drug 2 alone. (D)1 and (D)2 are the doses
in combination that inhibit cell growth by x%. A CI value of 1
indicates additive effects of the two agents, while a CI value
greater than 1 indicates antagonism effects, and less than 1
indicates synergism effects.
DAPI staining assay
Viable PANC-1 cells were plated in 6-well plates for
24 h, followed by treatment with indicated drugs. After 48 h, cells
were fixed with 4% paraformaldehyde for 20 min, followed by DAPI
staining for 10 min in the dark. Finally, cells were detected using
immunofluorescence microscopy (DSY5000X, OPPNO, Beijing,
China).
EdU (5-ethynyl-2′-deoxyuridine)
assay
Cell Light™ EdU kit was purchased from RiboBio Co.,
Ltd. (Guangzhou, China) and the experiment was conducted according
to the manufacturer's instructions. Briefly, prepared 50 μM
EdU DMEM medium solutions were added to drug treated PANC-1 cells
in 96-well plates, followed by incubation for 2 h and thereafter
washing with PBS, 4% paraformaldehyde was used to fix the cells for
30 min, and then 2 mg/ml glycine was given to neutralize the
remaining paraformaldehyde. Apollo® staining reaction
solution was used to incubate PANC-1 cells in the dark for 30 min,
and then washed with 0.5% TritonX-100 PBS 3 times. Finally, Hoechst
33342 was added for 30 min and images were taken from
immunofluorescence microscopy (DSY5000X, OPPNO).
Wound healing assay
PANC-1 cells were seeded on 6-well plates (Corning
Inc.). After incubation for 24 h, each well was initiated by
scratching with a sterile 10 μl pipette tip, followed by
washing with PBS three times, and treated with indicated drugs at
37°C. After 24 h, the distance between cell edges was analyzed
using the ImageJ software.
Flow cytometry
After treatment with drugs, PANC-1 cells were
digested by 0.25% trypsin from 6-well plates, and then collected
and incubated with 70% ethanol overnight. After fixation, cells
were stained with propidium iodide (PI) for 30 min at room
temperature. The cells were washed with ice cold PBS 3 times before
loaded to the flow cytometer (FACS Calibur, BD BioSciences). The
results were then analyzed with ModFit software according to the
manufacturer's instructions.
Transmission electron microscope
(TEM)
PANC-1 cell samples were digested by 0.25% trypsin
and centrifuged at 1500 rpm for 5 min and fixed overnight in 2.5%
glutaraldehyde at 4°C. Then the samples were fixed in 1% osmium
acid, dehydrated, and placed in embedding molds in a standard
fashion. Appropriate areas were selected and ultrathin sections of
0.08 μm were stained with lead citrate and uranyl acetate.
Those sections were then examined with a transmission electron
microscope (JEM1230, JEOL, Ltd., Tokyo, Japan).
Western blotting
PANC-1 cells were treated with drugs for 48 h and
lysed in RIPA buffer, followed by denaturation. Protein
concentration was measured by bicinchoninic acid assay system
(Beyotime, Shanghai, China). Protein samples were separated by
SDS-PAGE gel and electrophoretically transferred to nitrocellulose
membranes. The membranes were blocked with 5% non-fat milk for 30
min and incubated with anti-caspase-3, anti-cleaved-caspase-3,
anti-PARP and anti-cleaved-PARP antibodies (1:1,000; Cell Signaling
Technology, Danvers, MA, USA) at 4°C overnight. The membranes were
then incubated with goat anti-rabbit/anti-mouse secondary antibody
conjugated with horseradish peroxidase (1:3,000; Cell Signaling
Technology) and then membranes detected using an enhanced
chemiluminescence detection kit (Thermo Scientific). β-actin was
used as the internal control.
Statistical analysis
For statistical analyses, Prism 5 software was used.
Statistical analyses were performed using the Student's t-test. A
P-value of <0.05 was considered to indicate a statistically
significant difference.
Results
Baicalein induced morphologic changes of
pancreatic cancer cells and suppressed their proliferation
We determined the morphologic changes of PANC-1, MIA
PaCa-2 and HPAF-II cells after treatment with baicalein (200
μM) by inverted microscope. After treatment, cells exhibited
a more distinct cell profile and more shrunken morphology, while
DMSO-treated control group showed a blurred cell profile and
adhered tightly to the well (Fig.
