Introduction
Ovarian tumors are common with >200,000 women
estimated to be diagnosed with ovarian cancer annually worldwide
(1). Three main types are
recognized according to the type of cell origin. Epithelial cancer
is the most common form seen in the vast majority of cases. Germ
cell tumors (GCTs) develop in the egg cells (oocytes) of ovary.
Stromal cell tumors, arise from cells involved in ovarian steroid
production (e.g., granulosa, theca and Leydig cells) (2).
Although non-epithelial ovarian tumors are uncommon,
they are histologically, genetically and clinically heterogeneous
causing major challenges for treatment and clinical workup. Ovarian
GCTs (OGCTs) typically affect young women (<30 years) (3). It is further subclassified based on
histology and clinical behavior as: mature teratoma which is
benign, and the malignant form immature teratomas and malignant
OGCTs (including dysgerminoma, yolk sac tumor, embryonal carcinoma,
choriocarcinoma and mixed GCT). Mature teratoma, which is the most
common among all OGCTs, may contain a variety of
well-differentiated tissues, such as hair, fat, teeth and bone. In
rare cases, complex organs such as brain matter, eyes, torso,
hands, feet and other limbs are formed in teratomas (4–7).
Immature teratomas, on the other hand, contain incompletely
differentiated tissues in addition to mature elements, and have
worse prognosis (8). Malignant
OGCTs are thought to arise from the pluripotent primordial germ
cells, which can differentiate into various histologies. While
dysgerminoma is composed of primitive undifferentiated germ cells,
the other malignant OGCTs (non-dysgerminomas) are categorized based
on the degree of differentiation from their precursor cells that
mimic embryonic and extra-embryonic tissues (3).
Patients with sex cord stromal tumor (SCST) can
display various hormone-mediated syndromes and a variety of
clinical features depending on their cellular origin. Tumors
derived from ovarian cells (such as granulosa and theca cells)
usually exhibit clinical features associated with excessive
production of estrogen, while those derived from Sertoli and Leydig
cells exhibit phenotypes resulting from elevated levels of
androgens. The prognosis of SCST is generally good, although a
minority of patients develop metastasis (9).
The molecular basis underlying the non-epithelial
ovarian tumors is still poorly understood. However, accumulating
evidence indicate that both OGCTs and SCSTs may reflect the
developmental changes of the germ and stromal cells in the ovary.
For OGCTs, it is clear that most of the malignant tumors express
pluripotency markers. They generally express high level of POU
class 5 homeobox 1 (POU5F1), v-KIT proto-oncogene receptor tyrosine
kinase (KIT), Lin-28 homolog A (LIN28A) and Nanog homeobox (NANOG)
(10,11). On the other hand, the benign tumors
(mature teratomas) have complex expression patterns due to the cell
composition of multiple cell types in different tumors (2).
For SCSTs, they express transcription factors that
play important roles in sex determination, e.g., granulosa cell
tumors express Forkhead box L2 (FOXL2) and Sertoli-Leydig cell
tumors express sex determining region Y box 9 (SOX9), which
controls ovary and testis determination, respectively. The majority
(97%) of ovarian adult granulosa cell tumors harbor somatic
FOXL2 mutations (12),
which promote granulosa cell survival (13) and tumorigenesis (14). On the other hand, somatic missense
mutations of Dicer 1 ribonuclease III (DICER1) are found in
60% of the ovarian Sertoli-Leydig cell tumors (15). DICER1 plays an important role in
microRNA (miRNA) processing. Its mutations in SCSTs cause selective
processing of 3′-strand miRNAs (15). However, how these mutations result
in sex cell fate decision or tumorigenesis remain unknown.
miRNAs are single-stranded RNAs of ~22 nucleotides
in length, which regulate gene expression in many biological
processes, including cell growth, differentiation and tumorigenesis
(16). Distinct miRNA expression
profiles have been identified in a variety of tumor types
associated with histological subtypes and patient outcome,
suggesting their potential use as biomarkers for diagnosis and
prognosis (17). miRNA expression
profiles have been investigated in some ovarian germ cell tumors.
However, in all studies, the OGCTs were combined with their
testicular counterparts for analysis (18–21).
One of the reasons may due to the rarity of these tumor types and
very few samples were analyzed. Although DICER1 is
frequently mutated in SCSTs (15),
miRNA expression profiles of this tumor type and how their
expression profiles relate to OGCTs are yet to be investigated.
In this study, we characterized miRNA profiles of
OGCTs and SCSTs using small RNA (sRNA) sequencing, and compared the
expression differences between malignant and benign OGCTs, as well
as between SCSTs and OGCTs. We identified miRNA expression patterns
associated with different tumor types, suggesting their potential
use as a tool for defining their histological and biological
differences.
Materials and methods
Clinical samples
This study included 23 frozen non-epithelial ovarian
tumors, consisting of 16 ovarian germ cell tumors (OGCT1-16) and 7
sex cord stromal tumors (SCST1-7). The age at diagnosis and the
histological type of each tumor are given in Table I. All tumors were provided by the
Cooperative Human Tissue Network, which is funded by the National
Cancer Institute, USA. Other investigators may have received
specimens from the same subjects. The study was approved by the
Stanford Human Subjects Review Committee. All clinical samples were
de-identified, therefore no written informed consent was
required.
 | Table INon-epithelial ovarian tumors
included in the study. |
Table I
Non-epithelial ovarian tumors
included in the study.
| Sample no. | Age at diagnosis
(year) | Tumor
classification
| Analysed by
|
|---|
| Diagnosis |
Subclassification | Type | sRNA seq | RT-qPCR |
|---|
| OGCT1 | 19 | OGCT | Dysgerminoma | Malignant | – | RT-qPCR |
| OGCT2 | 21 | OGCT | Dysgerminoma | Malignant | – | RT-qPCR |
| OGCT3 | 41 | OGCT | Dysgerminoma | Malignant | – | RT-qPCR |
| OGCT4 | 73 | OGCT | Dysgerminoma | Malignant | sRNA seq | RT-qPCR |
| OGCT5 | 24 | OGCT | Yolk sac tumor | Malignant | – | RT-qPCR |
| OGCT6 | 17 | OGCT | Yolk sac tumor | Malignant | – | RT-qPCR |
| OGCT7 | 28 | OGCT | Primitive germ cell
tumor | Malignant | sRNA seq | RT-qPCR |
| OGCT8 | 26 | OGCT | Mature
teratoma | Benign | sRNA seq | RT-qPCR |
| OGCT9 | 64 | OGCT | Mature
teratoma | Benign | sRNA seq | RT-qPCR |
| OGCT10 | 47 | OGCT | Mature
teratoma | Benign | sRNA seq | RT-qPCR |
| OGCT11 | 58 | OGCT | Mature
teratoma | Benign | – | RT-qPCR |
| OGCT12 | 77 | OGCT | Mature
teratoma | Benign | sRNA seq | RT-qPCR |
| OGCT13 | 47 | OGCT | Mature
teratoma | Benign | sRNA seq | RT-qPCR |
| OGCT14 | 51 | OGCT | Mature
teratoma | Benign | sRNA seq | RT-qPCR |
| OGCT15 | 54 | OGCT | Mature
teratoma | Benign | sRNA seq | RT-qPCR |
| OGCT16 | 18 | OGCT | Mature
teratoma | Benign | – | RT-qPCR |
| SCST1 | 84 | SCST | Signet ring-stromal
tumor | Benign | sRNA seq | RT-qPCR |
| SCST2 | 45 | SCST | Fibrothecoma | Benign | sRNA seq | RT-qPCR |
| SCST3 | 46 | SCST | Sex cord stromal
tumor | Malignant | sRNA seq | RT-qPCR |
| SCST4 | 15 | SCST | Granulosa cell
tumor | Malignant | – | RT-qPCR |
| SCST5 | 48 | SCST | Granulosa cell
tumor | Malignant | – | RT-qPCR |
| SCST6 | 15 | SCST | Sertoli leydig cell
tumor | Malignant | – | RT-qPCR |
| SCST7 | 20 | SCST | Granulosa cell
tumor | Malignant | – | RT-qPCR |
RNA isolation and quantification
mirVana miRNA isolation kit (Thermo Fisher
Scientific, Waltham, MA, USA) was used to extract sRNA and total
RNA for cloning and reverse transcription-quantitative polymerase
chain reaction (RT-qPCR) analysis, respectively. RNA concentrations
were determined by the NanoDrop ND-1000 spectrophotometer (NanoDrop
Technologies, Wilmington, DE, USA).
sRNA library construction and
sequencing
sRNA cloning was performed on 9 OGCTs (7 benign and
2 malignant) and 3 SCSTs, using previously described methodologies
(22). Briefly, sRNAs were ligated
with adenylated 3′-adaptor and purified on 12% denaturing
polyacrylamide gel. Second ligation reaction was performed with
5′-adaptor, followed by gel purification. Complementary DNA (cDNA)
synthesis was performed using the reverse transcription enzyme
SuperScript II (Thermo Fisher Scientific) together with the reverse
transcription primer. cDNA was then PCR amplified for 16–20 cycles
with forward and reverse primers. sRNA-sequencing libraries were
sequenced by Solexa/Illumina sequencing platform (Illumina 1G
Genome Analyzer; Illumina Inc., San Diego, CA, USA). The sequence
of the adaptors and primers has been described previously (22). The sequencing data are available at
Gene Expression Omnibus (accession no. GSE98536).
miRNA analysis
sRNA sequencing reads from each of the pooled
libraries were separated based on their barcode sequence. Adaptor
sequences were trimmed and filtered using the package
Fastx-toolkit. The resulting dataset was aligned to miRBase
database version 21 (http://www.mirbase.org/) for mature miRNA using Bowtie
short read aligner version 1.1.1 (23) with settings -f -n 0 -l 15 -k 200 -S
-best -chunkmbs 128. All aligned reads were sorted and indexed by
SAMtools version 1.1 (24). For
miRNA quantification, the script HTSeq-count of the HTSeq version
0.6.1 was used (25). For
clustering analysis, the miRNA counts were normalized by TMM
(trimmed mean method) (26) and
the normalized expression values were clustered based on Euclidean
distance. For identification of differentially expressed miRNAs
between two groups, the miRNA counts were normalized and analyzed
by DESeq2 using default settings (27).
