Introduction
Breast cancer is the second leading cause of death
in women and has approximately 1 million new cases per year
worldwide (1,2). Breast cancer patients develop
metastasis eventually leading to poor prognosis (3). Triple-negative breast cancer (TNBC)
accounts for 12–20% of all breast cancer (4). It has more aggressive disease
progress and worse prognosis (5).
TNBC characteristics are the lack of expression of estrogen
receptor (ER), progesterone receptor (PR) and the lack of
overexpression of HER-2 (4,6).
TNBC is resistance to anti-hormone therapies and HER-2-aiming
target therapies (7,8). Treatment of TNBC remains a great
clinical challenge because of the lack of targeting agents and
limited therapeutic options (8,9).
Curcumin has been used in traditional Chinese
medicine for a long time in Taiwan, China and India (10). The pharmacological effects of
curcumin include anti-amyloid (11), anti-bacterial (12), anti-depressant (13), anti-inflammatory (14), anti-oxidant (15), anti-diabetes (16) and anticancer properties (17,18).
In addition, curcumin has been found to affect several anticancer
signaling pathways such as inhibition of cancer cell proliferation
(19,20) and induction of cell cycle arrest
(21), apoptosis (22) or autophagy (23). Specifically, the phase II and III
clinical trial of curcumin was advocated for use in patients with
colon and pancreatic cancers (24,25),
but its low water solubility exerts poor bioavailability and
primary limiting factors (low efficacy and safety) (26,27).
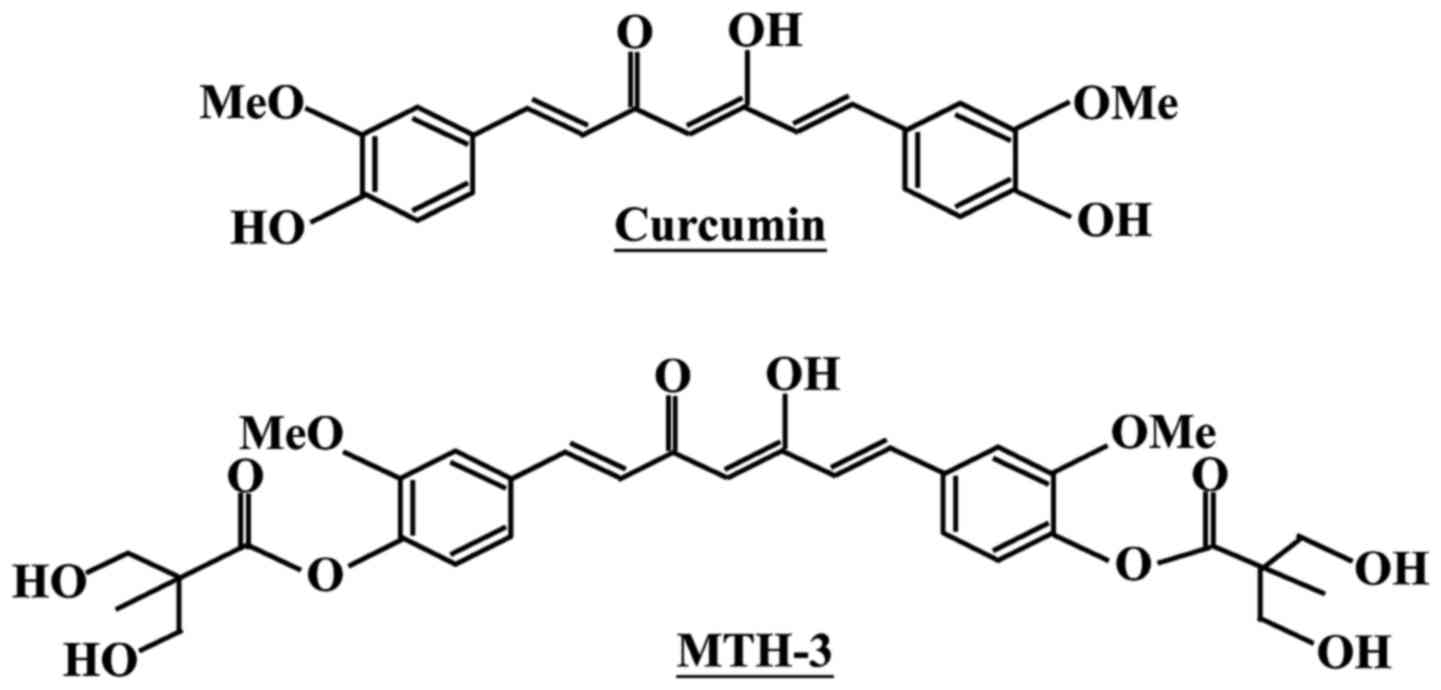
To improve these issues, we designed and developed a novel
bis(hydroxymethyl) alkanoate curcuminoid derivative, MTH-3
(Fig. 1). In our previous studies,
novel bis(hydroxymethyl) alkanoate curcuminoid derivatives were
shown to exhibit antitumor effects on triple-negative breast cancer
cells and in a xenograft animal experiment (28). The aim of the present study was to
characterize the property of MTH-3 and to clarify the molecular
mechanism of MTH-3 in human breast adenocarcinoma MDA-MB-231 cells
in vitro.
Materials and methods
Chemicals and reagents
MTH-3 was synthesized as previously described
(28) (patent pending). The purity
of MTH-3 is 98.7, and its molecular weight is 600.61. Leibovitz's
L-15 medium, fetal bovine serum (FBS), penicillin-streptomycin,
trypsin-EDTA, Premo Autophagy Sensor LC3B-GFP (BacMam 2.0) and
4′,6-diamidino-2-phenylindole (DAPI) were purchased from Thermo
Fisher Scientific (Waltham, MA, USA). The antibodies were purchased
from Cell Signaling Technology (Danvers, MA, USA). All chemicals
were obtained from Sigma-Aldrich (St. Louis, MO, USA) unless
otherwise stated.
Cell culture
The human breast adenocarcinoma cell line MDA-MB-231
was purchased from the Bioresource Collection and Research Center
(BCRC; Hsinchu, Taiwan). Cells were cultured in Leibovitz's L-15
medium with 10% FBS and 1% penicillin-streptomycin (100 Units/ml
penicillin and 100 μg/ml streptomycin) in an incubator under
95% air and 5% CO2 at 37°C.
Cell viability assay and morphologic
changes
Cell viability was evaluated by the reduction in MTT
to yield blue formazan. MDA-MB-231 cells (1×104
cells/well) in 96-well plates were allowed to attach overnight and
then treated with different concentrations (1, 3, 5 and 10
μM) of MTH-3 for 24 h. After treatments, MTT solution was
added to each well (a final concentration of 0.5 μg/ml), and
then the plates were incubated for another 4 h. The medium was
removed, blue formazan was dissolved in dimethyl sulfoxide (DMSO),
and the absorbance was read at 570 nm as previously described
(29). For trypan blue exclusion
assay, cells were collected after 1, 3, 5 and 10 μM of MTH-3
exposure, stained with 0.4% trypan blue and then counted on a
hemocytometer under a microscope. For morphological observation,
cells were visualized and photographed using a phase-contrast
microscope equipped with a digital camera (Leica Microsystems GmbH,
Wetzlar, Germany) as in previous reports (26,30).
Distribution of cell cycle analysis
MDA-MB-231 cells (2×105 cells/well) in
12-well plates were exposed to 10 μM MTH-3. After a 24-h
treatment, cells were harvested and fixed gently by putting 70%
ethanol at 4°C overnight before being stained with PI solution (40
μg/ml PI and 0.1 mg/ml RNase and 0.1% Triton X-100) in the
dark for 30 min as previously described (31). The cells were analyzed for the cell
cycle distribution with a flow cytometer (FACSCalibur; BD
Biosciences, San Jose, CA, USA).
CDK1 kinase assay
CDK1 kinase activity was analyzed according to the
manufacturer's protocol (CycLex Cdc2-Cyclin B Kinase Assay kit; MBL
International Corp., Woburn, MA, USA). The ability of CDK1 kinase
from MDA-MB-231 cell extracts prepared from each treatment of 10
μM MTH-3 for 4, 8, 16 and 24 h was measured as previously
described (32,33).
Apoptosis analysis
MDA-MB-231 cells (2×105 cells/well) into
12-well plates were incubated in the presence and absence of 10
μM MTH-3 for 24 and 48 h. Subsequently, cells were harvested
and stained with Annexin V and propidium iodide (PI) using the
Annexin V-FITC apoptosis detection kit (BD Biosciences, San Diego,
CA, USA) and subjected to flow cytometry (BD FACSCalibur; BD
Biosciences). The percentage of apoptotic cells were quantified
with BD CellQuest Pro software (BD Biosciences) (34,35).