1A–C). Then, we detected the effect of baicalein on cell
viability in vitro using MTT assay. Consistently, cell
viability of baicalein-treated PANC-1 was significantly suppressed
(Fig. 1D). As shown in Fig. 1D, 200 μM baicalein triggered
significant decrease in cell viability. Notably, the suppressive
effects of baicalein on cell viability of PANC-1, MIA PaCa-2 and
HPAF-II cells was in a dose-dependent manner.
Baicalein had synergistic effects with
gemcitabine/docetaxel on cell viability of pancreatic cancer
cells
To determine whether baicalein has a synergistic
effect with gemcitabine/docetaxel, we administered baicalein in
combination with different concentrations of gemcitabine/docetaxel
to PANC-1. As shown in Fig. 2A,
treatment with gemcitabine (2 μM) mildly affected cell
viability of PANC-1 with 82.03±5.033%. When it was combined with
baicalein (10 μM), cell viability decreased to 36.5±2.848%.
Similarly, baicalein (10 μM) plus gemcitabine (5 or 10
μM) remarkably affected cell viability compared with
gemcitabine (5 and 10 μM) alone (27.77±1.302% vs.
61.97±4.756%, P<0.01; 18.67±1.65% vs. 54.73±4.033%, P<0.01).
The CI values calculated by Calcusyn software were 0.191, 0.204 and
0.179 when baicalein and gemcitabine were concurrently administered
with the ratios of 5:1, 2:1 and 1:1, respectively (Fig. 2C). Similar to gemcitabine,
docetaxel also exhibited synergistic effect with baicalein.
Treatment with baicalein (10 μM) in combination with
docetaxel (2, 5, and 10 nM) resulted in remarkable decrease in cell
viability when compared with docetaxel alone (2, 5, and 10 nM)
(39.17±2.109% vs. 64.2±2.969%, P<0.01; 31.97±1.525% vs.
54.07±2.282%, P<0.01; 20.7±1.858% vs. 46.83±1.742%, P<0.001)
(Fig. 2B). The CI values were
0.201, 0.192 and 0.099, when baicalein (μM) and docetaxel
(nM) were administered with the ratios of 5:1, 2:1 and 1:1,
respectively (Fig. 2D).
Consistently, similar results were obtained from MIA PaCa-2 and
HPAF-II cells treatment with baicalein in combination with
gemcitabine/docetaxel (Fig. 2E–L),
suggesting that baicalein has synergistic effects with
gemcitabine/docetaxel on suppressing cell viability of PANC-1, MIA
PaCa-2 and HPAF-II cells.
 | Figure 2Effects of baicalein with gemcitabine
or docetaxel on cell viability of PANC-1, MIA PaCa-2 and HPAF-II
cells. (A and B) Combination effects of baicalein with
gemcitabine/docetaxel on cell viability of PANC-1 cells were
detected by MTT assay. Data are from three independent experiments.
*P<0.05, **P<0.01 and ***P<0.001
represent statistical significance. (C and D) Isobologram analysis
assessing the synergism of baicalein with gemcitabine/docetaxel on
PANC-1 cells; CI values depicting synergistic efficacy at indicated
combination groups. (E and F) Combination effects of baicalein with
gemcitabine/docetaxel on cell viability of MIA PaCa-2 cells were
detected by MTT assay. (G and H) Isobologram analysis assessing the
synergism of baicalein with gemcitabine/docetaxel on MIA PaCa-2
cells; CI values describing synergistic effects at indicated
combination groups. (I and J) Combinational effects of baicalein
with gemcitabine/docetaxel on cell viability of HPAF-II cells were
detected by MTT assay. (K and L) Isobologram analysis assessing the
synergism of baicalein with gemcitabine/docetaxel on HPAF-II cells;
CI values describing synergistic effects at indicated combination
groups. 10B, 10 μM baicalein; 2G, 2 μM gemcitabine;
5G, 5 μM gemcitabine; 10G, 10 μM gemcitabine; 10B+2G,
10 μM baicalein plus 2 μM gemcitabine; 10B+5G, 10
μM baicalein plus 5 μM gemcitabine; 10B+10G, 10
μM baicalein plus 10 μM gemcitabine; 2D, 2 nM
docetaxel; 5D, 5 nM docetaxel; 10D, 10 nM docetaxel; 10B+2D, 10
μM baicalein plus 2 nM docetaxel; 10B+5D, 10 μM
baicalein plus 5 nM docetaxel; 10B+10D, 10 μM baicalein plus
10 nM docetaxel. |
Combination treatment exhibited strong
suppressive effects on the proliferation of PANC-1 cells
It is well-known that gemcitabine and docetaxel
could inhibit proliferation of pancreatic cancer cells. However,
whether baicalein has synergistic effects with
gemcitabine/docetaxel on proliferation of pancreatic cancer cells
is unknown. Therefore, we used EdU assay to determine the effects
of cell proliferation by combination treatment. Relative
proliferation ability was normalized to DMSO treated group by the
ratios of proliferating cells which were stained red in the EdU
assay. The relative proliferation ability of baicalein (10
μM) treated group was lower than control, but no statistical
significance (Fig. 3A and B). The
relative proliferation ability of PANC-1 cells treated with
baicalein (10 μM) in combination with gemcitabine (2 and 10
μM) showed significantly decreased proliferation when
compared to gemcitabine alone (2 and 10 μM) (2.677±2.677%
vs. 17.48±3.243%, P<0.05; 1.496±0.8111% vs. 4.72±0.469%,
P<0.05) (Fig. 3A and B).
