Introduction
Breast cancer (BC) is thought to have the highest
diagnostic rate in cancer and is the principal cause of
cancer-related mortality in women worldwide (1). Thus far, the pathological diagnosis
has long been recognized to be the gold criteria for the diagnosis
and pathology-based classifications that are essential for guiding
the therapy of patients with BC. However, currently, gene
expression analyses have a considerable influence on our
understanding of the biology and molecular analysis of BC, thereby
providing clinically relevant information and targeted therapy
(2).
The 6-phosphofructo 2-kinase/fructose 2,
6-bisphospha-tase (PFKFB) family is composed of bifunctional
enzymes that control the level of fructose 2,6-bisphosphate (Fru-2,
6-BP), an essential allosteric activator in the glycolytic flux
(3). There are 4 isoforms of this
enzyme encoded by distinct genes, PFKFB1, PFKFB2,
PFKFB3 and PFKFB4, and each isozyme has been found to
be expressed in different tissues (4–6). The
third PFKFB isozyme encoded by the PFKFB3 gene has also been
termed PGR1, inducible PFK-2 (iPFK-2), placental PFK-2 and
ubiquitous PFK-2 (uPFK-2) (4).
Compared with the other isozymes, the PFKFB3 isozyme has a
very high kinase activity due to its crystal structure and has been
thoroughly researched (4,7). Previous findings have reported a
close correlation between the aberrant expression of PFKFBs and the
tumor aggressiveness grade, which demonstrates that PFKFBs
may play a crucial role in carcinogenesis (3). PFKFB has been found to be
overexpressed in various tumor types, particularly in cancers of
the colon (8), prostate (9), ovaries (9) and thyroid (9). Upregulated PFKFB3 levels have
been correlated with poorer survival statistics in patients with
human epidermal growth factor receptor 2 (HER2+) BC
(10), and its inhibition seems to
suppress glucose metabolism and the growth of HER2+ BC
(10). The overexpression of
microRNA (miRNA)-206 has been demonstrated to regulate PFKFB3
expression, possibly suppressing the proliferation and migration of
BC cells (11). Targeting
PFKFB3 regulates cell the proliferation and apoptosis of
bladder cancer cells by altering the tumor microenvironment
(12). Taken together, these
results suggest that PFKFB3 plays important roles in several
biological processes and in the progression of human cancers.
Glycolysis is important in the initiation of
angiogenesis in tumor vessels (13) and since PFKFB3 is a key
regulator of endothelial glycolysis metabolism, the inhibition or
deletion of endothelial PFKFB3 decreases angiogenesis
(13). Primary tumor growth and
metastasis is dependent on angiogenesis (14). Tumor cells preferentially
metabolize glucose through aerobic glycolysis, a phenomenon known
as the 'Warburg effect' (15).
There are many genes, molecular signals and metabolic pathways
involved in the movement and activity of angiogenesis in tumor
cells. PFKFB3 expression is upregulated by vascular
endothelial growth factor (VEGF), thereby increasing the rate of
glycolysis, and promoting the budding of blood vessels (16). Previous studies have determined
that PFKFB3 plays an important role in regulating tumor
glycolysis, cell proliferation and survival. AKT is a
serine-threonine-kinase that phosphorylates proteins in several
pathways regulating metabolism and proliferation in cancer cells
(17). Activated AKT regulates a
number of targets implicated in tumor progression (18) and PFKFB3 overexpression has
been reported to increase phosphorylated AKT (p-AKT) expression,
thereby preventing apoptosis in an osteoarthritic cartilage explant
(19). p27 is a cell cycle
inhibitor that regulates cell proliferation, motility and apoptosis
(20) and its loss may precede
tumor invasion (21). Increased
levels of p27 protein have been demonstrated in the nucleus and
cytoplasm after PFKFB3 knockdown in HeLa cells (22). The inhibition of the cell cycle and
the induction of apoptosis caused by the suppression of
PFKFB3 can be attenuated by p27 inhibition (23). Furthermore, since tumor vessel
formation is important for tumor progression, the inhibition of
VEGF, which is a main regulator of angiogenesis in vascular
endothelial cells (ECs), has provided benefits to a large number of
cancer patients (24,25).