Cells lysate preparation and western blot
analysis
After 10 μM MTH-3 treatments at indicated
intervals of time, MDA-MB-231 cells were harvested, washed and
suspended in the PRO-PREP Protein Extraction Solution (iNtRON
Biotechnology, Gyeonggi-do, Korea). Protein concentrations were
estimated using the Protein Assay kit (Bio-Rad Laboratories,
Hercules, CA, USA). The samples were resolved with SDS-PAGE and
transferred to a polyvinylidene difluo-ride membrane (PVDF) (EMD
Millipore, Billerica, MA, USA). Each membrane was blocked in 5%
non-fat milk in Tris-buffered saline with 0.1% Tween-20 for 1 h
followed by individual incubation with specific primary antibodies
[cyclin B1 (cat. no. 4138, 1:1,000), CDK1/Cdc2 (cat. no. 9116,
1:1,000), DR3 (cat. no. 4758, 1:1,000), DR5 (cat. no. 8074,
1:1,000), FADD (cat. no. 2782, 1:1,000), Bcl-2 (cat. no. 4223,
1:1,000), Bcl-xL (cat. no. 2764, 1:1,000), Ero1 (cat. no. 3264,
1:1,000), PDI (cat. no. 3501, 1:1,000), PERK (cat. no. 5683,
1:1,000), calnexin (cat. no. 2679, 1:1,000), IRE1α (cat. no. 3294,
1:1,000), CHOP (cat. no. 2895, 1:1,000), Bip (cat. no. 3177,
1:1,000), Atg5 (cat. no. 12994, 1:1,000), Atg7 (cat. no. 8558,
1:1,000), Atg12 (cat. no. 4180, 1:1,000), Beclin-1 (cat. no. 3495,
1:1,000), p62 (cat. no. 88588, 1:1,000), LC3A/B (cat. no. 12741,
1:1,000) and β-actin (cat. no. 3700, 1:5,000) (Cell Signaling
Technology, Danvers, MA, USA)] at 4°C overnight. Each membrane was
then incubated with anti-rabbit IgG (cat. no. 7074, 1:10,000) or
anti-mouse IgG (cat. no. 7076, 1:10,000) horseradish peroxidase
(HRP)-linked antibodies (Cell Signaling Technology) at room
temperature for 1 h. The signal was detected with the Immobilon
Western Chemiluminescent HRP substrate (EMD Millipore) and
visualized using the LAS 4000 imaging system (Fuji, Tokyo, Japan)
as previously described (36–38).
The quantitative densitometric analysis of immunoreactive band was
employed by ImageJ bundled with 64-bit Java 1.6.0_24 program for
Windows from the National Institutes of Health (NIH; Bethesda, MD,
USA).
Immunofluorescence staining
MDA-MB-231 cells (2×106 cells/dish) were
grown on sterile coverslips placed in a 10-cm dish. After 10
μM MTH-3 treatment, cells were fixed with 4%
paraformaldehyde and permeabilized with 0.2% Triton X-100 in
phosphate-buffered saline (PBS). After blocking with 2% bovine
serum albumin (BSA) in PBS, LC3B and p62 were detected using
anti-LC3B and anti-p62 antibody followed by reaction with FITC- or
PE-conjugated secondary antibody (BD Biosciences). Coverslips were
mounted on glass slides with ProLong Gold Antifade reagents (Thermo
Fisher Scientific) containing DAPI, and fluorescent image was taken
on a Leica Microsystems TCS SP2 Confocal Spectral microscope as
detailed by Lu et al (39).
cDNA microarray analysis
MDA-MB-231 cells were incubated with or without 10
μM MTH-3 for 24 h. After exposure, cell pellets were
collected, and the total RNA from each treatment was purified using
the Qiagen RNeasy Mini kit (Qiagen, Valencia, CA, USA). RNA purity
was determined to check the quality at 260/280 nm using a NanoDrop
1000 spectrophotometer (Thermo Fisher Scientific). mRNA was
amplified and labeled using the GeneChip WT Sense Target Labeling
and Control Reagents kit (Affymetrix, Santa Clara, CA, USA) for
expression analysis. The synthesized cDNA was labeled with
fluorescence and then hybridized for 17 h using GeneChip Human Gene
1.0 ST array (Affymetrix) to determine microarray hybridization
following the manufacturer's protocols. The arrays were
subsequently washed using GeneChip Fluidics Station 450
(Affymetrix), stained with streptavidin-phycoerythrin (GeneChip
Hybridization, Wash and Stain kit; Affymetrix) and scanned on a
GeneChip Scanner 3000 (Affymetrix). The localized concentrations of
fluorescent molecules were quantitated and analyzed using
Expression Console Software (Affymetrix) with default RMA
parameters as previously described (40). The gene expression level of a
2.5-fold change (log2 ratio) was considered a difference in
MTH-3-treated cells in vitro (41,42).
Statistical analysis
Data are presented as the mean ± SD for three
separate experiment. Differences among the groups were considered
to be significant at P<0.05 using ANOVA followed by the Duncan's
test.
Results
MTH-3 inhibits cell proliferation of
human breast adenocarcinoma MDA-MB-231 cells
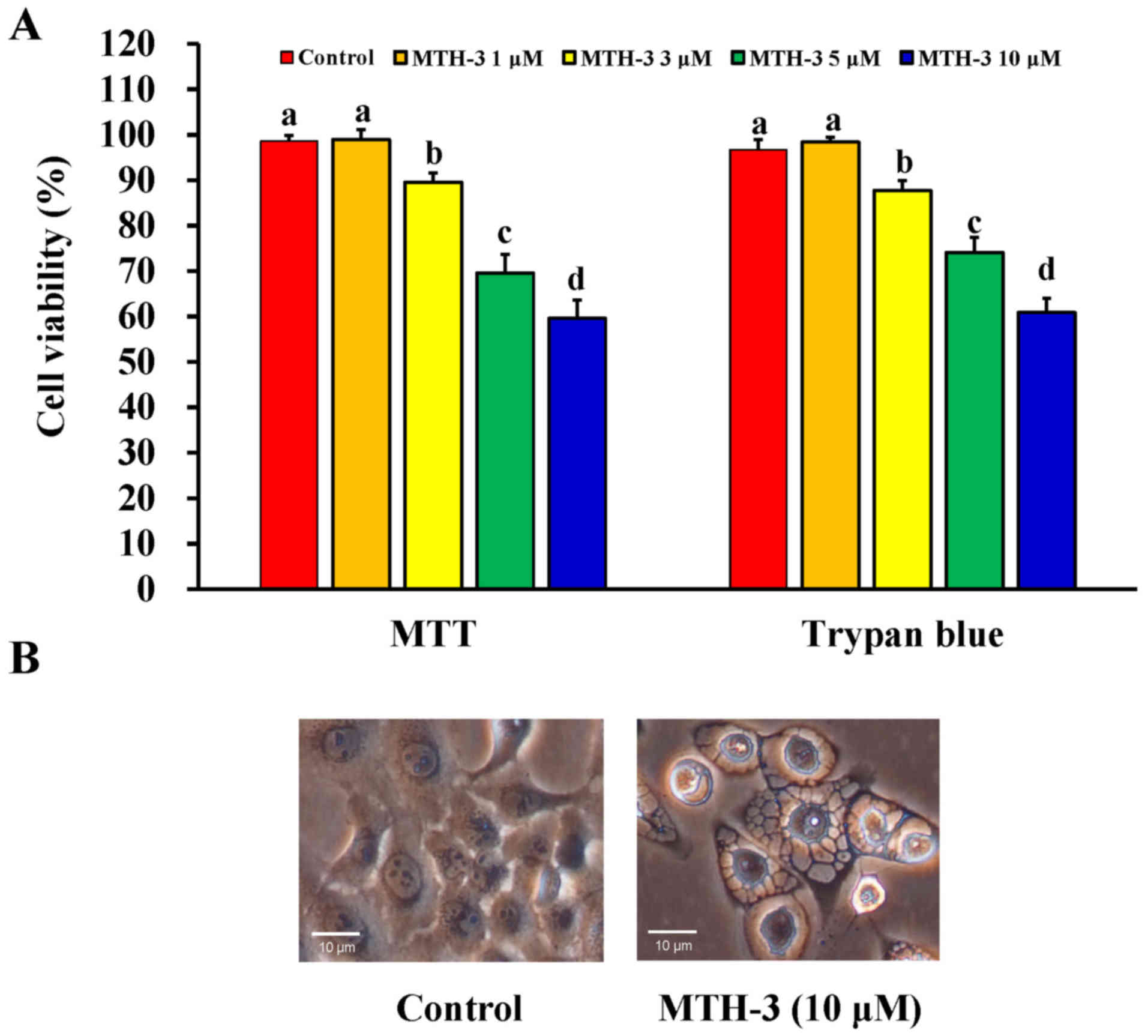
At first, the effect of MTH-3 on the viability of
MDA-MB-231 cells was investigated using the MTT and trypan blue
exclusion assays. MTH-3 at 1, 3, 5 and 10 μM significantly
reduced the viability of MDA-MB-231 cells by 98.94±2.26,
89.57±2.07, 69.57±4.13 and 59.6±4.04%, respectively (Fig. 2A). Importantly, the cell viability
reduction after 30 μM MTH-3 challenge is 34.23±3.31%. This
effect is in a concentration-dependent manner. Data from
morphological observation revealed that MTH-3 treatment at 10
μM caused obvious MDA-MB-231 cell apoptosis and autophagy
with characteristics, including cytoplasmic membrane blebbing, cell
shrinkage and autophagic vacuoles (Fig. 2B). Based on these findings and
gaining effective evidence of cell death, we selected MTH-3 at 10
μM for the majority of the experiments in MDA-MB-231
cells.