Similarly, the relative proliferation ability of cells treated with
baicalein (10 μM) in combination with docetaxel (2 and 10
nM) was significantly inhibited compared with docetaxel alone (2
and 10 nM) (29.36±5.822% vs. 45.11±8.995%; 9.051±3.642% vs.
23.91±3.953%), but no statistical significance (Fig. 3A and B). Our data suggest that
baicalein might be a potential adjuvant to strengthen the
anti-proliferation effects of the first-line clinical drugs on
pancreatic cancer cells.
Combination treatment triggered cell
cycle arrest of PANC-1 cells
Cell cycle blockade usually leads to growth
inhibition of cancer cells. To investigate the effects of baicalein
alone and combination treatment on cell cycle of PANC-1 cells, we
conducted flow cytometry to analyze the proportion of different
phases in cell cycle. The results showed that baicalein alone (50
and 100 μM) induced remarkable cell cycle arrest in the
S-phase (42.95 and 45.74%) compared with control (34.22%) (Fig. 4A and C). Combination treatment with
baicalein (10 μM) and gemcitabine (2 μM) resulted in
a significant obvious change than gemcitabine (2 μM) alone
(21.97 vs. 6.46%). Similar results were observed between
combination treatment with docetaxel (2 nM) plus baicalein (10
μM) and docetaxel (2 nM) alone (Fig. 4B and D). Therefore, baicalein might
curb cell growth of PANC-1 by blocking the cell cycle.
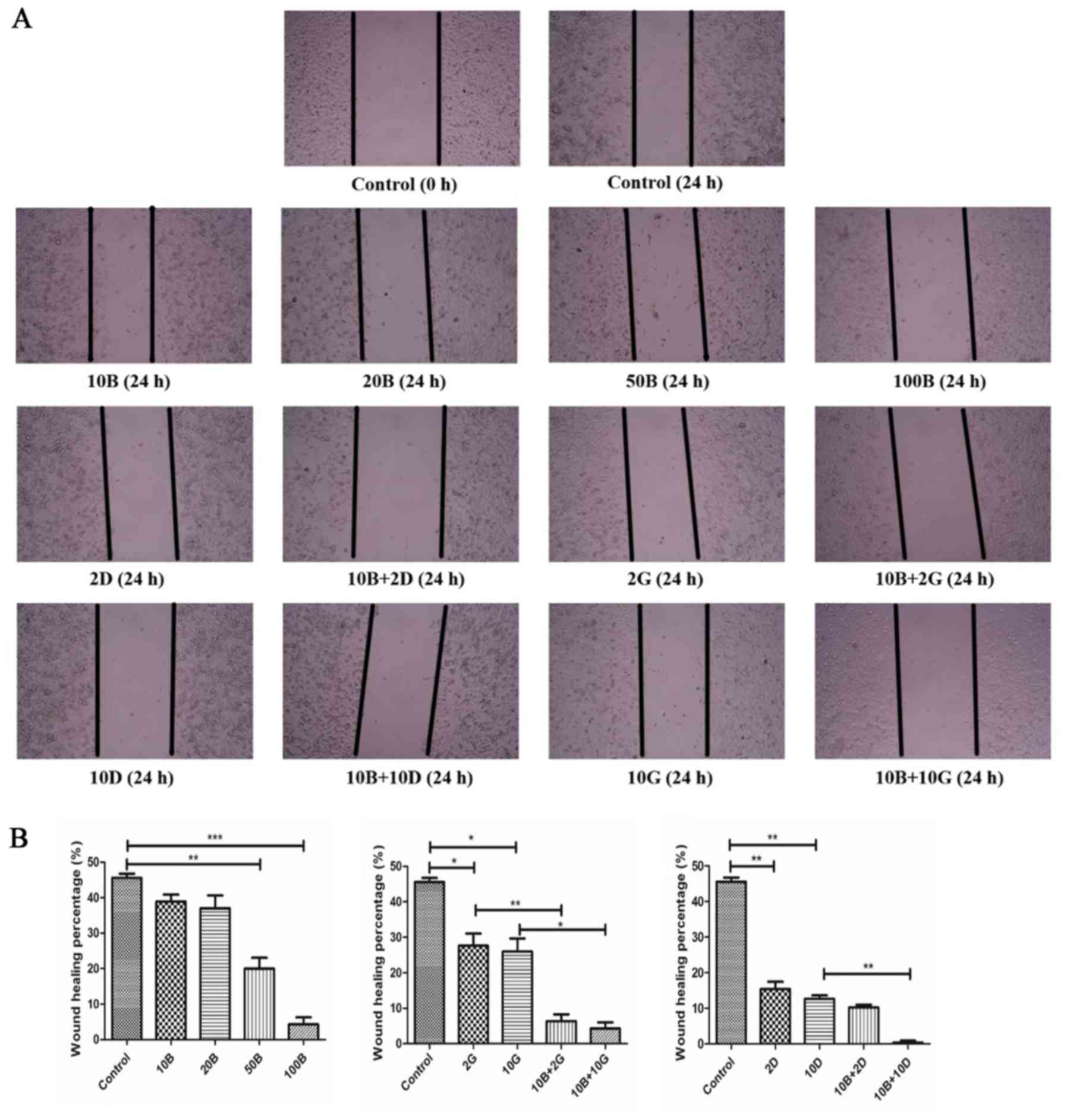
Baicalein strengthened the inhibitory
ability of gemcitabine/docetaxel on migration of PANC-1 cells
To further determine the effects of baicalein on
cell migration, we used wound healing assay to assess the migration
ability of PANC-1 cells. As expected, inhibitory effects of
baicalein on migration of PANC-1 cells was in a dose-dependent
manner. As shown in Fig. 5A and B,
either 10 or 20 μM baicalein alone exhibited no significant
effects. However, baicalein at 50 μM induced statistically
significant reduction in recovery ratio of PANC-1 cells compared
with control (20±3.225% vs. 45.6±0.665%; P<0.01). Next, we
investigated whether baicalein could reinforce the inhibitory
ability of gemcitabine/docetaxel on migration of PANC-1 cells in
vitro by using scratching assay. Treatment with baicalein (10