In the present study, in order to gain insight into
the molecular mechanisms behind the regulation of PFKFB3
expression in BC, we identified the expression status of
PFKFB3 in BC tissues and the correlation between the
PFKFB3 expression level and the prognosis of patients with
BC. We sequentially investigated the specific role of PFKFB3
in BC cell proliferation, migration, invasion, cell cycle
progression and angiogenesis, as well as whether the AKT-related
pathway, p27, and VEGF participate in the above-mentioned
process.
Materials and methods
Ethics statement
This study was approved by the Ethics Committee of
Sun Yat-Sen University (Guangzhou, China). The collection and
analysis of clinical specimens were sanctioned by the local Ethics
Committee of Sun Yat-sen University Cancer Center. Written informed
consent was obtained from each patient prior to participation. The
animal experiments were approved by the Animal Ethics and Welfare
Committee of Sun Yat-Sen University.
Clinical specimens and cell culture
Breast tissue specimens in this study were collected
from 74 patients with BC who had undergone surgery at the Sun
Yat-sen University Cancer Center from 2004 to 2012. These cases
included 49 women aged ≤50 years and 25 women aged >50 years.
The mean age of the study population was 47.83±10.37 years. None of
the patients had received pre-operative treatment, such as
chemotherapy or radiotherapy, prior to surgery. The normal breast
tissues were obtained with at least 5 cm clearance around the
tumor, and all samples were examined histologically. The clinical
pathological characteristics of all 74 patients and their
correlation with PFKFB3 expression are shown in Table I.
 | Table ICorrelation between PFKFB3
expression and clinicopathological characteristics of patients with
breast cancer. |
Table I
Correlation between PFKFB3
expression and clinicopathological characteristics of patients with
breast cancer.
|
Characteristics | PFKFB3
expression score
|
|---|
| All | 1 | 2 | 3 | P-value |
|---|
| Age (years) | | | | | |
| ≤50 | 49 | 13 | 22 | 14 | 0.928 |
| >50 | 25 | 10 | 5 | 10 | |
| ER status | | | | | |
| − | 74 | 16 | 29 | 29 | N/A |
| + | 0 | 0 | 0 | 0 | |
| PR status | | | | | |
| − | 74 | 16 | 29 | 29 | N/A |
| + | 0 | 0 | 0 | 0 | |
| HER-2 status | | | | | |
| − | 74 | 16 | 29 | 29 | N/A |
| + | 0 | 0 | 0 | 0 | |
| TNM stage | | | | | |
| I, II | 34 | 6 | 16 | 12 | 0.428 |
| III, IV | 40 | 10 | 13 | 17 | |
| Tumor size
(cm) | | | | | |
| ≤3 | 54 | 18 | 17 | 19 | 0.286 |
| >3 | 20 | 5 | 10 | 5 | |
| Distant
metastasis | | | | | |
| 0 (No) | 55 | 21 | 19 | 15 |
0.025a |
| 1 (Yes) | 19 | 2 | 8 | 9 | |
| Recurrence | | | | | |
| No | 66 | 22 | 24 | 20 | 0.179 |
| Yes | 8 | 1 | 3 | 4 | |
|
Differentiation | | | | | |
| Moderate | 66 | 20 | 24 | 22 | 0.608 |
| Well | 8 | 3 | 3 | 2 | |
The human BC cell lines, MDA-MB-231 and MDA-MB-468,
and human umbilical vein endothelial cells (HUVECs) were all
purchased from the Type Culture Collection of the Chinese Academy
of Sciences (Shanghai, China). The MDA-MB-231 and MDA-MB-468 cells
were cultured in RPMI-1640 medium (Gibco, Carlsbad, CA, USA), and
the HUVECs were cultured in RPMI-1640 medium with 10% fetal bovine
serum (FBS; Gibco). All cells were cultured in a humidified
incubator at 37°C in a 5% CO2 atmosphere.