MTH-3 triggers G2/M phase
arrest and reduces CDK1 activity in MDA-MB-231 cells
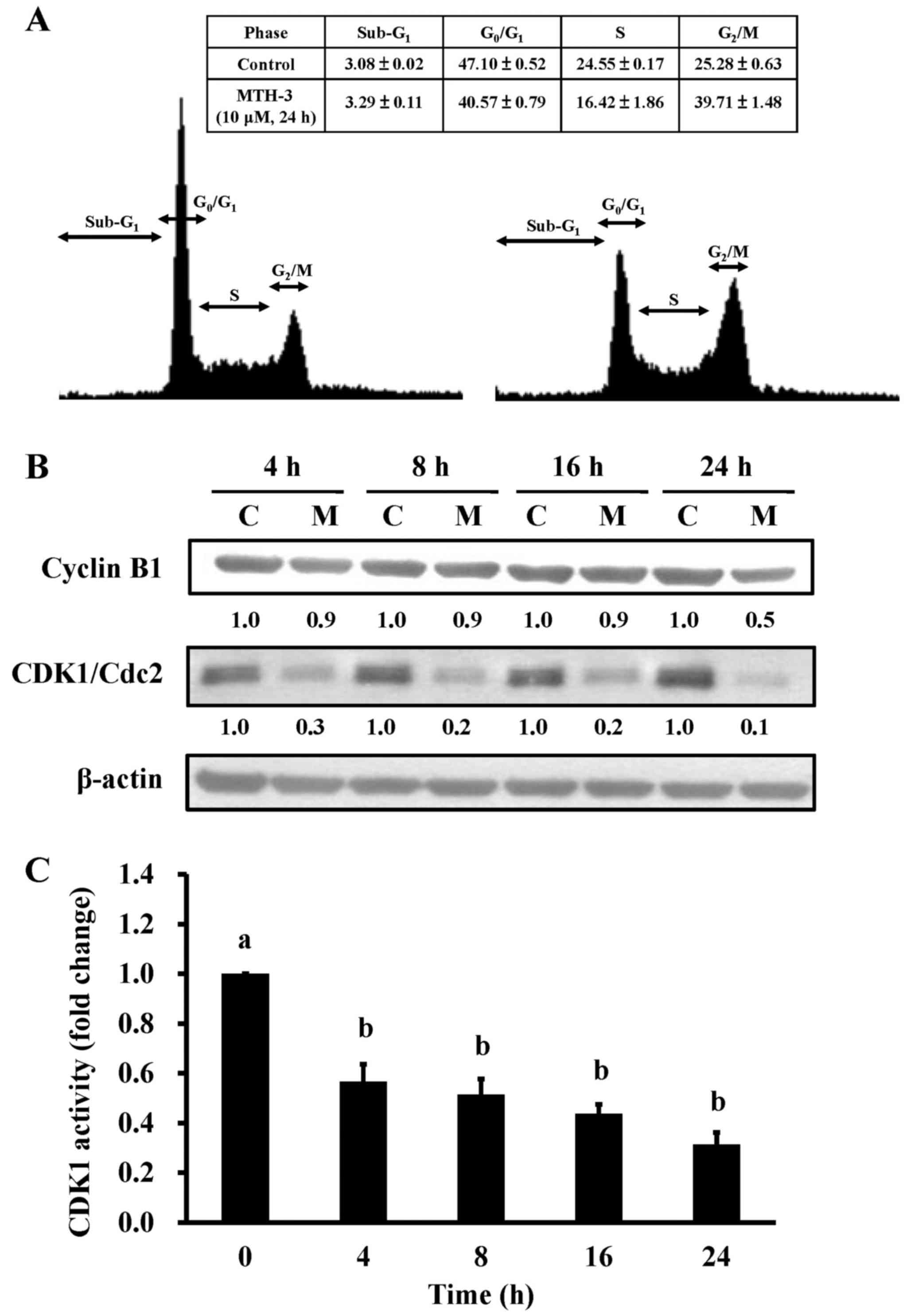
To investigate the cell cycle distribution of
treated and untreated MDA-MB-231 cells, cells were monitored after
10 μM MTH-3 challenge. Results from flow cytometric analysis
showed that MTH-3 treatment of MDA-MB-231 cells significantly
increased G2/M phase cell population at 24 h (Fig. 3A). The effects of MTH-3 on
G2/M phase-related proteins in MDA-MB-231 cells were
investigated. Our results showed that MTH-3 effectively
down-regulated the levels of cyclin B1 and CDK1 (Fig. 3B). We also tested the CDK1 kinase
activity in MDA-MB-231 cells prior to MTH-3 treatment. MTH-3
markedly reduced CDK1 kinase activity at 4, 8, 12 and 24 h of
treatment, respectively (Fig. 3C).
Therefore, the finding showed that downregulation of CDK1 activity
contributed to G2/M phase arrest caused by MTH-3 in
MDA-MB-231 cells.
MTH-3 elicits cell apoptosis of
MDA-MB-231 cells
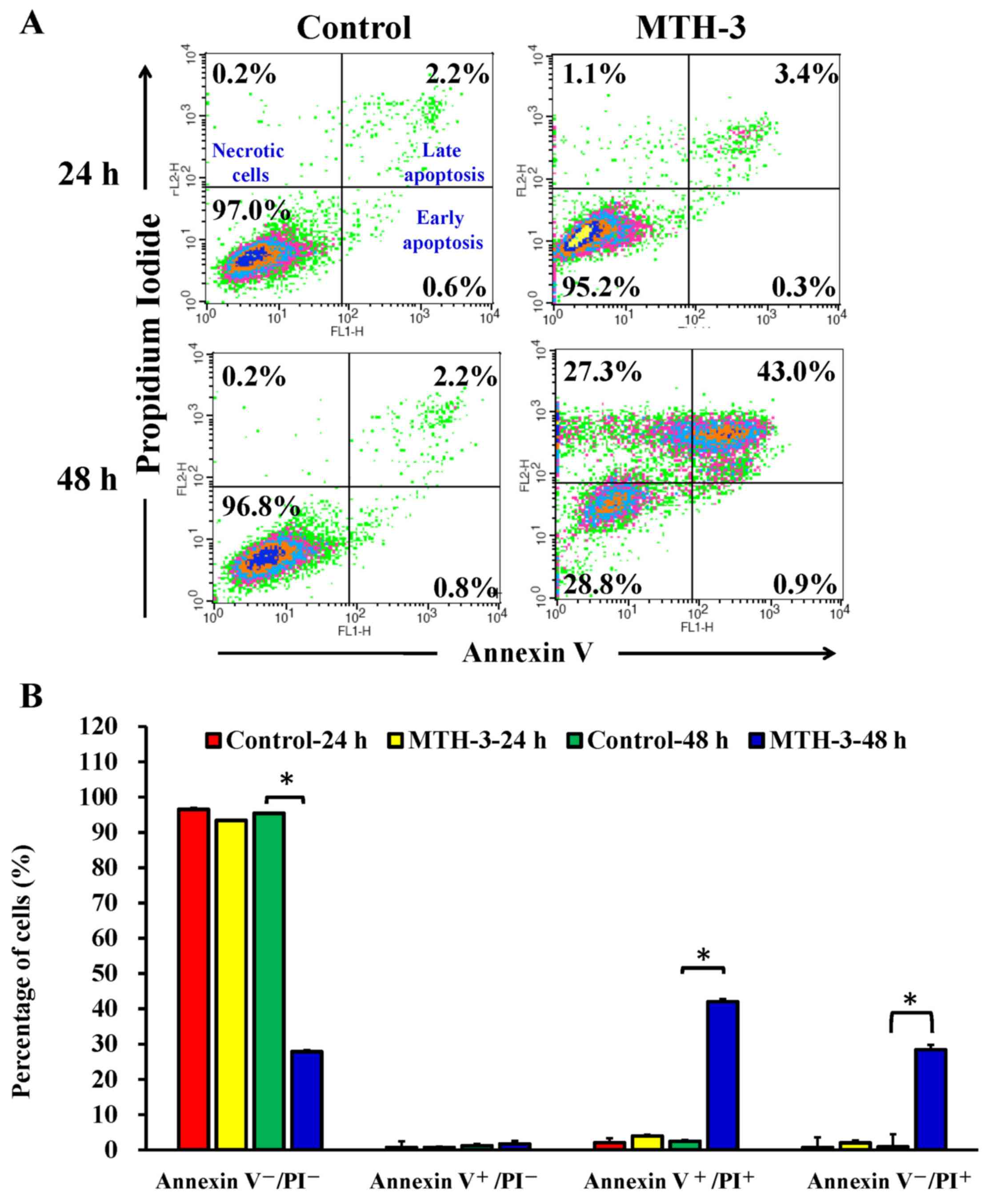
To further explore whether the inhibition of cell
viability results from the induction of apoptosis in MDA-MB-231
cells, MTH-3-treated cells were detected with Annexin V/PI double
staining (Fig. 4). Treatment with
10 μM MTH-3 for 48 h significantly increased the population
of Annexin V-positive cells (Fig.
4), indicating that MTH-3 induced apoptosis in MDA-MB-231
cells. However, the necrotic cells (Annexin
V+/PI+) increased rapidly after 48 h of 10
μM MTH-3 exposure.
MTH-3 activates death receptor,
mitochondrial and ER stress-mediated apoptotic pathways in
MDA-MB-231 cells
The effects of MTH-3 on apoptosis-related proteins
in MDA-MB-231 cells were investigated. Our results demonstrated
that MTH-3 upregulated the levels of DR5 and FADD, and it
downregulated the levels of Bcl-2 and Bcl-xL (Fig. 5A). Furthermore, our findings also
revealed that MTH-3 markedly increased the levels of CHOP and Bip,
as well as decreased the levels of Ero1, PDI, PERK, calnexin and
IRE1α (Fig. 5B). These results
suggest that MTH-3 induced apoptosis through death receptor
(extrinsic pathway) and mitochondria (intrinsic pathway)-dependent
pathways and possibly by modulating ER stress mechanism in
MDA-MB-231 cells.