μM) in combination with gemcitabine (2 μM) showed
more stronger ability of inhibiting cell migration compared with
gemcitabine alone (2 μM) (6.45±1.91% vs. 27.85±3.90%;
P<0.01). Similarly, combination of baicalein (10 μM) and
gemcitabine (10 μM) exhibited significantly stronger
suppressive effects on PANC-1 cells when compared with gemcitabine
alone (10 μM) (4.30±1.73% vs. 25.85±3.72%; P<0.05).
Baicalein (10 μM) plus docetaxel (10 nM) exhibited more
obvious inhibitory effects than docetaxel alone (10 nM) on
migration of PANC-1 cells (0.33±0.57% vs. 12.67±0.96%; P<0.01).
These results indicate that baicalein could enhance the capacity of
gemcitabine/docetaxel to inhibit migration of PANC-1 cells.
Baicalein induces apoptosis of PANC-1
cells and enhances the pro-apoptotic effects of docetaxel on PANC-1
cells
We further investigated whether baicalein-induced
suppression of cell growth of PANC-1 cells is associated with cell
apoptosis, we performed DAPI staining after treatment with drugs
for 48 h. As expected, treatment with baicalein at 10 μM
induced increased apoptosis in PANC-1 cells, compared with control
(1.922±0.551% vs. 0.377±0.132%, P<0.05; Fig. 6B). Then we further performed TEM to
confirm the above results. Consistently, apoptotic change in PANC-1
cells was found after treatment with baicalein alone at 50
μM (Fig. 6A). These data
suggest that baicalein treatment could induce apoptosis of PANC-1
cells in vitro. To further uncover the molecular mechanism
of apoptosis induced by baicalein, we performed western blot to
detect the expression level of caspase-3, cleaved-caspase-3, PARP
and cleaved-PARP, which are considered as classical
apoptosis-related molecules. The results showed that, after
treatment with baicalein, the expression level of caspase-3 was
significantly decreased, accompanied by increased expression of
cleaved-caspase-3. Immunoblotting for total PARP protein showed no
significant change after baicalein treatment, while protein level
of cleaved-PARP was markedly increased (Fig. 6E). Our data suggest that
apoptosis-related caspase-3/PARP signaling pathway might play an
important role in baicalein-induced apoptosis of PANC-1 cells.