TaqMan RT-qPCR
RT-qPCR was performed to quantify the expression
levels of miR-373-3p, miR-372-3p, miR-302c-3p, miR-199a-5p,
miR-125a-5p, miR-21, miR-34a, miR-202-5p, miR-513c-5p, miR-193a-3p,
miR-214-5p and let-7f, using TaqMan miRNA assays (Thermo
Fisher Scientific). Approximately 120 ng of total RNA was reversed
transcribed using the High-Capacity cDNA Reverse Transcription kit
(Thermo Fisher Scientific). The synthesized cDNA was then diluted
2- to 10-fold prior to RT-qPCR with TaqMan Universal PCR Master Mix
without AmpErase UNG (Thermo Fisher Scientific). The amplification
was performed using the Applied Biosystems 7500 Fast Real-time PCR
system (Thermo Fisher Scientific) with the following conditions: an
initial denaturation at 95°C for 10 min, 40 step cycles of
denaturing at 95°C for 15 sec and annealing at 60°C for 60 sec.
Each reaction was performed in triplicate. The average Ct-value of
each analyzed miRNA was normalized to RNU6B, and reported as
2−ΔCT. RNU6B was chosen as an endogenous control
due to its stability in the samples analyzed (standard deviation of
average Ct = 1.8; data not shown).
Western blotting
Western blotting was performed on 7 SCSTs, 9 benign
and 7 malignant OGCTs. Total protein lysates were prepared using
NP-40 lysis buffer (Thermo Fisher Scientific) with addition of 1 mM
phenylmethanesulfonyl (Sigma-Aldrich, St. Louis, MQ, USA) and
protease inhibitor (complete protease inhibitor cocktail;
Sigma-Aldrich). The concentrations were measured using the Pierce
BCA Protein assay kit (Thermo Fisher Scientific). Protein lysates
of 30 μg were separated in NuPAGE Novex 4–12% Bis-Tris gel
and transferred to nitrocellulose membranes. The membranes were
blocked with 5% skim milk diluted in Tris-buffered saline/0.05%
Tween-20 prior to incubation with anti-BECN1 rabbit polyclonal
(Novus Biologicals; dilution 1:1,000) or anti-GAPDH rabbit
monoclonal (Cell Signaling Technology; dilution at 1:1,000)
antibodies overnight at 4°C. Anti-rabbit IgG-HRP (Cell Signaling
Technology; dilution at 1:2,000) was used as a secondary antibody.
Signals were detected using the Novex ECL HRP chemiluminescent
substrate reagent (Thermo Fisher Scientific) and LI-COR Odyssey Fc
Imaging system (LI-COR Biosciences, Lincoln, NE, USA) and
quantified using Image Studio Lite version 5.2 (LI-COR
Biosciences).
Statistical analysis
All statistical tests were performed using
Statistical Package for the Social Sciences (SPSS) version 24 (IBM,
Armonk, NY, USA), unless specified otherwise. Kruskal-Wallis test
was used for comparisons among three sample groups, and
Mann-Whitney U test was used for two groups. A difference of
P<0.05 was considered to indicate a statistically significant
difference.
Results
miRNA expression profiles in
non-epithelial ovarian tumors by sRNA sequencing
To assess the miRNA profiles of non-epithelial
ovarian tumors, we performed sRNA sequencing of 9 OGCTs and 3
SCSTs. In total, we obtained 2,949,836 reads for OGCTs (benign,
919,886; malignant, 2,029,950) and 1,198,120 reads for SCSTs. On
average, 213,678 reads from the malignant OGCT libraries, 259,915
reads from the benign OGCT libraries and 262,386 reads from the
SCST libraries were mapped to mature miRNAs from miRBase release
21.
To determine whether miRNA expression profiles were
distinct among the three non-epithelial ovarian tumor groups, we
performed unsupervised clustering of the samples and miRNA
expression based on Euclidean distance. As shown in the dendrogram
in Fig. 1A, the benign OGCTs and
SCSTs were closely related but separated from the two malignant
OGCTs, suggesting distinct miRNA expression patterns in malignant
OGCTs and more similar miRNA expression profiles between benign
OGCTs and SCSTs.
To illustrate whether specific miRNA expression
signatures were distinct among these tumor groups, we performed
hierarchical clustering of the miRNA expressions (Fig. 1B). Notably, several miRNAs were
apparently unique to specific tumor types (Fig. 1C–E). For malignant OGCTs, several
miR-548 family, miR-302 and miR-371~373
clusters were more abundant, while several let-7 family
members were lower than the benign OGCTs and SCSTs (Fig. 1C). For benign OGCTs, the miRNA
expression patterns were not homogenous among the same tumor type
(Fig. 1D). However,
miR-193b-5p/3p, miR-320a/b and miR-22-5p were
frequently more abundant in the benign OGCTs as compared to the
malignant OGCTs and SCSTs. Similarly, we also observed
heterogeneous miRNA expression profiles among the three SCSTs.
However, miR-202 and miR-506~514 cluster were higher
in at least two SCSTs and absent or lower expression in the
malignant and benign OGCTs (Fig.
1E).
Differentially expressed miRNAs among the
three tumor types
We applied DESeq2 analysis to identify
differentially expressed miRNAs between malignant and benign OGCTs.
The analysis identified 128 and 59 miRNAs with higher and lower
expression respectively, in the malignant OGCTs compared to the
benign OGCTs (false discovery rate <0.5; data not shown).
Notably, miR-302~367 and miR-371~373 clusters were
among the differentially expressed miRNAs that had higher
expression in the malignant tumors compared to the benign tumors.
Additionally, expression of several let-7 family members was
lower in the malignant than the benign OGCTs.
For comparison between malignant OGCTs and SCSTs, we
identified 120 miRNAs with higher expression and 154 miRNAs with
lower expression in the malignant OGCTs compared to SCSTs (data not
shown). Among the differentially expressed miRNAs, lower expression
of miR-202-5p, miR-506~514 cluster and let-7 family,
as well as higher expression of miR-302 and
miR-371~373 cluster in the malignant OGCTs were also noted
in the clustering data (Fig. 1C and
E).