 | Figure 5MTH-3 activates death
receptor-mediated, mitochondrial and ER stress-regulated apoptosis
pathways in MDA-MB-231 cells. Cells were exposed to 10 μM
MTH-3 for 0, 4, 8, 16 and 24 h, and cell lysates were collected for
western blot analysis. (A) Death receptor-mediated (DR3, DR5 and
FADD) and mitochondrial (Bcl-2 and Bcl-xL) apoptosis pathways, and
(B) ER stress (Ero1, PDI, PERK, calnexin, IRE1α, CHOP and Bip) were
performed. β-actin served as an internal control. C, control; M,
MTH-3 exposure. |
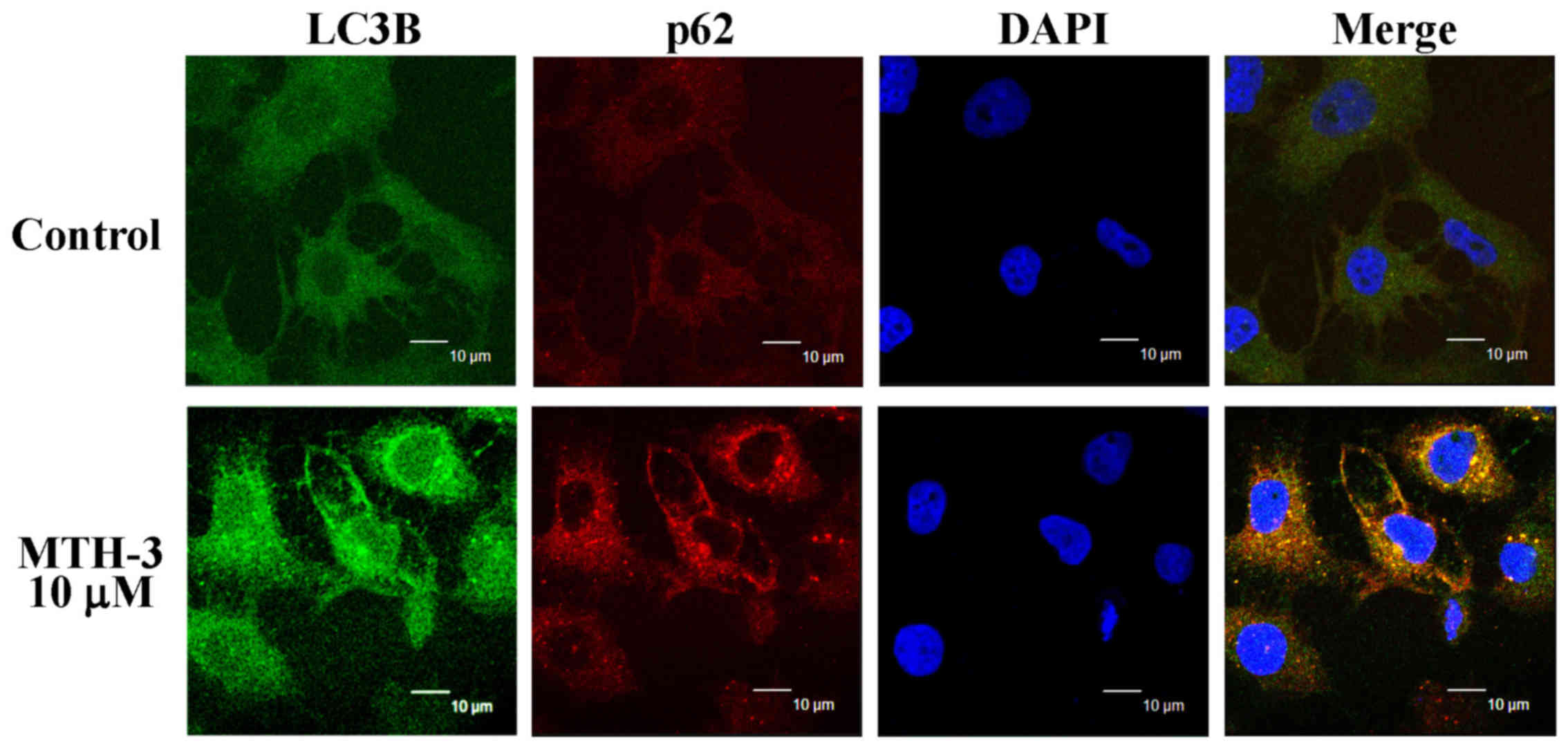
MTH-3 stimulates autophagy in MDA-MB-231
cells
To confirm if autophagy is involved in the
inhibition of MDA-MB-231 cell viability, cells with or without
MTH-3 exposure were detected with LC3B and p62 double
immunostaining. MTH-3 at 10 μM increased the LC3B (FITC;
green color) and p62 (PE; red color) protein expression (Fig. 6), indicating that MTH-3 induced
autophagy through increasing LC3B/p62 signaling in MDA-MB-231
cells.
MTH-3 alters the levels of
autophagy-associated proteins in MDA-MB-231 cells
Based on the results of autophagy, its related
signals were further employed by immunoblotting analysis. MTH-3
treatment induced the levels of Atg5, Atg7, Atg12, Beclin-1, p62
and LC3B in a time-dependent manner (Fig. 7). These data demonstrated that
MTH-3 induced autophagy by activating Atg family proteins in
MDA-MB-231 cells.
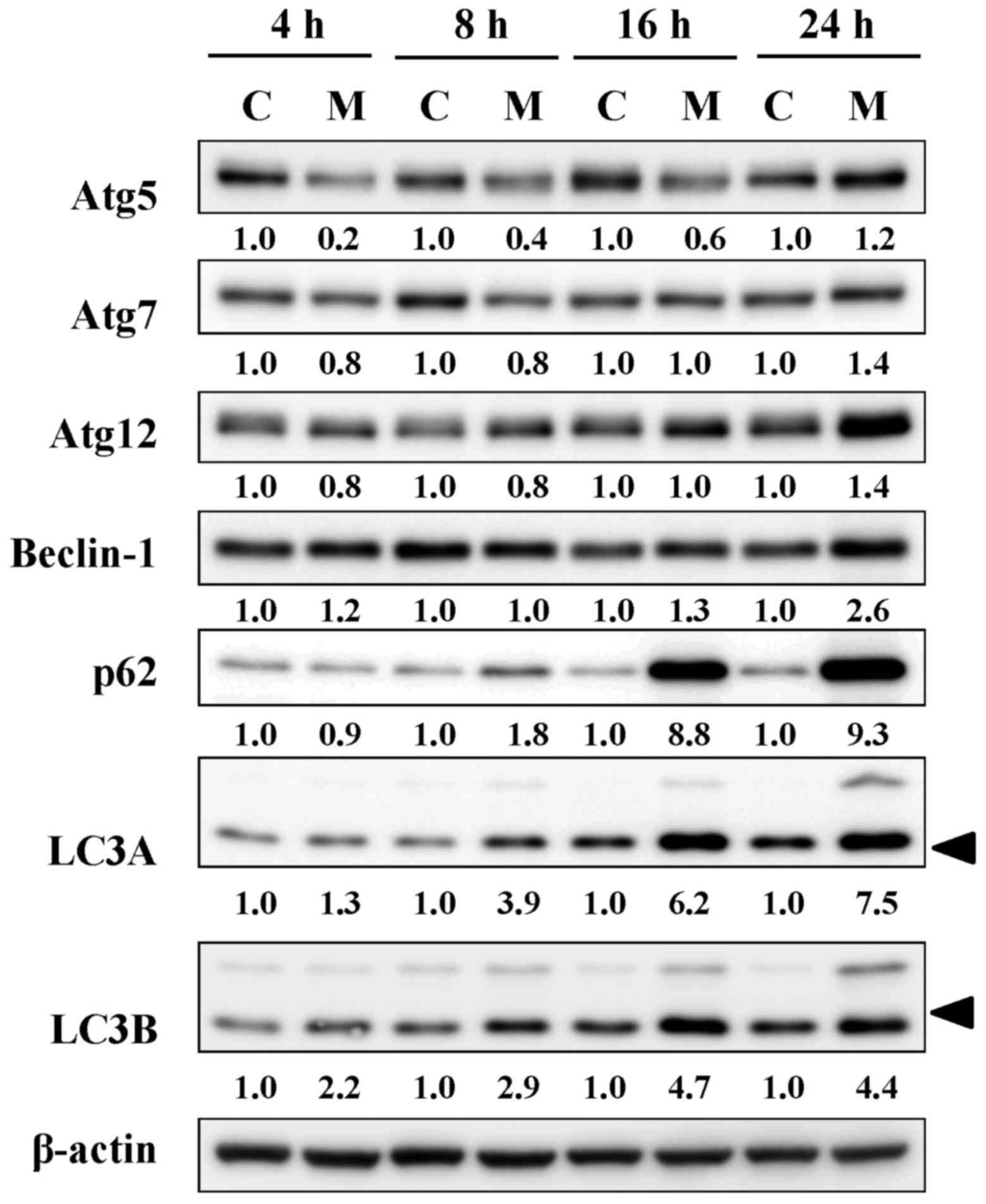 | Figure 7MTH-3 alters the protein levels of
autophagy-related proteins in MDA-MB-231 cells. Cells were
incubated with 10 μM MTH-3 for 4, 8, 16 and 24 h, and cell
lysates were collected for western blot analysis to probe
autophagic signals (Atg5, Atg7, Atg12, Beclin-1, p62, LC3A and
LC3B). β-actin was an internal control. C, control; M, MTH-3
exposure. |
MTH-3 modulates cell death-related gene
expression in MDA-MB-231 cells by cDNA microarray analysis
After treatment with 10 μM MTH-3 for 24 h,
cells were collected, and cDNA microarray analysis was performed.
The analysis showed that 97 genes (69 genes, upregulated; 28 genes,
down-regulated) were expressed at least by 2.5-fold compared with
the untreated control (Table I).