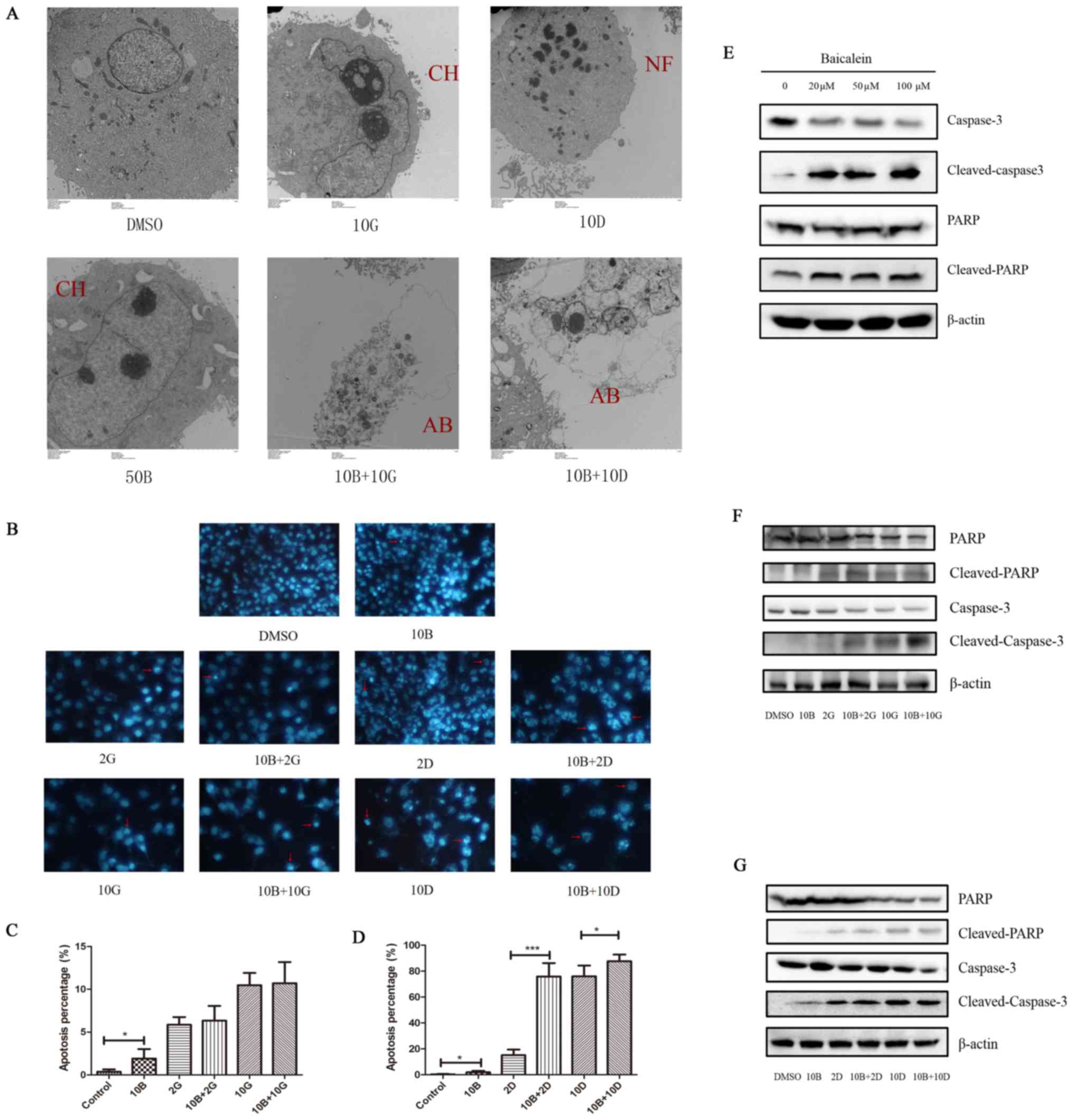 | Figure 6Effects of baicalein with gemcitabine
or docetaxel on apoptosis of PANC-1 cells. (A) Apoptotic changes
were observed using TEM in the indicated groups. AB, apoptotic
bodies; CH, chromatin condensation; NF, nucleus fragmentation. (B)
Apoptotic morphological change including nuclear condensation and
fragmentation observed by DAPI staining experiment. The red arrow
represents typical nuclear changes of apoptotic cells. (C and D)
Statistical analysis of percentage in cell apoptosis in different
treated groups (*P<0.05 and
***P<0.001). (E) Expression of caspase-3,
cleaved-caspase-3, PARP and cleaved-PARP detected by western
blotting. β-actin was used as internal control. (F) Immunoblot
analysis of caspase-3, cleaved-caspase-3, PARP and cleaved-PARP of
PANC-1 cells after treatment with solvent (DMSO), baicalein (10
μM) alone, gemcitabine (2 or 10 μM) alone and
combination treatment (10 μM baicalein + 2 μM
gemcitabine or 10 μM baicalein + 10 μM gemcitabine).