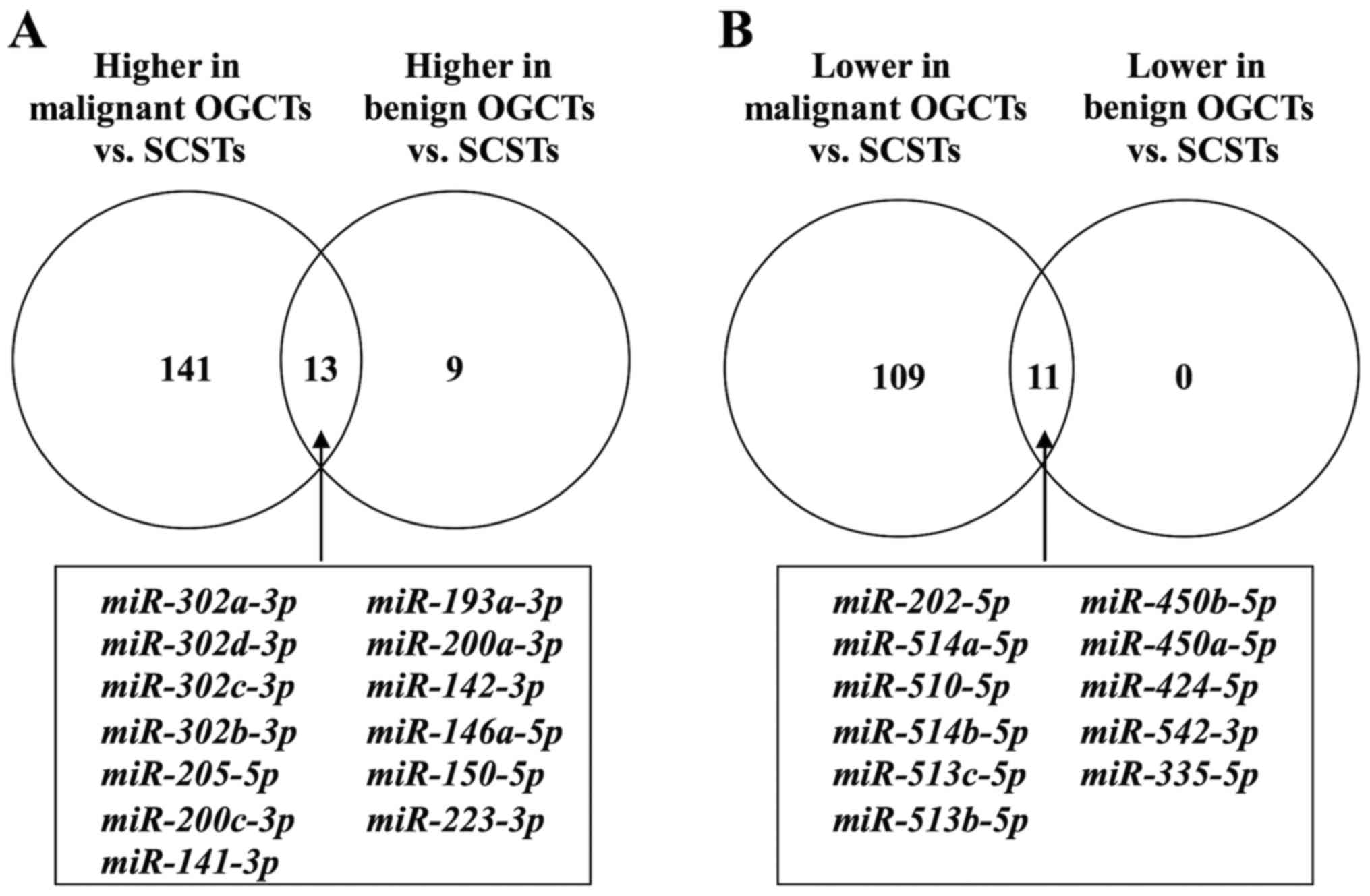
Strikingly, the number of differentially expressed
miRNAs between SCSTs and benign OGCTs was much fewer, i.e., 11
miRNAs had lower expression and 22 had higher expression in the
benign OGCTs than the SCSTs (data not shown). Notably, all 11
miRNAs with lower expression and 13 out of the 22 miRNAs with
higher expression in the benign OGCTs were overlapped in both
comparisons between SCSTs and malignant or benign OGCTs (Fig. 2), suggesting that these miRNAs may
be specific for SCSTs. Comparing with SCSTs, 141 miRNAs with higher
expression and 109 with lower expression were unique for malignant
OGCTs, while only 9 miRNAs with higher expression were unique for
benign OGCTs (Fig. 2 and Table II).
 | Table IIUnique differentially expressed
miRNAs in malignant and benign OGCT as compared to SCST. |
Table II
Unique differentially expressed
miRNAs in malignant and benign OGCT as compared to SCST.
| Differentially
expressed miRNAs compared to SCST |
|---|
| Higher in malignant
OGCT |
|
miR-373-3p |
miR-376b-3p |
miR-519e-5p |
miR-520c-3p |
miR-155-5p |
miR-148a-3p |
|
miR-372-3p |
miR-9-5p |
miR-654-3p |
miR-758-3p |
miR-544a |
miR-501-5p |
|
miR-182-5p |
miR-377-3p |
miR-18a-3p |
miR-1296-5p |
miR-190a-5p |
miR-185-5p |
|
miR-183-5p |
miR-363-3p |
miR-381-5p |
miR-519c-3p |
miR-505-3p |
miR-15b-5p |
|
miR-767-3p |
miR-629-3p |
miR-183-3p | miR-543 |
miR-93-3p |
miR-433-3p |
|
miR-371a-3p |
miR-18a-5p |
miR-361-3p |
miR-494-3p |
miR-149-5p |
miR-15a-5p |
|
miR-302b-5p |
miR-3180-3p |
miR-545-3p |
miR-146b-5p |
miR-769-5p |
miR-339-3p |
|
miR-7-5p |
miR-655-3p |
miR-345-5p |
miR-590-5p |
miR-20a-5p |
miR-340-3p |
|
miR-3529-3p |
miR-18b-5p |
miR-1277-3p |
miR-409-3p |
miR-92a-1-5p |
miR-93-5p |
|
miR-96-5p |
miR-373-5p |
miR-1248 |
miR-154-5p |
miR-134-5p |
miR-378a-5p |
|
miR-767-5p |
miR-136-5p |
miR-652-5p |
miR-625-3p |
miR-487b-3p |
miR-181d-5p |
|
miR-105-5p |
miR-5585-3p |
miR-210-3p |
miR-31-3p |
miR-30e-5p |
miR-505-5p |
|
miR-367-3p |
miR-493-3p |
miR-3679-5p |
miR-512-3p |
miR-16-2-3p |
miR-493-5p |
|
miR-135b-5p |
miR-372-5p |
miR-299-3p |
miR-377-5p |
miR-876-5p |
miR-130b-3p |
|
miR-380-3p |
miR-20b-5p |
miR-339-5p |
miR-324-5p |
miR-651-5p |
miR-92b-3p |
|
miR-154-3p |
miR-376a-5p |
miR-548b-5p |
miR-30d-5p |
miR-19a-3p |
miR-16-5p |
|
miR-539-3p |
miR-515-5p |
miR-382-5p | miR-421 |
miR-127-5p |
miR-301a-3p |
|
miR-381-3p |
miR-548d-5p |
miR-379-3p |
miR-519b-3p |
miR-342-3p |
miR-374a-5p |
|
miR-425-5p |
miR-329-3p |
miR-517a-3p |
miR-92b-5p |
miR-485-5p |
miR-34a-5p |
|
miR-495-3p |
miR-181c-5p |
miR-517b-3p |
miR-187-3p |
miR-423-3p |
miR-21-5p |
|
miR-302a-5p |
miR-302d-5p |
miR-103a-3p |
miR-519a-3p |
miR-550a-3p |
miR-19b-3p |
|
miR-485-3p |
miR-548y |
miR-103b |
miR-337-3p |
miR-550b-2-5p | |
|
miR-9-3p | miR-484 | miR-107 |
miR-454-3p |
miR-3065-5p | |
|
miR-409-5p |
miR-487a-3p |
miR-1323 | miR-941 |
miR-127-3p | |
| Lower in malignant
OGCT |
| miR-206 |
let-7b-5p |
miR-199a-3p |
miR-195-3p |
miR-200b-3p |
miR-502-3p |
|
miR-509-3p |
let-7c-5p |
miR-199b-3p |
miR-30c-1-3p |
miR-615-3p |
miR-125a-5p |
|
miR-508-3p |
let-7a-5p |
miR-1294 |
let-7f-5p |
miR-199b-5p |
miR-30a-5p |
|
miR-30c-2-3p |
let-7d-3p |
miR-98-3p |
miR-1299 |
miR-1270 |
miR-145-3p |
|
miR-542-5p |
miR-574-3p |
miR-508-5p |
miR-135a-5p |
miR-1180-3p |
miR-193a-5p |
|
miR-204-5p |
let-7f-2-3p |
miR-214-5p |
miR-664a-3p |
miR-744-5p |
miR-99b-5p |
|
miR-211-5p |
miR-874-5p |
miR-125b-1-3p |
miR-1271-5p |
miR-365a-3p |
miR-660-5p |
|
miR-1269a |
miR-143-3p |
miR-99a-5p |
miR-10b-5p |
miR-365b-3p |
miR-532-5p |
|
miR-450a-2-3p |
miR-133a-5p |
miR-30b-3p |
miR-95-3p |
miR-331-5p |
miR-29b-2-5p |
|
miR-506-5p |
let-7b-3p |
miR-196a-5p |
miR-196b-5p |
miR-140-5p |
miR-181a-5p |
|
miR-483-5p |
miR-214-3p |
miR-30a-3p |
miR-574-5p |
miR-26a-5p |
miR-29a-3p |
|
let-7c-3p |
miR-675-5p |
miR-100-5p |
miR-23a-3p |
let-7d-5p |
miR-29c-3p |
|
miR-133a-3p | miR-1 |
miR-509-3-5p |
miR-23b-3p |
miR-125a-3p |
miR-30b-5p |
| miR-375 |
miR-193b-5p |
miR-30e-3p |
miR-122-5p |
miR-191-5p |
miR-186-5p |
|
miR-133b |
miR-125b-5p |
miR-98-5p |
miR-664a-5p | miR-184 | |
|
miR-135a-3p |
miR-3609 |
miR-199a-5p |
miR-539-5p |
miR-221-5p | |
|
let-7e-5p |
miR-99a-3p |
miR-532-3p |
miR-26a-2-3p |
miR-423-5p | |
|
miR-4500 |
let-7f-1-3p |
miR-30d-3p |
miR-32-3p |
miR-3184-3p | |
|
miR-450a-1-3p |
let-7e-3p |
miR-708-3p |
miR-28-3p |
miR-1306-5p | |
| Higher in benign
OGCT |
|
miR-1247-5p |
miR-203a |
miR-338-3p |
miR-145-5p |
miR-10a-5p |
miR-22-3p |
|
miR-199b-5p |
miR-320a |
miR-320b | | | |
Validation of differentially expressed
miRNAs by RT-qPCR
To validate the sRNA sequencing findings, we
selected 12 miRNAs (i.e., miR-373-3p, miR-372-3p, miR-302c-3p,
miR-199a-5p, miR-125a-5p, miR-21, miR-34a, miR-202-5p, miR-513c-5p,
miR-193a-3p, miR-214-5p and let-7f) for RT-qPCR in an
extended cohort of samples, which consisted of 16 OGCTs (9 benign
and 7 malignant) and 7 SCSTs. These miRNAs were selected from
different comparisons, and because of their involvement in
testicular GCTs (miR-373-3p, miR-372-3p,
miR-302c-3p, miR-21, miR-513c-5p, miR-199a-5p,
miR-214-5p and let-7f) (11,19,20,28–32)
or other tumor types (miR-125a-5p, miR-34a, miR-193a-3p and
miR-202-5p) (33–35).