The top alteration in gene expression scored by the number of
pathway networks from GeneGo analysis program (Fig. 8). These genes may also be involved
in cell death and cytotoxic responses in MTH-3-treated MDA-MB-231
cells.
 | Table IThe >2.5-fold changes in mRNA
levels in MDA-MB-231 cells following a 24-h treatment with 10
μM MTH-3 as identified using DNA microarray. |
Table I
The >2.5-fold changes in mRNA
levels in MDA-MB-231 cells following a 24-h treatment with 10
μM MTH-3 as identified using DNA microarray.
| ID | log2 (ratio) | Gene_symbol | Description |
|---|
| PH_hs_0049600 | 6.643856 | HSPA6 | Heat shock 70 kDa
protein 6 (HSP70B') |
| PH_hs_0006387 | 6.274261 | ZFAND2A | zinc finger,
AN1-type domain 2A |
| PH_hs_0004421 | 5.381376 | PPP1R15A | Protein phosphatase
1, regulatory subunit 15A |
| PH_hs_0000305 | 4.941673 | MMP10 | Matrix
metallopeptidase 10 (stromelysin 2) |
| PH_hs_0046245 | 4.763129 | RN7SK | RNA, 7SK small
nuclear |
| PH_hs_0000076 | 4.587356 | IL12A | Interleukin
12A |
| PH_hs_0027902 | 4.286664 | ABL2 | v-abl Abelson
murine leukemia viral oncogene homolog 2 |
| PH_hs_0010276 | 4.189167 | DUSP1 | Dual specificity
phosphatase 1 |
| PH_hs_0031719 | 4.146525 | CCL26 | Chemokine (C-C
motif) ligand 26 |
| PH_hs_0000156 | 4.093858 | DUSP2 | Dual specificity
phosphatase 2 |
| PH_hs_0011943 | 4.063702 | HMOX1 | Heme oxygenase
(decycling) 1 |
| PH_hs_0045501 | 4.039442 | EID3 | EP300 interacting
inhibitor of differentiation 3 |
| PH_hs_0004561 | 3.997336 | GEM | GTP binding protein
overexpressed in skeletal muscle |
| PH_hs_0042334 | 3.931415 | MT4 | Metallothionein
4 |
| PH_hs_0048553 | 3.866096 | MYCT1 | myc target 1 |
| PH_hs_0000684 | 3.853854 | DNAJB9 | DnaJ (Hsp40)
homolog, subfamily B, member 9 |
| PH_hs_0035404 | 3.763571 | SAT1 | Spermidine/spermine
N1-acetyltransferase 1 |
| PH_hs_0000057 | 3.698185 | ATF3 | Activating
transcription factor 3 |
| PH_hs_0025319 | 3.562429 | C3orf52 | Chromosome 3 open
reading frame 52 |
| PH_hs_0033101 | 3.555868 | DDIT3 |
DNA-damage-inducible transcript 3
(CHOP) |
| PH_hs_0002700 | 3.513438 | OSGIN1 | Oxidative stress
induced growth inhibitor 1 |
| PH_hs_0037472 | 3.480422 | MALAT1 | Metastasis
associated lung adenocarcinoma transcript 1 |
| PH_hs_0035765 | 3.427173 | GDF15 | Growth
differentiation factor 15 |
| PH_hs_0002492 | 3.366024 | SAT1 | Spermidine/spermine
N1-acetyltransferase 1 |
| PH_hs_0062199 | 3.356707 |
AKR1C1|LOC101060798 | Aldo-keto reductase
family 1, member C1|aldo-keto reductase family 1 member
C2-like |
| PH_hs_0000852 | 3.324182 | SESN2 | Sestrin 2 |
| PH_hs_0023008 | 3.242113 | FRS2 | Fibroblast growth
factor receptor substrate 2 |
| PH_hs_0004751 | 3.219326 | MMP1 | Matrix
metallopeptidase 1 (interstitial collagenase) |
| PH_hs_0031143 | 3.213328 | VIMP | VCP-interacting
membrane protein |
| PH_hs_0025525 | 3.198476 | CLU | Clusterin |
| PH_hs_0024315 | 3.075314 | DNAJB4 | DnaJ (Hsp40)
homolog, subfamily B, member 4 |
| PH_hs_0035614 | 3.062771 | RC3H1 | Ring finger and
CCCH-type domains 1 |
| PH_hs_0027152 | 3.037995 | RMND5A | Required for
meiotic nuclear division 5 homolog A (S. cerevisiae) |
| PH_hs_0021974 | 3.010862 | DNAJC3 | DnaJ (Hsp40)
homolog, subfamily C, member 3 |
| PH_hs_0061784 | 2.967357 | CDKN1A | Cyclin-dependent
kinase inhibitor 1A (p21, Cip1) |
| PH_hs_0035466 | 2.962064 | AKR1C3|AKR1C1 | Aldo-keto reductase
family 1, member C3|aldo-keto reductase family 1, member C1 |
| PH_hs_0027162 | 2.960759 | SLC3A2 | Solute carrier
family 3 (activators of dibasic and neutral amino acid transport),
member 2 |
| PH_hs_0022919 | 2.960552 | CLCF1 | Cardiotrophin-like
cytokine factor 1 |
| PH_hs_0000255 | 2.916655 | SRGN | Serglycin |
| PH_hs_0024155 | 2.904033 | CDKN1A | Cyclin-dependent
kinase inhibitor 1A (p21, Cip1) |
| PH_hs_0043719 | 2.894684 | HMGCS1 |
3-hydroxy-3-methylglutaryl-CoA synthase 1
(soluble) |
| PH_hs_0045838 | 2.838192 | SLC6A6 | Solute carrier
family 6 (neurotransmitter transporter, taurine), member 6 |
| PH_hs_0014155 | 2.836392 | HSPA1B | Heat shock 70 kDa
protein 1B |
| PH_hs_0044272 | 2.829317 | CLK1 | CDC-like kinase
1 |
| PH_hs_0048881 | 2.809371 | FKBP4 | FK506 binding
protein 4, 59 kDa |
| PH_hs_0020147 | 2.803912 | CLK1 | CDC-like kinase
1 |
| PH_hs_0028987 | 2.768552 | TCF21 | Transcription
factor 21 |
| PH_hs_0042409 | 2.76703 | DNAJB1 | DnaJ (Hsp40)
homolog, subfamily B, member 1 |
| PH_hs_0001262 | 2.748306 | SENP5 | SUMO1/sentrin
specific peptidase 5 |
| PH_hs_0060828 | 2.734692 | TRIB3 | Tribbles homolog 3
(Drosophila) |
| PH_hs_0023556 | 2.733421 | C21orf91 | Chromosome 21 open
reading frame 91 |
| PH_hs_0061012 | 2.731293 | ZBTB21 | Zinc finger and BTB
domain containing 21 |
| PH_hs_0029660 | 2.695316 | AKR1C1 | Aldo-keto reductase
family 1, member C1|aldo-keto reductase family 1 |
| PH_hs_0037242 | 2.683231 | MALAT1 | Metastasis
associated lung adenocarcinoma transcript 1 (non-protein
coding) |
| PH_hs_0002812 | 2.667718 | C18orf25 | Chromosome 18 open
reading frame 25 |
| PH_hs_0027209 | 2.665362 | GADD45B | Growth arrest and
DNA-damage-inducible, β |
| PH_hs_0002971 | 2.664712 | ZNF77 | Zinc finger protein
77 |
| PH_hs_0003180 | 2.646292 | SMIM13 | Small integral
membrane protein 13 |
| PH_hs_0000694 | 2.625719 | RND3 | Rho family GTPase
3 |
| PH_hs_0023711 | 2.599232 | HSPA5 | Heat shock 70 kDa
protein 5 |
| PH_hs_0023894 | 2.583817 | TRIB3 | Tribbles homolog 3
(Drosophila) |
| PH_hs_0060053 | 2.574976 | ZNF121 | Zinc finger protein
121 |
| PH_hs_0014119 | 2.571605 | BRF2 | BRF2, subunit of
RNA polymerase III transcription initiation factor, BRF1-like |
| PH_hs_0033027 | 2.547837 | SIK1 | Salt-inducible
kinase 1 |
| PH_hs_0024236 | 2.547678 | ATP2A2 | ATPase, Ca++
transporting, cardiac muscle, slow twitch 2 |
| PH_hs_0042225 | 2.541029 | DUSP5 | Dual specificity
phosphatase 5 |
| PH_hs_0044921 | 2.534876 | HSPA1A | Heat shock 70 kDa
protein 1A |
| PH_hs_0000566 | 2.528881 | SLC25A25 | Solute carrier
family 25, member 25 |
| PH_hs_0030976 | 2.516291 | NFKBIB | Nuclear factor of
kappa light polypeptide gene enhancer in B-cells inhibitor, β |
| PH_hs_0014995 | −3.653241 | METTL7A | Methyltransferase
like 7A |
| PH_hs_0023845 | −3.269308 | BBS2 | Bardet-Biedl
syndrome 2 |
| PH_hs_0009437 | −3.05235 | TOP2A | Topoisomerase (DNA)
II α 170 kDa |
| PH_hs_0047352 | −3.043277 | MARCKS | Myristoylated
alanine-rich protein kinase C substrate |
| PH_hs_0047965 | −2.959225 | PHLDA1 | Pleckstrin
homology-like domain, family A, member 1 |
| PH_hs_0040619 | −2.891495 | MXD3 | MAX dimerization
protein 3 |
| PH_hs_0012629 | −2.890238 | H1F0 | H1 histone family,
member 0 |
| PH_hs_0004988 | −2.878231 | LMNB1 | Lamin B1 |
| PH_hs_0035609 | −2.788184 | ETV1 | Ets variant 1 |
| PH_hs_0049449 | −2.729758 | GPR39 | G protein-coupled
receptor 39 |
| PH_hs_0027843 | −2.724437 | FAM20C | FAmily with
sequence similarity 20, member C |
| PH_hs_0027863 | −2.718276 | LRRC45 | Leucine rich repeat
containing 45 |
| PH_hs_0007383 | −2.717289 | F2R | Coagulation factor
II (thrombin) receptor |
| PH_hs_0036878 | −2.71449 | PIF1 | PIF1 5′-to-3′ DNA
helicase homolog (S. cerevisiae) |
| PH_hs_0047697 | −2.688182 | ARF6 | ADP-ribosylation
factor 6 |
| PH_hs_0048993 | −2.677322 | NRP1 | Neuropilin 1 |
| PH_hs_0031540 | −2.66121 | GNG2 | Guanine nucleotide
binding protein (G protein), gamma 2 |
| PH_hs_0010634 | −2.659899 |
TXNIP|LOC101060503 | Thioredoxin
interacting protein|thioredoxin-interacting protein-like |
| PH_hs_0028935 | −2.621805 | CCDC85B | Coiled-coil domain
containing 85B |
| PH_hs_0000866 | −2.612763 | OMA1 | OMA1 zinc
metallopeptidase homolog (S. cerevisiae) |
| PH_hs_0030800 | −2.552826 | FANCF | Fanconi anemia,
complementation group F |
| PH_hs_0025966 | −2.55207 | CTDSP1 | CTD small
phosphatase 1 |
| PH_hs_0023862 | −2.551096 | CBY1 | Chibby homolog 1
(Drosophila) |
| PH_hs_0047571 | −2.546813 | PDP1 | Pyruvate
dehyrogenase phosphatase catalytic subunit 1 |
| PH_hs_0028200 | −2.537288 | CENPI | Centromere protein
I |
| PH_hs_0003147 | −2.533627 | PDGFC | Platelet derived
growth factor C |
| PH_hs_0035337 | −2.514458 | OMA1 | OMA1 zinc
metallopeptidase homolog (S. cerevisiae) |
| PH_hs_0038982 | −2.502536 | LOC100134259 | Uncharacterized
LOC100134259 |
Discussion
Previous studies have demonstrated the anticancer
potential of curcumin in regulating cell cycle, autophagy,
apoptosis and survival, proliferation, angiogenesis, invasion and
metastasis (19–23). Guan et al (43) demonstrated that curcumin reduced
Akt kinase in MDA-MB-231 cells accompanied by a decrease in cell
proliferation and migration as well as an increase in autophagic
activity; moreover, AMPK-mediated activation of autophagy
contributes to anticancer effects through Akt degradation. In the
present study, we also checked the growth inhibition effect of
curcumin on MDA-MB-231 cells. Our data indicated that the half
maximal inhibitory concentration (IC50) value of
curcumin on MDA-MB-231 cells is 38.77±3.35 μM. Strikingly,
the IC50 value of MTH-3 on MDA-MB-231 cells is 5.37±1.22
μM (data not shown). Our results demonstrated that the MTH-3
had highly cytotoxic effects on MDA-MB-231 cells. Moreover, we also
found that MTH-3 was non-cytotoxic on non-tumorigenic epithelial
mammary MCF10A cells and human skin fibroblast Detroit 551 cells,
respectively (data not shown). These are only preliminary data and
further study is needed to validate the findings.