(G) Immunoblot analysis of caspase-3, cleaved-caspase-3, PARP and
cleaved-PARP of PANC-1 cells after treatment with solvent (DMSO),
baicalein (10 μM) alone, docetaxel (2 or 10 nM) alone and
combination treatment (10 μM baicalein + 2 nM docetaxel or
10 μM baicalein + 10 nM docetaxel). |
Next, we further investigated whether baicalein
could enhance pro-apoptotic effects of gemcitabine/docetaxel on
PANC-1 cells using DAPI staining. Interestingly, treatment with 10
μM baicalein in combination with 2 nM docetaxel resulted in
significantly increased apoptosis of PNCA-1 cells compared with 2
nM docetaxel alone (75.86±5.184% vs. 15.13±2.169%; P<0.001).
Similar results were observed at high concentrations of docetaxel.
Docetaxel at 10 nM triggered significant apoptosis of PNCA-1 cells
with the apoptosis rate of 76.07±9.61%, while 10 nM docetaxel
together with 10 μM baicalein induced more severe cell
apoptosis of 87.63±5.19% (P<0.05) (Fig. 6B and D). In addition, TEM showed
that apoptosis changes existed in all drug treated groups (Fig. 6A). These results demonstrate that
baicalein has a potential to enhance pro-apoptotic effects of
docetaxel on PANC-1 cells in vitro. However, treatment with
10 μM baicalein plus 2 μM gemcitabine did not induce
significant change in apoptosis rate of PANC-1 cells when compared
with 2 μM gemcitabine alone. Similarly, there was also no
significant difference in apoptosis of PNCA-1 cells treated with 10
μM baicalein plus 10 μM gemcitabine compared with 10
μM gemcitabine alone (Fig. 6B
and C). Moreover, caspase-3/PARP signaling pathway was
significantly activated when treated with baicalein in combination
with gemcitabine/docetaxel (Fig. 6F
and G). These data suggest that baicalein might exert different
effects on pro-apoptosis effect of different drugs and the
underlying mechanism might be complicated.
Discussion
As an enigmatic and aggressive malignancy with a
dismal prognosis, PC is the fourth leading cause of cancer-related
death in the United States. The current first-line chemotherapy
regimens, including gemcitabine and paclitaxel remain
unsatisfactory to OS of PC patients (18,19).
Gemcitabine and docetaxel have been widely used in PC, but the
median OS under gemcitabine therapy was only 5.65 months (18–20).
Therefore, an alternative approach to the treatment for PC is
urgently needed. Baicalein, a kind of low toxic natural compound,
has been reported to exert antitumor effects on many types of
cancer including PC. However, effects of combination therapy of
baicalein with gemcitabine/docetaxel on PC is unclear. In the
current study, we are the first to report that baicalein has
synergistic effects with gemcitabine/docetaxel on PANC-1, MIA
PaCa-2 and HPAF-II cells, suggesting that baicalein might be an
alternative choice in combination treatment for PC.
Although 10 μM baicalein alone exhibited mild
impact on the proliferation of PC cells, combination treatment of
baicalein with gemcitabine/docetaxel resulted in obvious
suppression of cell proliferation. Cell cycle arrest is one of the
most important cellular mechanisms leading to proliferation
inhibition of tumor cells. Our study showed that high dose of
baicalein alone (50 or 100 μM) could result in cell cycle
arrest of PC cells in S phase. This is in accordance with previous
studies in which baicalein alone could lead to G0/G1, G2/M and
S-phase arrest in various cancer cells (14,21–24).