Immunohistochemistry
Subsequently, the tissues were embedded in paraffin
and cut into 4-µm-thick sections, followed by treatment with
xylene to remove the paraffin, then rehydrated and treated with
0.3% hydrogen peroxide for 10 min to eliminate endogenous
peroxidase activity. The sections were blocked with 5% goat serum
and incubated for 20 min at room temperature. After washing, the
sections were treated with rabbit polyclonal anti-PFKFB3
antibody raised to the recombinant protein for 30 min (1:200
dilution, ab96699; Abcam, Cambridge, MA, USA). The primary antibody
was replaced with 1% non-immune rabbit serum (negative control),
followed by incubation with an anti-PFKFB3 antibody in the
presence of excess recombinant PFKFB3. The sections were
subsequently incubated with biotinylated goat anti-rabbit
immunoglobulin (Vector Laboratories, Burlingame, CA, USA) and were
developed with the Vectastain Elite BCA kit (Vector Laboratories)
as chromogen, according to the manufacturer's recommendations.
Following counterstaining with Mayer's hematoxylin (Sigma-Aldrich,
St. Louis, MO, USA), the sections were dehydrated and coverslips
were attached with neutral resins (Thermo Fisher Scientific,
Pittsburgh, PA, USA). Each experiment was performed twice. The
expression of PFKFB3 protein was assessed by a proportion
score of the tumor cells and was categorized into scores of 1
(<5% of tumor cells stained positive), 2 (5–25% of tumor cells
stained positive) and 3 (>25% of tumor cells stained positive),
as previously described (26),
with higher scores indicating a greater proportion of positive
cells. In order to investigate the association of PFKFB3
expression with the overall survival (OS) of patients, the survival
analysis was performed using the Kaplan-Meier method.
To further investigate the prognostic value of
PFKFB3 expression in BC, an online survival analysis was
performed via the online database Kaplan-Meier Plotter (http://kmplot.com/analysis/), which contained
expression data from 54,675 genes and survival information from
5,143 breast cancer patients. In order to analyze the OS (n=1,402),
recurrence-free survival (RFS) (n=3,951), distant metastasis-free
survival (DMFS) (n=1,746), and post-progression survival (PPS)
(n=414) of the patients with BC, the patient samples were split
into 2 groups by median expression (high or low expression), with a
hazard ratios (HR), 95% confidence intervals (CI) and log-rank
P-values. The gene symbol PFKFB3 (202464_s_at) was selected
to obtain Kaplan-Meier plots in which the number at risk was
indicated below the main plot.
Lentiviral vector construction and
transduction
The PFKFB3 full-length cDNA was amplified by
RT-PCR using mRNA from the MDA-MB-231 cells with specifically
designed primers (forward primer, 5′-TAG GAT CCA TGG ACT ACA AGG
ACG ACG ACG ACA AGT TGG AAC TGA CGC AGA GCC GA-3′; and reverse
primer, 5′-TGA AGC TTG GAA ATG GAA TGG AAC CGA C-3′). The PCR
products were then digested with BamHI (R3136; New England
BioLabs, Inc., Ipswich, MA, USA) and HindIII I (D6389;
Beyotime Institute of Biotechnology, Shanghai, China) prior to
insertion into a lentiviral vector. A lentiviral vector expressing
enhanced green fuorescent protein (EGFP) was used as the control.
Furthermore, we designed a short-hairpin RNA (shRNA) to target
human PFKFB3 (Guangzhou RiboBio Co., Ltd., Guangzhou,
China), and cloned the shRNA into a human U6 promoter-containing
pBluescript SK (+) plasmid (pU6). The U6-shRNA cassettes were then
sub-cloned into a lentiviral vector. Lentivirus carrying shRNA
targeting firefly luciferase (shNC) was used as the control.
Lentiviral vectors carrying shRNA targeting PFKFB3 were
generated. A total of 5×104 BC cells (MDA-MB-231 and
MDA-MB-468) were then transduced with the lentiviruses
(shPFKFB3 or NC) as previously described (27). Lentiviruses were harvested 3 days
after purification and precipitation.