There are no reports regarding that the effects of
MTH-3 on cell cycle arrest, autophagy and apoptosis and associated
gene expression in human breast cancer cells. This study is first
to demonstrate that MTH-3 induced cytotoxic effect on induction of
G2/M phase arrest, autophagy and apoptosis in human
breast adenocarcinoma MDA-MB-231 cells. The data demonstrated that
MTH-3 induced growth inhibitory effects through G2/M
phase arrest, apoptosis and autophagy in MDA-MB-231 cells. Our
results showed that MTH-3 induced G2/M phase arrest
through regulating cyclin B1 and CDK1 signaling. G2/M
phase progression has been reported to regulate CDK1 and CDK2
kinases that are activated primarily in association with cyclins A
and B (44). Furthermore, MTH-3
inhibited the CDK1 activity and the protein expression of CDK1 in
MDA-MB-231 cells. However, neither effect is positively correlated
because CDK1 activity might be involved in kinase activation rather
than CDK1/cdc2 protein level (32,33).
Previous studies also demonstrated that curcumin inhibited cell
proliferation through induction of G0/G1 phase arrest of
cancer cells (45,46), but our finding indicated that MTH-3
induced G2/M phase arrest upon different types of cancer
cell lines. However, the results are in agreement with previous
studies to show that curcumin inhibited cell proliferation by
inducing G2/M phase arrest in human glioblastoma U87
cells (47) and in Bcl-2
overex-pressed MCF-7 cells (48).
Further research is required to verify the mechanism of MTH-3
action in different breast cancer cell lines (such as MCF-7 and
MDA-MB-453 cells).
It is well documented that apoptosis plays an
important role in the maintenance of tissue homeostasis for the
elimination of excessive cells (49,50).
Induction of apoptosis of cancer cells by anticancer drugs such as
etoposide, cisplatin and paclitaxel have been used for treatment of
cancer in target cells (51).
Apoptosis-associated signaling pathways include extrinsic (death
receptor), intrinsic (mitochondria-dependent) and ER stress
(unfolded protein response) signals (52,53).
Our results demonstrated that MTH-3 promoted the protein levels of
DR5, and FADD and downregulated the levels of Bcl-2 and Bcl-xL in
MDA-MB-231 cells. MTH-3 also promoted the protein levels of CHOP
and Bip, and it reduced the levels of Ero1, PDI, PERK, calnexin and
IRE1α in MDA-MB-231 cells. Our novel findings suggest that both
extrinsic and intrinsic pathways, and ER stress signals were
involved in MTH-3-treated cells in vitro. This agrees with a
previous study reporting that the major targets of apoptotic
initiation are mediated by dysfunction of cellular organelles
(mitochondria, ER, lysosomes and golgi apparatus) (54).
Autophagy is another major clearance route for
intracellular protein (55).
Recently, curcumin can induce autophagy in cancer cells (56,57).
Our results showed that MTH-3 significantly increased protein
expression of autophagy markers LC3B, Atg complex (Atg5, Atg7 and
Atg12) and Beclin-1, as well as GFP-LC3 puncta formation,
suggesting that LC3B was recruited to the autophagosomal membrane
during autophagosome formation. Our data strongly suggest that
MTH-3 activated autophagy in MDA-MB-231 cells.
From gene expression profiles by DNA microarray, we
found that cellular and molecular responses to MTH-3 treatment are
multi-faceted and mediated by various regulatory pathways in
MDA-MB-231 cells. MTH-3 regulated the expression of important genes
in cell cycle, pathways in cancer, MAPK signaling, base excision
repair, DNA replication, p53 signaling, homologous recombination,
TGF-β signaling, G2/M checkpoint, pyrimidine metabolism,
Jak-STAT signaling, focal adhesion, endocytosis and mismatch repair
pathways. The gene regulation may be responsible for inhibiting the
proliferation of MDA-MB-23 cells. Cyclins associate with
cyclin-dependent protein kinases (CDKs) and CDK inhibitor (CKI) can
control the procedure of cell cycle to arrest the cell cycle and
inhibit the cell growth of cancer cells (44,58).
Our results from gene expression profiles indicated that MTH-3
changed the expression of cyclin and cyclin-dependent kinase
inhibitor gene CDKN1A, suggesting a change in cyclin,
cyclin-dependent kinase inhibitors which could finally lead to cell
cycle G2/M phase arrest.
Heme oxygenase-1 (HO-1) has been implicated in
cellular defense against oxidative stress and has anti-inflammation
function (59,60). A recent study has demonstrated that
curcumin inhibits appoptosin-induced apoptosis by upregulating HO-1
expression in SH-SY5Y cells (61).
Curcumin-induced HO-1 expression also prevents
H2O2-induced cell death in wild-type and HO-2
knockout adipose-derived mesenchymal stem cells (62). In this study of the gene expression
profiles, MTH-3 upregulated the expression of heme oxygenase 1
(HMOX1) gene, suggesting that MTH-3 might have
anti-inflammation and cell protection function.