In addition, combination treatment of baicalein with docetaxel
increased approximately 15% ratio of S-phase of PC cells. The cell
cycle change caused by baicalein gave further explanations to its
capacity to inhibit cell growth of PC cells.
Metastasis is another critical factor associated
with the poor OS in PC patients. Therefore, investigators have
tried new combination drug therapy regimens in order to prolong OS
of metastatic PC patients (5,6,25,26).
We conducted wound healing assay to evaluate migration ability of
PC cells in the presence of baicalein at different concentrations
or in combination treatment. The results not only revealed the
potential of baicalein at high concentrations to inhibit migration
of PC cells, but also indicated that it might be a potent adjuvant
benefiting the inhibitory ability of gemcitabine/docetaxel on
migration of PC cells. Therefore, baicalein might be a promising
drug to improve the outcome of current chemotherapy regimen for
metastatic PC.
It has been reported that baicalein can induce
apoptosis in many types of cancer (19,27–29).
Consistently, in the current study, PC cells treated with baicalein
suffered from obvious apoptotic change. This effect might be
dependent on caspase-3/PARP signaling pathway. Furthermore,
baicalein was found to strengthen the pro-apoptotic effects of
docetaxel on PC cells. Curiously, the synergistic effects of
baicalein with gemcitabine on PC cells seem to be independent on
caspase-3/PARP signaling pathway. Therefore, its underlying
mechanism needs to be further investigated.
In conclusion, our study demonstrate that baicalein
has synergistic effects with gemcitabine/docetaxel on the
proliferation, cell cycle, migration and apoptosis of PC cells.
These data suggest that combination treatment containing baicalein
might be a promising strategy to improve the outcome of clinical
treatment for PC.
Acknowledgments
We would like to thank Dr Panwen Wang (Mayo Clinics,
Rochester, MN, USA) for the revision of the manuscript. This study
was supported by National Natural Foundation of China (no.
81500151), Wuhan Program (no. WX15Z03 and A2011-13) and
Undergraduate Training Program for Innovation and Entrepreneurship
by Wuhan University (no. S2016861).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hidalgo M: Pancreatic cancer. N Engl J
Med. 362:1605–1617. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Humphris JL, Johns AL, Simpson SH, Cowley
MJ, Pajic M, Chang DK, Nagrial AM, Chin VT, Chantrill LA, Pinese M,
et al Australian Pancreatic Cancer Genome Initiative: Clinical and
pathologic features of familial pancreatic cancer. Cancer.
120:3669–3675. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hidalgo M: New insights into pancreatic
cancer biology. Ann Oncol. 23(Suppl 10): x135–x138. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Garrido-Laguna I and Hidalgo M: Pancreatic
cancer: From state-of-the-art treatments to promising novel
therapies. Nat Rev Clin Oncol. 12:319–334. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Von Hoff DD, Ervin T, Arena FP, Chiorean
EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et
al: Increased survival in pancreatic cancer with nab-paclitaxel
plus gemcitabine. N Engl J Med. 369:1691–1703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yassine F, Salibi E and Gali-Muhtasib H:
Overview of the formulations and analogs in the taxanes' story.
Curr Med Chem. 23:4540–4558. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mantripragada KC and Safran H: Optimizing
initial chemotherapy for metastatic pancreatic cancer. Future
Oncol. 12:1125–1133. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu H, Dong Y, Gao Y, Du Z, Wang Y, Cheng
P, Chen A and Huang H: The fascinating effects of baicalein on
cancer: A review. Int J Mol Sci. 17:172016. View Article : Google Scholar
|
|
10
|
Donald G, Hertzer K and Eibl G: Baicalein
- an intriguing therapeutic phytochemical in pancreatic cancer.
Curr Drug Targets. 13:1772–1776. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu JY, Tsai KW, Li YZ, Chang YS, Lai YC,
Laio YH, Wu JD and Liu YW: Anti-bladder-tumor effect of baicalein
from Scutellaria baicalensis Georgi and its application in vivo.
Evid Based Complement Alternat Med. 2013:5797512013.PubMed/NCBI
|
|
12
|
Taniguchi H, Yoshida T, Horinaka M, Yasuda
T, Goda AE, Konishi M, Wakada M, Kataoka K, Yoshikawa T and Sakai
T: Baicalein overcomes tumor necrosis factor-related
apoptosis-inducing ligand resistance via two different
cell-specific pathways in cancer cells but not in normal cells.
Cancer Res. 68:8918–8927. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang L, Ling Y, Chen Y, Li CL, Feng F, You
QD, Lu N and Guo QL: Flavonoid baicalein suppresses adhesion,
migration and invasion of MDA-MB-231 human breast cancer cells.