MTS cell proliferation assay
Cell proliferation was determined by MTS assay with
the CellTiter 96® AQueous One Solution Cell
Proliferation Assay (Promega, Madison, WI, USA). Briefly, 3,000
cells (100 µl/well) were seeded into 96-well plates at a
density of 3×103 cells/well and cultured at 37°C for 1,
2 or 3 days. At these time-points, 20 µl of CellTiter 96®
AQueous One Solution Reagent were added to each well, and the cells
were then incubated for an additional 4 h at 37°C in a 5%
CO2 atmosphere. An ELx800 microplate reader (Bio-Tek
Instruments, Inc., Winooski, VT, USA) was subsequently used to
measure the corresponding absorbance at 490 nm. Each condition was
determined in quintuplicate, and all experiments were repeated
thrice.
Quantitative PCR (qPCR)
Total RNA was extracted from the BC cells using
TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer's instructions and PFKFB3 cDNA synthesis was
performed with a random RT primer using the RevertAid First Strand
cDNA Synthesis kit (Thermo Fisher Scientific, Inc., Rockford, IL,
USA). The PFKFB3 expression level was evaluated by qPCR
using SYBR-Green PCR Master Mix (Applied Biosystems, Warrington,
UK) and an ABI 7500 real-time PCR system (Applied Biosystems).
Glyceraldehyde 3-phosphate dehydrogenase (GAPDH), an endogenous
housekeeping gene, was used to normalize the relative mRNA
expression level of PFKFB3 with the following primers:
PFKFB3 forward, 5′-GG CCGCATCGGGGGCGACTC-3′ and reverse,
5′-TTGCGTCT CAGCTCAGGGAC-3′; and GAPDH forward, 5′-CCGAGA
ATGGGAAGCTTGTC-3′ and reverse, 5′-AAGCACCAACA GAGGAGAA-3′. The
results were normalized to GAPDH expression and RNA enrichments
were calculated using the 2−ΔΔCq method (28).
Cell cycle analysis
Cell cycle analysis was performed with propidium
iodide (PI; Sigma-Aldrich) staining, as previously described
(29). The cells were collected
and fixed in 70% (v/v) ethanol on ice, then washed with
phosphate-buffered saline (PBS) and suspended in the propidium
iodide staining solution (50 mg/l) supplemented with 0.1% Triton
X-100 and RNase (100 mg/l). Following 30 min of incubation, cell
fluorescence was measured using a FACSCalibur flow cytometer (BD
Biosciences, San Jose, CA, USA), and cell cycle analysis was
performed with the ModFit LT 2.0 software (Verity Software House
Inc., Topsham, ME, USA). Each experiment was performed in
triplicate.
Cell migration and invasion assays
The cell migration and invasion assays were
evaluated using Transwell cell culture chambers with 8 µm
pores (Corning Costar Corp., Cambridge, MA, USA) according to the
manufacturer's instructions. For the migration assay, the
MDA-MB-231 and MDA-MB-468 cells incubated in serum-free medium
(Gibco) were separately seeded into the upper chambers without
Matrigel, and the complete medium was supplemented with 10% FBS and
was used in the lower chambers as a chemoattractant. For invasion
assays, Transwell membranes were pre-coated with 10 µl
Matrigel (diluted 1:3; BD Biosciences). Following incubation at
37°C for 24 h, the migratory and invasive cells on the bottom
surface were fixed using 4% paraformaldehyde and stained with a
0.1% crystal violet solution (Sangon Biotech, Co., Ltd., Shanghai,
China). The cells that did not migrate were removed from the upper
membrane surface with a cotton swab. Five randomly selected fields
from each membrane were counted using a light microscope (Nikon
Eclipse E600; Nikon, Tokyo, Japan) with a X20 objective. Each
experiment was performed in triplicate.
Tube formation assay
The effect of the silencing of PFKFB3 using a
lentiviral vector carrying shPFKFB3 on the angiogenesis of
BC cells was evaluated in vitro with a tube formation assay.