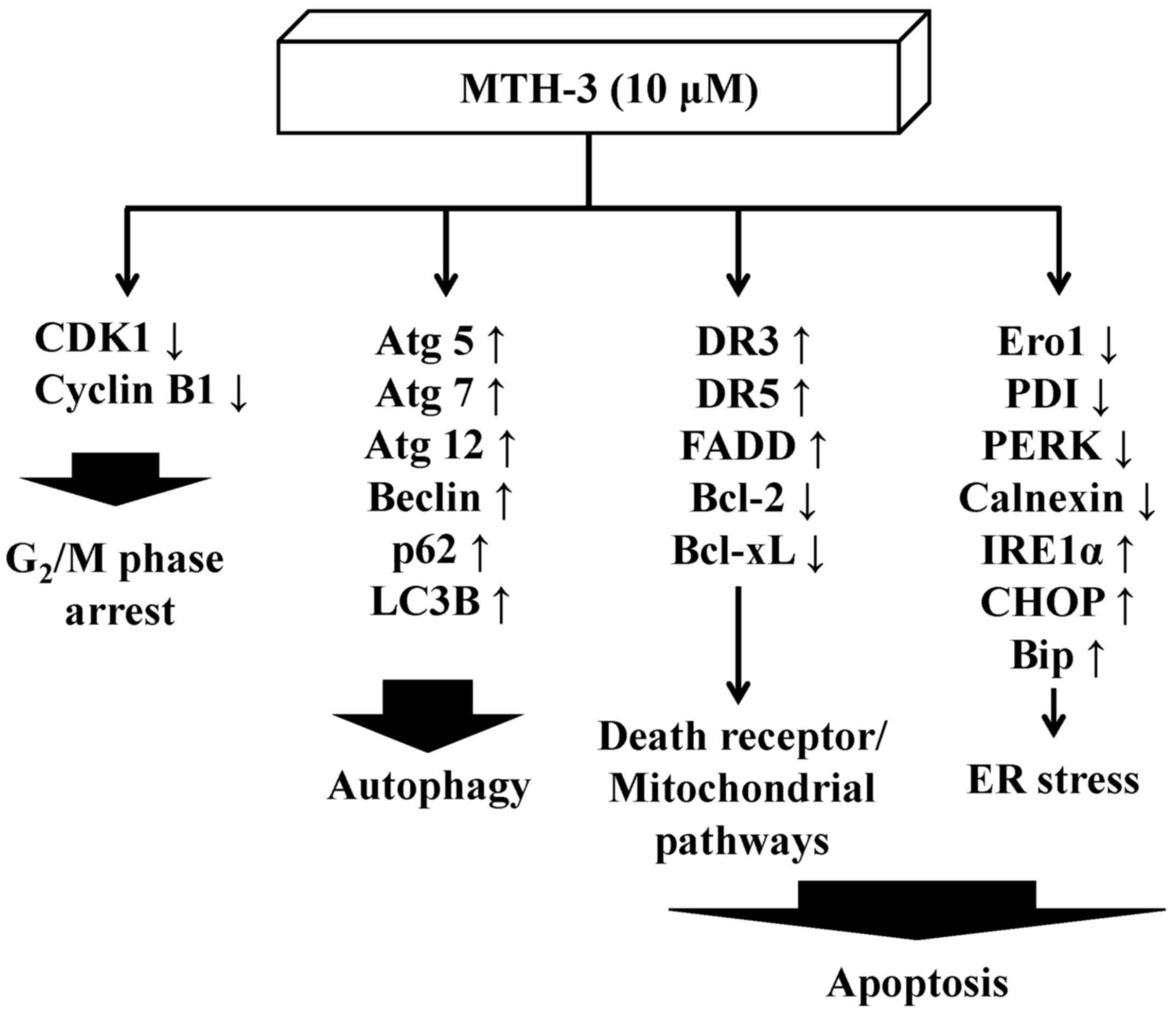
In conclusion, the molecular signaling pathways are
summarized in Fig. 9. This study
is the first report to provide an approach regarding the
bis(hydroxymethyl) alkanoate curcuminoid derivative, MTH-3 tends to
inhibit human breast adenocarcinoma MDA-MB-231 cells. Based on the
presented novel findings, the efficacy of MTH-3 might be sufficient
to further investigate the potential of breast cancer
treatment.
Acknowledgments
The present study was supported by research grants
from the National Science Council of the Republic of China awarded
to S.-C.K. and by China Medical University under the Aim for Top
University Plan of the Ministry of Education, Taiwan
(CHM106-2).
References
|
1
|
Aseyev O, Ribeiro JM and Cardoso F: Review
on the clinical use of eribulin mesylate for the treatment of
breast cancer. Expert Opin Pharmacother. 17:589–600. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cornejo-Moreno BA, Uribe-Escamilla D and
Salamanca-Gómez F: Breast cancer genes: Looking for BRACA's lost
brother. Isr Med Assoc J. 16:787–792. 2014.
|
|
3
|
Koo T and Kim IA: Brain metastasis in
human epidermal growth factor receptor 2-positive breast cancer:
From biology to treatment. Radiat Oncol J. 34:1–9. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mouh FZ, Mzibri ME, Slaoui M and Amrani M:
Recent progress in triple negative breast cancer research. Asian
Pac J Cancer Prev. 17:1595–1608. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hurvitz S and Mead M: Triple-negative
breast cancer: Advancements in characterization and treatment
approach. Curr Opin Obstet Gynecol. 28:59–69. 2016.
|
|
6
|
Zeichner SB, Terawaki H and Gogineni K: A
review of systemic treatment in metastatic triple-negative breast
cancer. Breast Cancer (Auckl). 10:25–36. 2016.
|
|
7
|
Wang Y, Cao S and Chen Y: Molecular
treatment of different breast cancers. Anticancer Agents Med Chem.
15:701–720. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tomao F, Papa A, Zaccarelli E, Rossi L,
Caruso D, Minozzi M, Vici P, Frati L and Tomao S: Triple-negative
breast cancer: New perspectives for targeted therapies. Onco
Targets Ther. 8:177–193. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gilani RA, Phadke S, Bao LW, Lachacz EJ,
Dziubinski ML, Brandvold KR, Steffey ME, Kwarcinski FE, Graveel CR,
Kidwell KM, et al: UM-164: A potent c-Src/38 kinase inhibitor with
in vivo activity against triple-negative breast cancer. Clin Cancer
Res. 22:5087–5096. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zheng B, Yang L, Wen C, Huang X, Xu C, Lee
KH and Xu J: Curcumin analog L3 alleviates diabetic atherosclerosis
by multiple effects. Eur J Pharmacol. 775:22–34. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ferreira N, Saraiva MJ and Almeida MR:
Natural polyphenols as modulators of TTR amyloidogenesis: in vitro
and in vivo evidences towards therapy. Amyloid. 19(Suppl 1): 39–42.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vilekar P, King C, Lagisetty P, Awasthi V
and Awasthi S: Antibacterial activity of synthetic curcumin
derivatives: 3,5-bis(benzylidene)-4-piperidone (EF24) and
EF24-dimer linked via diethylenetriaminepentacetic acid (EF2DTPA).
Appl Biochem Biotechnol. 172:3363–3373. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bhutani MK, Bishnoi M and Kulkarni SK:
Anti-depressant like effect of curcumin and its combination with
piperine in unpredictable chronic stress-induced behavioral,
biochemical and neurochemical changes. Pharmacol Biochem Behav.
92:39–43. 2009. View Article : Google Scholar
|
|
14
|
Cianciulli A, Calvello R, Porro C, Trotta
T, Salvatore R and Panaro MA: PI3k/Akt signalling pathway plays a
crucial role in the anti-inflammatory effects of curcumin in
LPS-activated microglia. Int Immunopharmacol. 36:282–290. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Choudhury AK, Raja S, Mahapatra S,
Nagabhushanam K and Majeed M: Synthesis and evaluation of the
anti-oxidant capacity of curcumin glucuronides, the major curcumin
metabolites. Antioxidants. 4:750–767. 2015. View Article : Google Scholar
|
|
16
|
Yang H, Xu W, Zhou Z, Liu J, Li X, Chen L,
Weng J and Yu Z: Curcumin attenuates urinary excretion of albumin
in type II diabetic patients with enhancing nuclear factor
erythroid-derived 2-like 2 (Nrf2) system and repressing
inflammatory signaling efficacies. Exp Clin Endocrinol Diabetes.
123:360–367. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee D, Kim IY, Saha S and Choi KS:
Paraptosis in the anti-cancer arsenal of natural products.
Pharmacol Ther. 162:120–133. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Borges GA, Rêgo DF, Assad DX, Coletta RD,
De Canto Luca G and Guerra EN: In vivo and in vitro effects of
curcumin on head and neck carcinoma: A systematic review. J Oral
Pathol Med. 46:3–20. 2017. View Article : Google Scholar
|
|
19
|
Sordillo PP and Helson L: Curcumin and
cancer stem cells: Curcumin has asymmetrical effects on cancer and
normal stem cells. Anticancer Res. 35:599–614. 2015.PubMed/NCBI
|
|
20
|
Iqbal B, Ghildiyal A, Sahabjada, Singh S,
Arshad M, Mahdi AA and Tiwari S: Antiproliferative and apoptotic
effect of curcumin and TRAIL (TNF related apoptosis inducing
ligand) in chronic myeloid leukaemic cells. J Clin Diagn Res.
10:XC01–XC05. 2016.PubMed/NCBI
|
|
21
|
Zhang L, Cheng X, Gao Y, Bao J, Guan H, Lu
R, Yu H, Xu Q and Sun Y: Induction of ROS-independent DNA damage by
curcumin leads to G2/M cell cycle arrest and apoptosis
in human papillary thyroid carcinoma BCPAP cells. Food Funct.
7:315–325. 2016. View Article : Google Scholar
|
|
22
|
Huang YT, Lin YW, Chiu HM and Chiang BH:
Curcumin induces apoptosis of colorectal cancer stem cells by
coupling with CD44 marker. J Agric Food Chem. 64:2247–2253. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kantara C, O'Connell M, Sarkar S, Moya S,
Ullrich R and Singh P: Curcumin promotes autophagic survival of a
subset of colon cancer stem cells, which are ablated by
DCLK1-siRNA. Cancer Res. 74:2487–2498. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ji JL, Huang XF and Zhu HL: Curcumin and
its formulations: Potential anti-cancer agents. Anticancer Agents
Med Chem. 12:210–218. 2012. View Article : Google Scholar
|
|
25
|
Shehzad A, Wahid F and Lee YS: Curcumin in
cancer chemo-prevention: Molecular targets, pharmacokinetics,
bioavailability, and clinical trials. Arch Pharm (Weinheim).
343:489–499. 2010. View Article : Google Scholar
|
|
26
|
Chang PY, Peng SF, Lee CY, Lu CC, Tsai SC,
Shieh TM, Wu TS, Tu MG, Chen MY and Yang JS: Curcumin-loaded
nanoparticles induce apoptotic cell death through regulation of the
function of MDR1 and reactive oxygen species in cisplatin-resistant
CAR human oral cancer cells. Int J Oncol. 43:1141–1150. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Douglass BJ and Clouatre DL: Beyond yellow
curry: Assessing commercial curcumin absorption technologies. J Am
Coll Nutr. 34:347–358. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hsieh MT, Chang LC, Hung HY, Lin HY, Shih
MH, Tsai CH, Kuo SC and Lee KH: New bis(hydroxymethyl) alkanoate
curcuminoid derivatives exhibit activity against triple-negative
breast cancer in vitro and in vivo. Eur J Med Chem. 131:141–151.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Peng SF, Lee CY, Hour MJ, Tsai SC, Kuo DH,
Chen FA, Shieh PC and Yang JS: Curcumin-loaded nanoparticles
enhance apoptotic cell death of U2OS human osteosarcoma cells
through the Akt-Bad signaling pathway. Int J Oncol. 44:238–246.