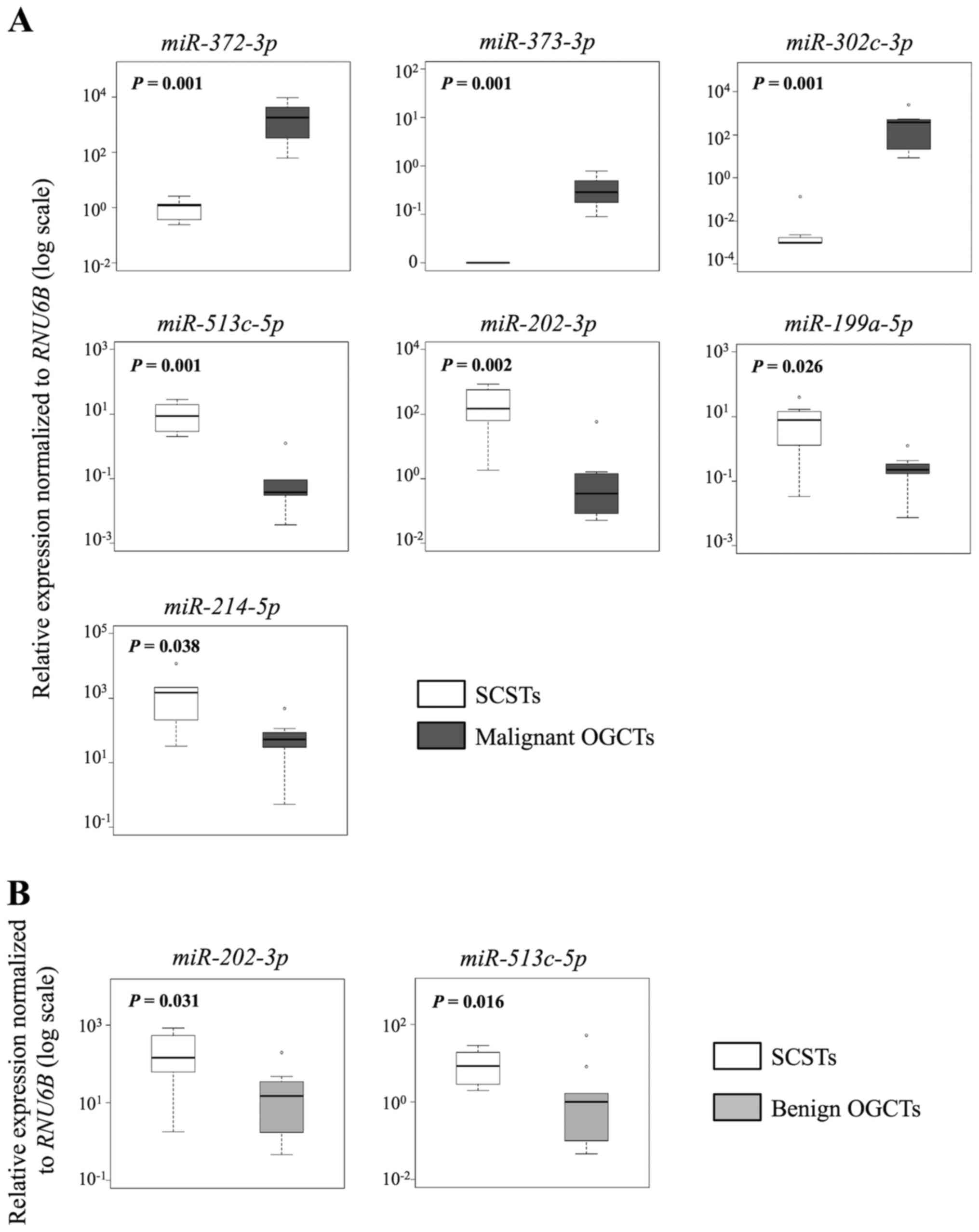
In the comparison between malignant and benign
OGCTs, 8 of the 12 miRNAs were concordant between the sRNA
sequencing and the RT-qPCR results (Table III and Fig. 3). miR-373-3p,
miR-372-3p and miR-302c-3p had higher expression,
whereas miR-199a-5p, miR-214-5p and miR-202-3p
had lower expression in the malignant OGCTs compared to the benign
OGCTs (P<0.05; Mann-Whitney U test). miR-21 and
miR-125a-5p were not differentially expressed between the
two groups using both sRNA sequencing and RT-qPCR.
 | Table IIIEvaluation of 12 selected miRNAs
using sRNA sequencing and RT-qPCR methods. |
Table III
Evaluation of 12 selected miRNAs
using sRNA sequencing and RT-qPCR methods.
| miRNA | Malignant vs.
benign OGCTsa
| Malignant OGCTs vs.
SCSTsb
| Benign OGCTs vs.
SCSTsb
|
|---|
| sRNA seq | RT-qPCR | Concordance | sRNA seq | RT-qPCR | Concordance | sRNA seq | RT-qPCR | Concordance |
|---|
|
miR-372-3p | Higher | Higher | Yes | Higher | Higher | Yes | Equal | Equal | Yes |
|
miR-373-3p | Higher | Higher | Yes | Higher | Higher | Yes | Equal | Equal | Yes |
|
miR-302c-3p | Higher | Higher | Yes | Higher | Higher | Yes | Higher | Equal | No |
|
miR-199a-5p | Lower | Lower | Yes | Lower | Lower | Yes | Equal | Equal | Yes |
|
miR-214-5p | Lower | Lower | Yes | Lower | Lower | Yes | Equal | Equal | Yes |
|
miR-202-3p | Lower | Lower | Yes | Lower | Lower | Yes | Lower | Lower | Yes |
|
miR-513c-5p | Equal | Lower | No | Lower | Lower | Yes | Lower | Lower | Yes |
| let-7f | Lower | Equal | No | Lower | Equal | No | Equal | Equal | Yes |
| miR-21 | Equal | Equal | Yes | Higher | Equal | No | Equal | Equal | Yes |
| miR-34a | Higher | Equal | No | Higher | Equal | No | Equal | Equal | Yes |
|
miR-125a-5p | Equal | Equal | Yes | Lower | Equal | No | Equal | Equal | Yes |
|
miR-193a-3p | Higher | Equal | No | Higher | Equal | No | Higher | Equal | No |
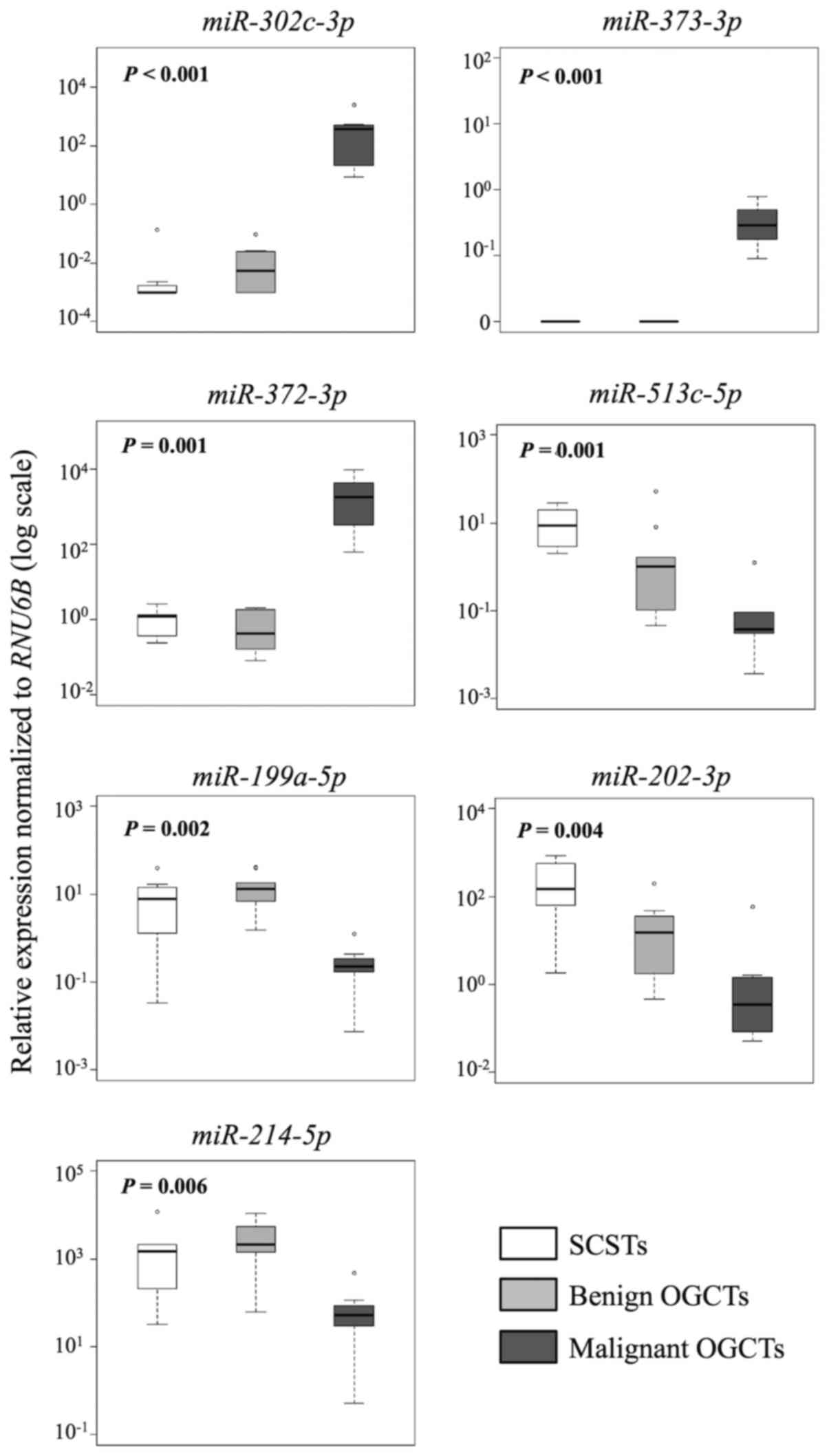
Similarly, 7 and 10 out of the 12 miRNAs were
concordant in both methods for the comparisons between SCSTs and
malignant or benign OGCTs, respectively (Table III and Figs. 4 and 5). Three miRNAs (miR-372-3p,
miR-373-3p and miR-302c-3p) were higher and four
miRNAs (miR-513c-5p, miR-202-3p, miR-199a-5p
and miR-214-5p) were lower in the malignant OGCTs compared
to the SCSTs (Fig. 4A), while only
miR-202c-3p and miR-513c-5p were lower in the benign