Cancer Lett. 297:42–48. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang CZ, Zhang CF, Chen L, Anderson S, Lu
F and Yuan CS: Colon cancer chemopreventive effects of baicalein,
an active enteric microbiome metabolite from baicalin. Int J Oncol.
47:1749–1758. 2015.PubMed/NCBI
|
|
15
|
Li-Weber M: New therapeutic aspects of
flavones: The anticancer properties of Scutellaria and its main
active constituents Wogonin, Baicalein and Baicalin. Cancer Treat
Rev. 35:57–68. 2009. View Article : Google Scholar
|
|
16
|
Lu QY, Zhang L, Moro A, Chen MC, Harris
DM, Eibl G and Go VL: Detection of baicalin metabolites baicalein
and oroxylina in mouse pancreas and pancreatic xenografts.
Pancreas. 41:571–576. 2012. View Article : Google Scholar :
|
|
17
|
Takahashi H, Chen MC, Pham H, Angst E,
King JC, Park J, Brovman EY, Ishiguro H, Harris DM, Reber HA, et
al: Baicalein, a component of Scutellaria baicalensis, induces
apoptosis by Mcl-1 down-regulation in human pancreatic cancer
cells. Biochim Biophys Acta. 1813:1465–1474. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Caparello C, Meijer LL, Garajova I,
Falcone A, Le Large TY, Funel N, Kazemier G, Peters GJ, Vasile E
and Giovannetti E: FOLFIRINOX and translational studies: Towards
personalized therapy in pancreatic cancer. World J Gastroenterol.
22:6987–7005. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Goel G and Sun W: Novel approaches in the
management of pancreatic ductal adenocarcinoma: Potential promises
for the future. J Hematol Oncol. 8:442015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ghosn M, Ibrahim T, Assi T, El Rassy E,
Kourie HR and Kattan J: Dilemma of first line regimens in
metastatic pancreatic adenocarcinoma. World J Gastroenterol.
22:10124–10130. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mu J, Liu T, Jiang L, Wu X, Cao Y, Li M,
Dong Q, Liu Y and Xu H: The traditional Chinese medicine baicalein
potently inhibits gastric cancer cells. J Cancer. 7:453–461. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zheng YH, Yin LH, Grahn TH, Ye AF, Zhao YR
and Zhang QY: Anticancer effects of baicalein on hepatocellular
carcinoma cells. Phytother Res. 28:1342–1348. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Seo MJ, Choi HS, Jeon HJ, Woo MS and Lee
BY: Baicalein inhibits lipid accumulation by regulating early
adipogenesis and m-TOR signaling. Food Chem Toxicol. 67:57–64.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang CZ, Calway TD, Wen XD, Smith J, Yu C,
Wang Y, Mehendale SR and Yuan CS: Hydrophobic flavonoids from
Scutellaria baicalensis induce colorectal cancer cell apoptosis
through a mitochondrial-mediated pathway. Int J Oncol.
42:1018–1026. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kosmidis C, Sapalidis K, Kotidis E,
Mixalopoulos N, Zarogoulidis P, Tsavlis D, Baka S, Man YG and
Kanellos J: Pancreatic cancer from bench to bedside: Molecular
pathways and treatment options. Ann Transl Med. 4:1652016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lemstrova R, Melichar B and
Mohelnikova-Duchonova B: Therapeutic potential of taxanes in the
treatment of metastatic pancreatic cancer. Cancer Chemother
Pharmacol. 78:1101–1111. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gong WY, Zhao ZX, Liu BJ, Lu LW and Dong
JC: Exploring the chemopreventive properties and perspectives of
baicalin and its aglycone baicalein in solid tumors. Eur J Med
Chem. 126:844–852. 2017. View Article : Google Scholar
|
|
28
|
Li J, Ma J, Wang KS, Mi C, Wang Z, Piao
LX, Xu GH, Li X, Lee JJ and Jin X: Baicalein inhibits TNF-α-induced
NF-κB activation and expression of NF-κB-regulated target gene
products. Oncol Rep. 36:2771–2776. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Choi EO, Park C, Hwang HJ, Hong SH, Kim
GY, Cho EJ, Kim WJ and Choi YH: Baicalein induces apoptosis via
ROS-dependent activation of caspases in human bladder cancer 5637
cells. Int J Oncol. 49:1009–1018. 2016. View Article : Google Scholar : PubMed/NCBI
|