2014. View Article : Google Scholar
|
|
30
|
Liao CL, Lai KC, Huang AC, Yang JS, Lin
JJ, Wu SH, Gibson Wood W, Lin JG and Chung JG: Gallic acid inhibits
migration and invasion in human osteosarcoma U-2 OS cells through
suppressing the matrix metalloproteinase-2/-9, protein kinase B
(PKB) and PKC signaling pathways. Food Chem Toxicol. 50:1734–1740.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tsai SC, Lu CC, Lee CY, Lin YC, Chung JG,
Kuo SC, Amagaya S, Chen FN, Chen MY, Chan SF, et al: AKT
serine/threonine protein kinase modulates bufalin-triggered
intrinsic pathway of apoptosis in CAL 27 human oral cancer cells.
Int J Oncol. 41:1683–1692. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu CY, Yang JS, Huang SM, Chiang JH, Chen
MH, Huang LJ, Ha HY, Fushiya S and Kuo SC: Smh-3 induces
G2/M arrest and apoptosis through calcium-mediated
endoplasmic reticulum stress and mitochondrial signaling in human
hepatocellular carcinoma Hep3B cells. Oncol Rep. 29:751–762. 2013.
View Article : Google Scholar
|
|
33
|
Yang JS, Hour MJ, Huang WW, Lin KL, Kuo SC
and Chung JG: MJ-29 inhibits tubulin polymerization, induces
mitotic arrest, and triggers apoptosis via cyclin-dependent kinase
1-mediated Bcl-2 phosphorylation in human leukemia U937 cells. J
Pharmacol Exp Ther. 334:477–488. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang JS, Lin CA, Lu CC, Wen YF, Tsai FJ
and Tsai SC: Carboxamide analog ITR-284 evokes apoptosis and
inhibits migration ability in human lung adenocarcinoma A549 cells.
Oncol Rep. 37:1786–1792. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ho CC, Huang AC, Yu CS, Lien JC, Wu SH,
Huang YP, Huang HY, Kuo JH, Liao WY, Yang JS, et al: Ellagic acid
induces apoptosis in TSGH8301 human bladder cancer cells through
the endoplasmic reticulum stress- and mitochondria-dependent
signaling pathways. Environ Toxicol. 29:1262–1274. 2014.
|
|
36
|
Yuan CH, Horng CT, Lee CF, Chiang NN, Tsai
FJ, Lu CC, Chiang JH, Hsu YM, Yang JS and Chen FA: Epigallocatechin
gallate sensitizes cisplatin-resistant oral cancer CAR cell
apoptosis and autophagy through stimulating AKT/STAT3 pathway and
suppressing multidrug resistance 1 signaling. Environ Toxicol.
32:845–855. 2017. View Article : Google Scholar
|
|
37
|
Chiang JH, Yang JS, Lu CC, Hour MJ, Chang
SJ, Lee TH and Chung JG: Newly synthesized quinazolinone HMJ-38
suppresses angiogenetic responses and triggers human umbilical vein
endo-thelial cell apoptosis through p53-modulated Fas/death
receptor signaling. Toxicol Appl Pharmacol. 269:150–162. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Huang WW, Chiu YJ, Fan MJ, Lu HF, Yeh HF,
Li KH, Chen PY, Chung JG and Yang JS: Kaempferol induced apoptosis
via endoplasmic reticulum stress and mitochondria-dependent pathway
in human osteosarcoma U-2 OS cells. Mol Nutr Food Res.
54:1585–1595. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lu CC, Yang JS, Chiang JH, Hour MJ, Lin
KL, Lee TH and Chung JG: Cell death caused by quinazolinone HMJ-38
challenge in oral carcinoma CAL 27 cells: Dissections of
endoplasmic reticulum stress, mitochondrial dysfunction and tumor
xenografts. Biochim Biophys Acta. 1840:2310–2320. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
King YA, Chiu YJ, Chen HP, Kuo DH, Lu CC
and Yang JS: Endoplasmic reticulum stress contributes to arsenic
trioxide-induced intrinsic apoptosis in human umbilical and bone
marrow mesenchymal stem cells. Environ Toxicol. 31:314–328. 2016.
View Article : Google Scholar
|
|
41
|
Kuo YJ, Yang JS, Lu CC, Chiang SY, Lin JG
and Chung JG: Ethanol extract of Hedyotis diffusa willd upregulates
G0/G1 phase arrest and induces apoptosis in human leukemia cells by
modulating caspase cascade signaling and altering associated genes
expression was assayed by cDNA microarray. Environ Toxicol.
30:1162–1177. 2015. View Article : Google Scholar
|
|
42
|
Wu RS, Liu KC, Tang NY, Chung HK, Ip SW,
Yang JS and Chung JG: cDNA microarray analysis of the gene
expression of murine leukemia RAW 264.7 cells after exposure to
propofol. Environ Toxicol. 28:471–478. 2013. View Article : Google Scholar
|
|
43
|
Guan F, Ding Y, Zhang Y, Zhou Y, Li M and
Wang C: Curcumin suppresses proliferation and migration of
MDA-MB-231 breast cancer cells through autophagy-dependent Akt
degradation. PLoS One. 11:e01465532016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Dorée M and Hunt T: From Cdc2 to Cdk1:
When did the cell cycle kinase join its cyclin partner? J Cell Sci.
115:2461–2464. 2002.PubMed/NCBI
|
|
45
|
Chen ZQ, Jie X and Mo ZN: Curcumin
inhibits growth, induces G1 arrest and apoptosis on human prostatic
stromal cells by regulating Bcl-2/Bax. Zhongguo Zhong Yao Za Zhi.
33:2022–2025. 2008.In Chinese. PubMed/NCBI
|
|
46
|
Srivastava RK, Chen Q, Siddiqui I, Sarva K
and Shankar S: Linkage of curcumin-induced cell cycle arrest and
apoptosis by cyclin-dependent kinase inhibitor p21(/WAF1/CIP1).
Cell Cycle. 6:2953–2961. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cheng C, Jiao JT, Qian Y, Guo XY, Huang J,
Dai MC, Zhang L, Ding XP, Zong D and Shao JF: Curcumin induces G2/M
arrest and triggers apoptosis via FoxO1 signaling in U87 human
glioma cells. Mol Med Rep. 13:3763–3770. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Berrak O, Akkoc Y, Arisan ED, Coker-Gurkan
A, Obakan-Yerlikaya P and Palavan-Unsal N: The inhibition of PI3K
and NFkappaB promoted curcumin-induced cell cycle arrest at G2/M
via altering polyamine metabolism in Bcl-2 overexpressing MCF-7
breast cancer cells. Biomed Pharmacother. 77:150–160. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Negroni A, Cucchiara S and Stronati L:
Apoptosis, necrosis, and necroptosis in the gut and intestinal
homeostasis. Mediators Inflamm. 2015:2507622015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yang Y, Jiang G, Zhang P and Fan J:
Programmed cell death and its role in inflammation. Mil Med Res.
2:122015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Friesen C, Fulda S and Debatin KM:
Cytotoxic drugs and the CD95 pathway. Leukemia. 13:1854–1858. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhang YS, Shen Q and Li J: Traditional
Chinese medicine targeting apoptotic mechanisms for esophageal
cancer therapy. Acta Pharmacol Sin. 37:295–302. 2016. View Article : Google Scholar :
|
|
53
|
Tameire F, Verginadis II and Koumenis C:
Cell intrinsic and extrinsic activators of the unfolded protein
response in cancer: Mechanisms and targets for therapy. Semin
Cancer Biol. 33:3–15. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ferri KF and Kroemer G: Organelle-specific
initiation of cell death pathways. Nat Cell Biol. 3:E255–E263.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Dong Z, Liang S, Hu J, Jin W, Zhan Q and
Zhao K: Autophagy as a target for hematological malignancy therapy.
Blood Rev. 30:369–380. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Gupta SC, Kismali G and Aggarwal BB:
Curcumin, a component of turmeric: From farm to pharmacy.
Biofactors. 39:2–13. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ye MX, Li Y, Yin H and Zhang J: Curcumin:
Updated molecular mechanisms and intervention targets in human lung
cancer. Int J Mol Sci. 13:3959–3978. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Sánchez-Martínez C, Gelbert LM, Lallena MJ
and de Dios A: Cyclin dependent kinase (CDK) inhibitors as
anticancer drugs. Bioorg Med Chem Lett. 25:3420–3435. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Kozakowska M, Szade K, Dulak J and
Józkowicz A: Role of heme oxygenase-1 in postnatal differentiation
of stem cells: a possible cross-talk with microRNAs. Antioxid Redox
Signal. 20:1827–1850. 2014. View Article : Google Scholar :
|
|
60
|
Murakami A: Dose-dependent functionality
and toxicity of green tea polyphenols in experimental rodents. Arch
Biochem Biophys. 557:3–10. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zheng KM, Zhang J, Zhang CL, Zhang YW and
Chen XC: Curcumin inhibits appoptosin-induced apoptosis via
upregulating heme oxygenase-1 expression in SH-SY5Y cells. Acta
Pharmacol Sin. 36:544–552. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Cremers NA, Lundvig DM, van Dalen SC,
Schelbergen RF, van Lent PL, Szarek WA, Regan RF, Carels CE and
Wagener FA: Curcumin-induced heme oxygenase-1 expression prevents
H2O2 induced cell death in wild type and heme
oxygenase-2 knockout adipose-derived mesenchymal stem cells. Int J
Mol Sci. 15:17974–17999. 2014. View Article : Google Scholar : PubMed/NCBI
|