OGCTs than the SCSTs (Fig. 4B).
Eight miRNAs (miR-372-3p, miR-373-3p,
miR-199a-5p, miR-214-5p, let-7f, miR-21, miR-34a and
miR-125a-5p) were not differentially expressed between
benign OGCTs and SCSTs using both methods. Comparing the three
groups, 7 miRNAs (miR-302c-3p, miR-373-3p,
miR-372-3p, miR-513c-5p, miR-199a-5p,
miR-202-3p and miR-214-5p) were significant using
Kruskal-Wallis test (P<0.01; Fig.
5), suggesting their diagnostic potential in ovarian
non-epithelial tumors.
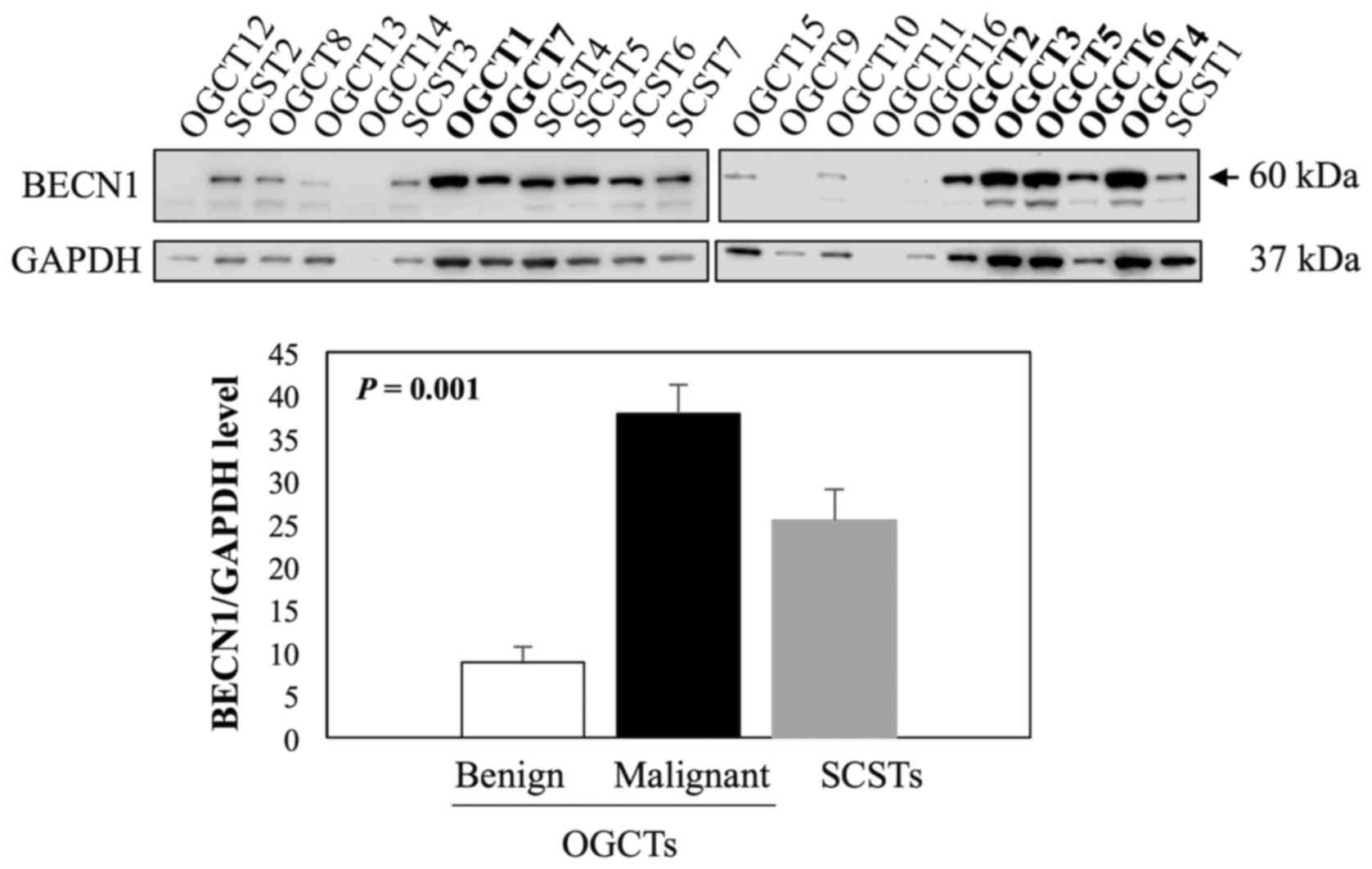
Evaluation of BECN1 expression in
non-epithelial ovarian tumors
Beclin 1 (BECN1) is one of the known direct
targets of miR-199a-5p (36,37)
and it plays an important role in germ cell survival and
proliferation (38,39). We therefore examined BECN1
expression in 16 OGCTs (7 malignant and 9 benign) and 7 SCSTs using
western blot analysis. The results showed that BECN1 was the most
abundant in all malignant OGCTs analyzed, moderate to high levels
in SCSTs, and very low or undetectable levels in benign OGCTs
(Fig. 6). For quantification, we
excluded two samples, OGCT14 and OGCT11, due to low concentrations
of the protein lysates. BECN1 expression was significantly
different among the three groups (Kruskal-Wallis test, P<0.001),
and the significant difference was observed in the comparison
between malignant and benign OGCTs.
Discussion
miRNAs play important roles in gene regulation of
many cellular processes that contribute to cancer development and
progression. Despite miRNA expression and function have been
characterized in a broad range of tumor types, miRNA profiles of
non-epithelial ovarian tumors are still scarce. In this study, we
characterized miRNA expression pattern of OGCTs and SCSTs using
sRNA sequencing. Our analysis of miRNA profiles indicated
similarities and differences of expression level in these tumor
types.
We observed higher expression of miR-302 and
miR-371~373 cluster in malignant OGCTs, which is consistent
with previous studies (18–21,40).
Although the clinical significance of these miRNAs in OGCT has not
been investigated, these miRNAs appear as potential serum
biomarkers for diagnosis and follow-up of malignant testicular GCTs
(TGCTs) (29,30,41,42).
Importantly, miR-372 and miR-373 have been
demonstrated as oncogenes in TGCTs (28), and miR-302 can regulate
stemness, differentiation and tumorigenesis (43–47).
Similarly, lower expressions of miR-199a and
miR-214 were also observed in malignant TGCTs (32,48).
miR-199a-2 (one of the two loci encoding miR-199a)
and miR-214 are derived from the same cluster located on
chromosome 1q24.3, and their expressions are regulated by the same
promoter and transcription factor (49). Previous studies have shown that the
promoter of this locus is frequently hypermethylated in malignant
testicular tumors leading to decreased expressions of
miR-199a and miR-214 (48,50,51).
miR-199a-5p has been reported to act as a tumor suppressor
in TGCTs by suppressing cell proliferation, migration, invasion and
metastasis, possibly through its targets podocalyxin-like
(PODXL) and MAF BZIP transcription factor B (MAFB)
(48,51). Although miR-214 has been
demonstrated to directly regulate proteasome 26S subunit,
non-ATPase 10 (PSMD10) in TGCT (32), its functional role remains
undetermined. Contrary to their reduced expression in OGCTs,
increased expression of miR-199a and miR-214 is found
in epithelial ovarian cancer (52). miR-199a has been shown to
regulate nuclear factor κB (NF-κB) activity via targeting inhibitor
of nuclear factor κB kinase subunit beta (IKKβ) (53), while miR-214 can induce cell
survival and cisplatin resistance by targeting phosphatase and
tensin homolog (PTEN) in ovarian carcinoma cells (52,54).
Comparing with both malignant and benign OGCTs, two
miRNAs (miR-202-3p and miR-513c-5p) are significantly
higher in SCSTs. Expression of miR-202-3p is detected
specifically in gonads, and predominantly in the granulosa
(55) and Sertoli cells (56), supporting that this miRNA is
specific for SCSTs. Additionally, miR-202-3p is tightly
linked to sex hormone secretion and sex differentiation (57–59),
suggesting its important role in gonadal development and
differentiation. Indeed, miR-202-3p prevents spermatogonial
stem cell differentiation by suppressing multiple cell cycle
regulators and RNA binding proteins (60). miR-202-3p expression is also
correlated with the expression level of testis-associated (e.g.,
SOX9) and ovary-associated genes (e.g., FOXL2) (57), in which both SOX9 and FOXL2 are
commonly expressed in SCSTs. FOXL2 is a transcription factor
required for granu-losa cell differentiation and ovary development
(61), and its somatic mutation
has been linked to the development of adult granulosa cell tumors
of the ovary (12). The mutation
can protect granulosa cells from apoptosis (13) and promote tumorigenesis through
enhanced glycogen synthase kinase 3β (GSK3β)-mediated S33
phosphorylation (14). Further
studies are yet to be conducted to determine the expression
relationship between miR-202-3p and FOXL2 and the functional
role of miR-202-3p in the development of SCST.
miR-513c-5p is one of the miR-513
subfamily that belongs to the miR-506~514 cluster. Although
hardly anything is known about the functional role of
miR-513c-5p, the miRNA cluster has been demonstrated as an
oncogene or a tumor suppressor depending on cellular context. In
melanoma, this cluster can promote melanocyte transformation and
melanoma growth (62). On the
other hand, it has been shown to suppress cell growth and induce
senescence in ovarian carcinoma (63), and inhibit NF-κB pathway in TGCT
(31). Notably, the
miR-506~514 cluster can be induced by Forkhead box protein
O1 (FOXO1) (64). FOXO1 is a
transcription factor that plays important roles in regulation of
apoptosis, cell cycle progression, insulin signaling and metabolic
homeostasis in response to oxidative stress (65). FOXO1 is expressed in granulosa
cells of growing follicles (66),
and is critical in granulosa cell fate decisions and follicle
growth (67,68). Importantly, Liu et al,
showed that inactivation of the two FOX proteins FOXO1 and FOXO3
expression in mouse granulosa cells can promote ovarian granulosa
cell tumor development, and coordinate PTEN depletion
enhances the granulosa cell tumor occurrence in FOXO1/3
depletion mice (69). Together, it
is tempting to speculate that miR-506~514 cluster may
contribute to the development of SCST (in particular the granulosa
cell tumor subtype) through FOXO1/PTEN pathways.
We also observed increased expression of BECN1, a
target of miR-199a-5p, in malignant OGCTs, suggesting its
importance in ovarian germ cell maintenance and/or tumorigenesis.
In line with such speculation, BECN1 is required to control germ
cell proliferation/survival (38,39)
and cell cycle progression (38,70).
BECN1 is one of the key regulators of autophagy, an evolutionally
conserved regulatory pathway of cellular degradation and recycling
(71). Autophagy has been shown to
play important roles in germ cell function in multiple organisms,
including mouse (72), fish
(73), worm (74,75),
moss (76) and sea urchin
(77). Although the role of BECN1
or autophagy in human germ cell tumorigenesis remains unclear,
Rossi et al demonstrated that mitogen-activated protein
kinase 15 (MAPK15)-mediated autophagy can promote cell
proliferation and prevent DNA damage accumulation in TGCTs
(78). On the other hand, BECN1
expression is lower in malignant epithelial ovarian cancers than
their non-malignant counterparts (79), and induction of autophagy in
ovarian carcinoma cells can lead to tumor suppression (80). Together, autophagy may function as
a tumor suppressor in ovarian epithelial tumors and an oncogene in
OGCTs.
In conclusion, we showed that different
non-epithelial ovarian tumors have distinct miRNA expression
pattern, implying their role in tumorigenesis and their potential
values as diagnostic markers. Our data also provide the starting
points to elucidate the molecular mechanisms underlying the
pathogenesis of these tumor types.
Acknowledgments
We thank the Cooperative Human Tissue Network for
frozen tissue samples, the Stanford Sequencing Facility for sRNA
sequencing and Dr Andrew Fire and the members of the sRNA group for
their help and suggestions. This study was supported by Swedish
Research Council, Cancer Research Funds of Radiumhemmet, Swedish
Cancer Society, Karolinska Institutet and Stockholm County Council.
R.K. Chang was supported by the Karolinska Institutet PhD program
(KID).
References
|
1
|
Curado MP, Edwards B, Shin HR, Storm H,
Ferlay J, Heanue M and Boyle P: Cancer incidence in five continents
Volume IX. IARC Sci Publ. 160:1–837. 2008.
|
|
2
|
Colombo N and Peiretti M: Non-epithelial
ovarian cancer: ESMO clinical recommendations for diagnosis,
treatment and follow-up. Ann Oncol. 20(Suppl 4): 24–26. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kraggerud SM, Hoei-Hansen CE, Alagaratnam
S, Skotheim RI, Abeler VM, Rajpert-De Meyts E and Lothe RA:
Molecular characteristics of malignant ovarian germ cell tumors and
comparison with testicular counterparts: Implications for
pathogenesis. Endocr Rev. 34:339–376. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chi JG, Lee YS, Park YS and Chang KY:
Fetus-in-fetu: Report of a case. Am J Clin Pathol. 82:115–119.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sergi C, Ehemann V, Beedgen B, Linderkamp
O and Otto HF: Huge fetal sacrococcygeal teratoma with a completely
formed eye and intratumoral DNA ploidy heterogeneity. Pediatr Dev
Pathol. 2:50–57. 1999. View Article : Google Scholar
|
|
6
|
Kuno N, Kadomatsu K, Nakamura M,
Miwa-Fukuchi T, Hirabayashi N and Ishizuka T: Mature ovarian cystic
teratoma with a highly differentiated homunculus: A case report.
Birth Defects Res A Clin Mol Teratol. 70:40–46. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Arlikar JD, Mane SB, Dhende NP, Sanghavi
Y, Valand AG and Butale PR: Fetus in fetu: Two case reports and
review of literature. Pediatr Surg Int. 25:289–292. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Scully RE: Classification of human ovarian
tumors. Environ Health Perspect. 73:15–25. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schultz KA, Harris AK, Schneider DT, Young
RH, Brown J, Gershenson DM, Dehner LP, Hill DA, Messinger YH and
Frazier AL: Ovarian sex cord-stromal tumors. J Oncol Pract.
12:940–946. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hoei-Hansen CE, Kraggerud SM, Abeler VM,
Kaern J, Rajpert-De Meyts E and Lothe RA: Ovarian dysgerminomas are
characterised by frequent KIT mutations and abundant expression of
pluripotency markers. Mol Cancer. 6:122007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Murray MJ, Saini HK, Siegler CA, Hanning
JE, Barker EM, van Dongen S, Ward DM, Raby KL, Groves IJ, Scarpini
CG, et al CCLG: LIN28 expression in malignant germ cell tumors
downregulates let-7 and increases oncogene levels. Cancer Res.
73:4872–4884. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shah SP, Köbel M, Senz J, Morin RD, Clarke
BA, Wiegand KC, Leung G, Zayed A, Mehl E, Kalloger SE, et al:
Mutation of FOXL2 in granulosa-cell tumors of the ovary. N Engl J
Med. 360:2719–2729. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim JH, Yoon S, Park M, Park HO, Ko JJ,
Lee K and Bae J: Differential apoptotic activities of wild-type
FOXL2 and the adult-type granulosa cell tumor-associated mutant
FOXL2 (C134W). Oncogene. 30:1653–1663. 2011. View Article : Google Scholar
|
|
14
|
Kim JH, Kim YH, Kim HM, Park HO, Ha NC,
Kim TH, Park M, Lee K and Bae J: FOXL2 posttranslational
modifications mediated by GSK3β determine the growth of granulosa
cell tumours. Nat Commun. 5:29362014.
|
|
15
|
Heravi-Moussavi A, Anglesio MS, Cheng SW,
Senz J, Yang W, Prentice L, Fejes AP, Chow C, Tone A, Kalloger SE,
et al: Recurrent somatic DICER1 mutations in nonepithelial ovarian
cancers. N Engl J Med. 366:234–242. 2012. View Article : Google Scholar
|
|
16
|
Bushati N and Cohen SM: microRNA
functions. Annu Rev Cell Dev Biol. 23:175–205. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Croce CM: Causes and consequences of
microRNA dysregulation in cancer. Nat Rev Genet. 10:704–714. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gillis AJ, Stoop HJ, Hersmus R, Oosterhuis
JW, Sun Y, Chen C, Guenther S, Sherlock J, Veltman I, Baeten J, et
al: High-throughput microRNAome analysis in human germ cell
tumours. J Pathol. 213:319–328. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Palmer RD, Murray MJ, Saini HK, van Dongen
S, Abreu-Goodger C, Muralidhar B, Pett MR, Thornton CM, Nicholson
JC, Enright AJ, et al: Children's Cancer and Leukaemia Group:
Malignant germ cell tumors display common microRNA profiles
resulting in global changes in expression of messenger RNA targets.
Cancer Res. 70:2911–2923. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Murray MJ, Saini HK, van Dongen S, Palmer
RD, Muralidhar B, Pett MR, Piipari M, Thornton CM, Nicholson JC,
Enright AJ, et al: The two most common histological subtypes of
malignant germ cell tumour are distinguished by global microRNA
profiles, associated with differential transcription factor
expression. Mol Cancer. 9:2902010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fustino N, Rakheja D, Ateek CS, Neumann JC
and Amatruda JF: Bone morphogenetic protein signalling activity
distinguishes histological subsets of paediatric germ cell tumours.
Int J Androl. 34:e218–e233. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Witten D, Tibshirani R, Gu SG, Fire A and
Lui WO: Ultra-high throughput sequencing-based small RNA discovery
and discrete statistical biomarker analysis in a collection of
cervical tumours and matched controls. BMC Biol. 8:582010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Langmead B, Trapnell C, Pop M and Salzberg
SL: Ultrafast and memory-efficient alignment of short DNA sequences
to the human genome. Genome Biol. 10:R252009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li H, Handsaker B, Wysoker A, Fennell T,
Ruan J, Homer N, Marth G, Abecasis G and Durbin R; 1000 Project
Genome Data Processing Subgroup: The Sequence Alignment/Map format
and SAMtools. Bioinformatics. 25:2078–2079. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Anders S, Pyl PT and Huber W: HTSeq - a
Python framework to work with high-throughput sequencing data.
Bioinformatics. 31:166–169. 2015. View Article : Google Scholar
|
|
26
|
Robinson MD and Oshlack A: A scaling
normalization method for differential expression analysis of
RNA-seq data. Genome Biol. 11:R252010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Love MI, Huber W and Anders S: Moderated
estimation of fold change and dispersion for RNA-seq data with
DESeq2. Genome Biol. 15:5502014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Voorhoeve PM, le Sage C, Schrier M, Gillis
AJ, Stoop H, Nagel R, Liu YP, van Duijse J, Drost J, Griekspoor A,
et al: A genetic screen implicates miRNA-372 and miRNA-373 as
oncogenes in testicular germ cell tumors. Cell. 124:1169–1181.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gillis AJ, Rijlaarsdam MA, Eini R,
Dorssers LC, Biermann K, Murray MJ, Nicholson JC, Coleman N,
Dieckmann KP, Belge G, et al: Targeted serum miRNA (TSmiR) test for
diagnosis and follow-up of (testicular) germ cell cancer patients:
A proof of principle. Mol Oncol. 7:1083–1092. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Murray MJ, Halsall DJ, Hook CE, Williams
DM, Nicholson JC and Coleman N: Identification of microRNAs From
the miR-371~373 and miR-302 clusters as potential serum biomarkers
of malignant germ cell tumors. Am J Clin Pathol. 135:119–125. 2011.
View Article : Google Scholar
|
|
31
|
Özata DM, Li X, Lee L, Liu J, Warsito D,
Hajeri P, Hultman I, Fotouhi O, Marklund S, Ährlund-Richter L, et
al: Loss of miR-514a-3p regulation of PEG3-activates the NF-kappa B
pathway in human testicular germ cell tumors. Cell Death Dis.
8:e27592017. View Article : Google Scholar
|
|
32
|
Chen BF, Suen YK, Gu S, Li L and Chan WY:
A miR-199a/miR-214 self-regulatory network via PSMD10, TP53 and
DNMT1 in testicular germ cell tumor. Sci Rep. 4:64132014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhong X, Li N, Liang S, Huang Q, Coukos G
and Zhang L: Identification of microRNAs regulating reprogramming
factor LIN28 in embryonic stem cells and cancer cells. J Biol Chem.
285:41961–41971. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nakano H, Yamada Y, Miyazawa T and Yoshida
T: Gain-of-function microRNA screens identify miR-193a regulating
proliferation and apoptosis in epithelial ovarian cancer cells. Int
J Oncol. 42:1875–1882. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhao Y, Li C, Wang M, Su L, Qu Y, Li J, Yu
B, Yan M, Yu Y, Liu B, et al: Decrease of miR-202-3p expression, a
novel tumor suppressor, in gastric cancer. PLoS One. 8:e697562013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yi H, Liang B, Jia J, Liang N, Xu H, Ju G,
Ma S and Liu X: Differential roles of miR-199a-5p in
radiation-induced autophagy in breast cancer cells. FEBS Lett.
587:436–443. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li Y, Jiang W, Hu Y, Da Z, Zeng C, Tu M,
Deng Z and Xiao W: MicroRNA-199a-5p inhibits cisplatin-induced drug
resistance via inhibition of autophagy in osteosarcoma cells. Oncol
Lett. 12:4203–4208. 2016.PubMed/NCBI
|
|
38
|
Ames K, Da Cunha DS, Gonzalez B, Konta M,
Lin F, Shechter G, Starikov L, Wong S, Bülow HE and Meléndez A: A
non-cell-autonomous role of BEC-1/BECN1/Beclin1 in coordinating
cell-cycle progression and stem cell proliferation during germline
development. Curr Biol. 27:905–913. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gawriluk TR, Hale AN, Flaws JA, Dillon CP,
Green DR and Rucker EB III: Autophagy is a cell survival program
for female germ cells in the murine ovary. Reproduction.
141:759–765. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rounge TB, Furu K, Skotheim RI, Haugen TB,
Grotmol T and Enerly E: Profiling of the small RNA populations in
human testicular germ cell tumors shows global loss of piRNAs. Mol
Cancer. 14:1532015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dieckmann KP, Spiekermann M, Balks T, Flor
I, Löning T, Bullerdiek J and Belge G: MicroRNAs miR-371-3 in serum
as diagnostic tools in the management of testicular germ cell
tumours. Br J Cancer. 107:1754–1760. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Syring I, Bartels J, Holdenrieder S,
Kristiansen G, Müller SC and Ellinger J: Circulating serum miRNA
(miR-367-3p, miR-371a-3p, miR-372-3p and miR-373-3p) as biomarkers
in patients with testicular germ cell cancer. J Urol. 193:331–337.
2015. View Article : Google Scholar
|
|
43
|
Subramanyam D, Lamouille S, Judson RL, Liu
JY, Bucay N, Derynck R and Blelloch R: Multiple targets of miR-302
and miR-372 promote reprogramming of human fibroblasts to induced
pluripotent stem cells. Nat Biotechnol. 29:443–448. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fareh M, Turchi L, Virolle V, Debruyne D,
Almairac F, de-la-Forest Divonne S, Paquis P, Preynat-Seauve O,
Krause KH, Chneiweiss H, et al: The miR 302–367 cluster drastically
affects self-renewal and infiltration properties of
glioma-initiating cells through CXCR4 repression and consequent
disruption of the SHH-GLI-NANOG network. Cell Death Differ.
19:232–244. 2012. View Article : Google Scholar
|
|
45
|
Yang CM, Chiba T, Brill B, Delis N, von
Manstein V, Vafaizadeh V, Oellerich T and Groner B: Expression of
the miR-302/367 cluster in glioblastoma cells suppresses
tumorigenic gene expression patterns and abolishes transformation
related phenotypes. Int J Cancer. 137:2296–2309. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gu KL, Zhang Q, Yan Y, Li TT, Duan FF, Hao
J, Wang XW, Shi M, Wu DR, Guo WT, et al: Pluripotency-associated
miR-290/302 family of microRNAs promote the dismantling of naive
pluripotency. Cell Res. 26:350–366. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li HL, Wei JF, Fan LY, Wang SH, Zhu L, Li
TP, Lin G, Sun Y, Sun ZJ, Ding J, et al: miR-302 regulates
pluripotency, teratoma formation and differentiation in stem cells
via an AKT1/OCT4-dependent manner. Cell Death Dis. 7:e20782016.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Cheung HH, Davis AJ, Lee TL, Pang AL,
Nagrani S, Rennert OM and Chan WY: Methylation of an intronic
region regulates miR-199a in testicular tumor malignancy. Oncogene.
30:3404–3415. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lee YB, Bantounas I, Lee DY, Phylactou L,
Caldwell MA and Uney JB: Twist-1 regulates the miR-199a/214 cluster
during development. Nucleic Acids Res. 37:123–128. 2009. View Article : Google Scholar :
|
|
50
|
Cheung HH, Lee TL, Davis AJ, Taft DH,
Rennert OM and Chan WY: Genome-wide DNA methylation profiling
reveals novel epigenetically regulated genes and non-coding RNAs in
human testicular cancer. Br J Cancer. 102:419–427. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Gu S, Cheung HH, Lee TL, Lu G, Poon WS and
Chan WY: Molecular mechanisms of regulation and action of
microRNA-199a in testicular germ cell tumor and glioblastomas. PLoS
One. 8:e839802013. View Article : Google Scholar
|
|
52
|
Yang H, Kong W, He L, Zhao JJ, O'Donnell
JD, Wang J, Wenham RM, Coppola D, Kruk PA, Nicosia SV, et al:
MicroRNA expression profiling in human ovarian cancer: miR-214
induces cell survival and cisplatin resistance by targeting PTEN.
Cancer Res. 68:425–433. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chen R, Alvero AB, Silasi DA, Kelly MG,
Fest S, Visintin I, Leiser A, Schwartz PE, Rutherford T and Mor G:
Regulation of IKKbeta by miR-199a affects NF-kappaB activity in
ovarian cancer cells. Oncogene. 27:4712–4723. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yin G, Chen R, Alvero AB, Fu HH, Holmberg
J, Glackin C, Rutherford T and Mor G: TWISTing stemness,
inflammation and proliferation of epithelial ovarian cancer cells
through MIR199A2/214. Oncogene. 29:3545–3553. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sontakke SD, Mohammed BT, McNeilly AS and
Donadeu FX: Characterization of microRNAs differentially expressed
during bovine follicle development. Reproduction. 148:271–283.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wainwright EN, Jorgensen JS, Kim Y, Truong
V, Bagheri-Fam S, Davidson T, Svingen T, Fernandez-Valverde SL,
McClelland KS, Taft RJ, et al: SOX9 regulates microRNA
miR-202-5p/3p expression during mouse testis differentiation. Biol
Reprod. 89:342013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Bannister SC, Smith CA, Roeszler KN, Doran
TJ, Sinclair AH and Tizard ML: Manipulation of estrogen synthesis
alters MIR202* expression in embryonic chicken gonads. Biol Reprod.
85:22–30. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Tao W, Sun L, Shi H, Cheng Y, Jiang D, Fu
B, Conte MA, Gammerdinger WJ, Kocher TD and Wang D: Integrated
analysis of miRNA and mRNA expression profiles in tilapia gonads at
an early stage of sex differentiation. BMC Genomics. 17:3282016.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Bizuayehu TT, Babiak J, Norberg B,
Fernandes JM, Johansen SD and Babiak I: Sex-biased miRNA expression
in Atlantic halibut (Hippoglossus hippoglossus) brain and gonads.
Sex Dev. 6:257–266. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Chen J, Cai T, Zheng C, Lin X, Wang G,
Liao S, Wang X, Gan H, Zhang D, Hu X, et al: MicroRNA-202 maintains
spermatogonial stem cells by inhibiting cell cycle regulators and
RNA binding proteins. Nucleic Acids Res. 45:4142–4157. 2017.
|
|
61
|
Eggers S, Ohnesorg T and Sinclair A:
Genetic regulation of mammalian gonad development. Nat Rev
Endocrinol. 10:673–683. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Streicher KL, Zhu W, Lehmann KP,
Georgantas RW, Morehouse CA, Brohawn P, Carrasco RA, Xiao Z, Tice
DA, Higgs BW, et al: A novel oncogenic role for the miRNA-506-514
cluster in initiating melanocyte transformation and promoting
melanoma growth. Oncogene. 31:1558–1570. 2012. View Article : Google Scholar
|
|
63
|
Liu G, Sun Y, Ji P, Li X, Cogdell D, Yang
D, Parker Kerrigan BC, Shmulevich I, Chen K, Sood AK, et al:
MiR-506 suppresses proliferation and induces senescence by directly
targeting the CDK4/6-FOXM1 axis in ovarian cancer. J Pathol.
233:308–318. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Singhal R, Bard JE, Nowak NJ, Buck MJ and
Kandel ES: FOXO1 regulates expression of a microRNA cluster on X
chromosome. Aging (Albany NY). 5:347–356. 2013. View Article : Google Scholar
|
|
65
|
Gross DN, van den Heuvel AP and Birnbaum
MJ: The role of FoxO in the regulation of metabolism. Oncogene.
27:2320–2336. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Richards JS, Sharma SC, Falender AE and Lo
YH: Expression of FKHR, FKHRL1, and AFX genes in the rodent ovary:
Evidence for regulation by IGF-I, estrogen, and the gonadotropins.
Mol Endocrinol. 16:580–599. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Liu Z, Castrillon DH, Zhou W and Richards
JS: FOXO1/3 depletion in granulosa cells alters follicle growth,
death and regulation of pituitary FSH. Mol Endocrinol. 27:238–252.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Shen M, Liu Z, Li B, Teng Y, Zhang J, Tang
Y, Sun SC and Liu H: Involvement of FoxO1 in the effects of
follicle-stimulating hormone on inhibition of apoptosis in mouse
granulosa cells. Cell Death Dis. 5:e14752014. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Liu Z, Ren YA, Pangas SA, Adams J, Zhou W,
Castrillon DH, Wilhelm D and Richards JS: FOXO1/3 and PTEN
depletion in granulosa cells promotes ovarian granulosa cell tumor
development. Mol Endocrinol. 29:1006–1024. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
You SY, Park YS, Jeon HJ, Cho DH, Jeon HB,
Kim SH, Chang JW, Kim JS and Oh JS: Beclin-1 knockdown shows
abscission failure but not autophagy defect during oocyte meiotic
maturation. Cell Cycle. 15:1611–1619. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Hale AN, Ledbetter DJ, Gawriluk TR and
Rucker EB III: Autophagy: Regulation and role in development.
Autophagy. 9:951–972. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Song ZH, Yu HY, Wang P, Mao GK, Liu WX, Li
MN, Wang HN, Shang YL, Liu C, Xu ZL, et al: Germ cell-specific Atg7
knockout results in primary ovarian insufficiency in female mice.
Cell Death Dis. 6:e15892015. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Herpin A, Englberger E, Zehner M, Wacker
R, Gessler M and Schartl M: Defective autophagy through epg5
mutation results in failure to reduce germ plasm and mitochondria.
FASEB J. 29:4145–4161. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zhang Y, Yan L, Zhou Z, Yang P, Tian E,
Zhang K, Zhao Y, Li Z, Song B, Han J, et al: SEPA-1 mediates the
specific recognition and degradation of P granule components by
autophagy in C elegans. Cell. 136:308–321. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Wang H, Lu Q, Cheng S, Wang X and Zhang H:
Autophagy activity contributes to programmed cell death in
Caenorhabditis elegans. Autophagy. 9:1975–1982. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Vera VS, Kenchappa CS, Landberg K,
Bressendorff S, Schwarzbach S, Martin T, Mundy J, Petersen M,
Thelander M and Sundberg E: Autophagy is required for gamete
differentiation in the moss Physcomitrella patens. Autophagy. Aug
24–2017.Epub ahead of print. View Article : Google Scholar
|
|
77
|
Agnello M, Chiarelli R, Martino C, Bosco L
and Roccheri MC: Autophagy is required for sea urchin oogenesis and
early development. Zygote. 24:918–926. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Rossi M, Colecchia D, Ilardi G, Acunzo M,
Nigita G, Sasdelli F, Celetti A, Strambi A, Staibano S, Croce CM,
et al: MAPK15 upregulation promotes cell proliferation and prevents
DNA damage in male germ cell tumors. Oncotarget. 7:20981–20998.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Shen Y, Li DD, Wang LL, Deng R and Zhu XF:
Decreased expression of autophagy-related proteins in malignant
epithelial ovarian cancer. Autophagy. 4:1067–1068. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Kandala PK and Srivastava SK: Regulation
of macroautophagy in ovarian cancer cells in vitro and in vivo by
controlling glucose regulatory protein 78 and AMPK. Oncotarget.
3:435–449. 2012. View Article : Google Scholar : PubMed/NCBI
|