The cell supernatant was obtained from the MDA-MB-231 and
MDA-MB-468 transfected with shPFKFB3 cells by centrifugation
at 3,000 × g for 10 min at 4°C. A total of 30,000 HUVECs were
seeded in Matrigel (BD Biosciences) with serum-free medium (Gibco)
in the 24-well plates, in triplicate, co-incubated with the above
cell supernatant at 37°C for 12 h, and fixed with 4%
paraformaldehyde at room temperature. The tubules were visualized
under light microscopy (Nikon Eclipse E600; Nikon) at low
magnification (×40). Photomicrographs from each well were captured,
and the number of tubules was analyzed using ImageJ software,
version 2.02 (National Institutes of Health, Bethesda, MD,
USA).
Western blot analysis
The cells were lysed in Radio-Immunoprecipitation
assay (RIPA) buffer (Thermo Fisher Scientific) supplemented with
protease inhibitor cocktail (Thermo Fisher Scientific). The BCA
protein assay kit (Qcbio Science Technologies, Co., Ltd., Shanghai,
China) was used for the detection of protein concentrations.
Proteins (40 µg) were separated using 10% polyacrylamide
gels, electrophoresed and transferred to a polyvinylidene fluoride
membrane (PVDF; Millipore, Billerica, MA, USA). All the membranes
were blocked with 5% skim milk for 2 h at room temperature, and
then incubated overnight with the respective primary antibodies at
4°C. The following day, the membranes were washed using TBST
(Tris-buffered saline and Tween-20), and incubated with horseradish
peroxidase (HRP)-labeled secondary anti-mouse (ab157532, 1:500) or
anti-rabbit IgG antibody (ab6728, 1:500) at room temperature for 1
h. After washing again with TBST, all blots were visualized using
an enhanced chemiluminescence substrate kit (Amersham Biosciences
Inc., Piscataway, NY, USA). GAPDH was used as the loading control.
The related primary antibodies were rabbit anti-GAPDH antibody
(sc-47724, 1:200; Santa Cruz Biotechnology, Santa Cruz, CA, USA),
rabbit anti-PFKFB3 antibody (ab96699, 1:100), rabbit anti-AKT
antibody (ab8805, 1:100), rabbit anti-p-AKT antibody (ab38449,
1:100), mouse anti-p27 antibody (ab215434, 1:100) and mouse
anti-VEGFα antibody (ab42228, 1:100) (all from Abcam, Cambridge,
MA, USA).
Tumor xenograft growth in nude mice
A total of 10 BALB/c nude mice (male, 5–6 weeks old,
weighing 18–22 g) were purchased from the Shanghai LAC Laboratory
Animal Co., Ltd. (Shanghai, China). The nude mice were housed in a
specific-pathogen-free (SPF) grade animal center. The housing
environment was maintained at 25±2°C, 45–55% humidity, and a
standard 12-h dark/12-h light cycle, and the mice were fed an
autoclaved diet with free access to water. At the beginning of the
experiment, the mice were subcutaneously injected with
2.5×106 MDA-MB-231 cells transduced with either NC or
shPFKFB3 in the front right legs (n=5 mice per group). The tumor
sizes were recorded on days 10, 20 and 30 after the injection and
the tumor volume (mm3) was calculated as follows: Volume
= 0.5 × length × width2. The maximum diameter of the
tumor was approximately 1.0 cm. On the 30th day post-injection, all
mice (weighing 25–30 g) were sacrificed by CO2
inhalation and the tumor nodules were dissected and weighed.
Statistical analysis
All experiments were performed in triplicate, and
data are presented as the means ± SD. Statistical analysis was
performed using SPSS 19.0 (IBM, Armonk, NY, USA). The comparison of
continuous values between 2 groups was performed by means of an
independent-samples t-test. The association between PFKFB3
expression and the clinicopathological characteristics was assessed
using Chi-square tests. Survival was estimated by the Kaplan-Meier
method, and differences between groups were assessed by the
log-rank test. P-values of <0.05 were considered to indicate
statistically significant differences.
Results
PFKFB3 expression and prognosis in BC
tissues
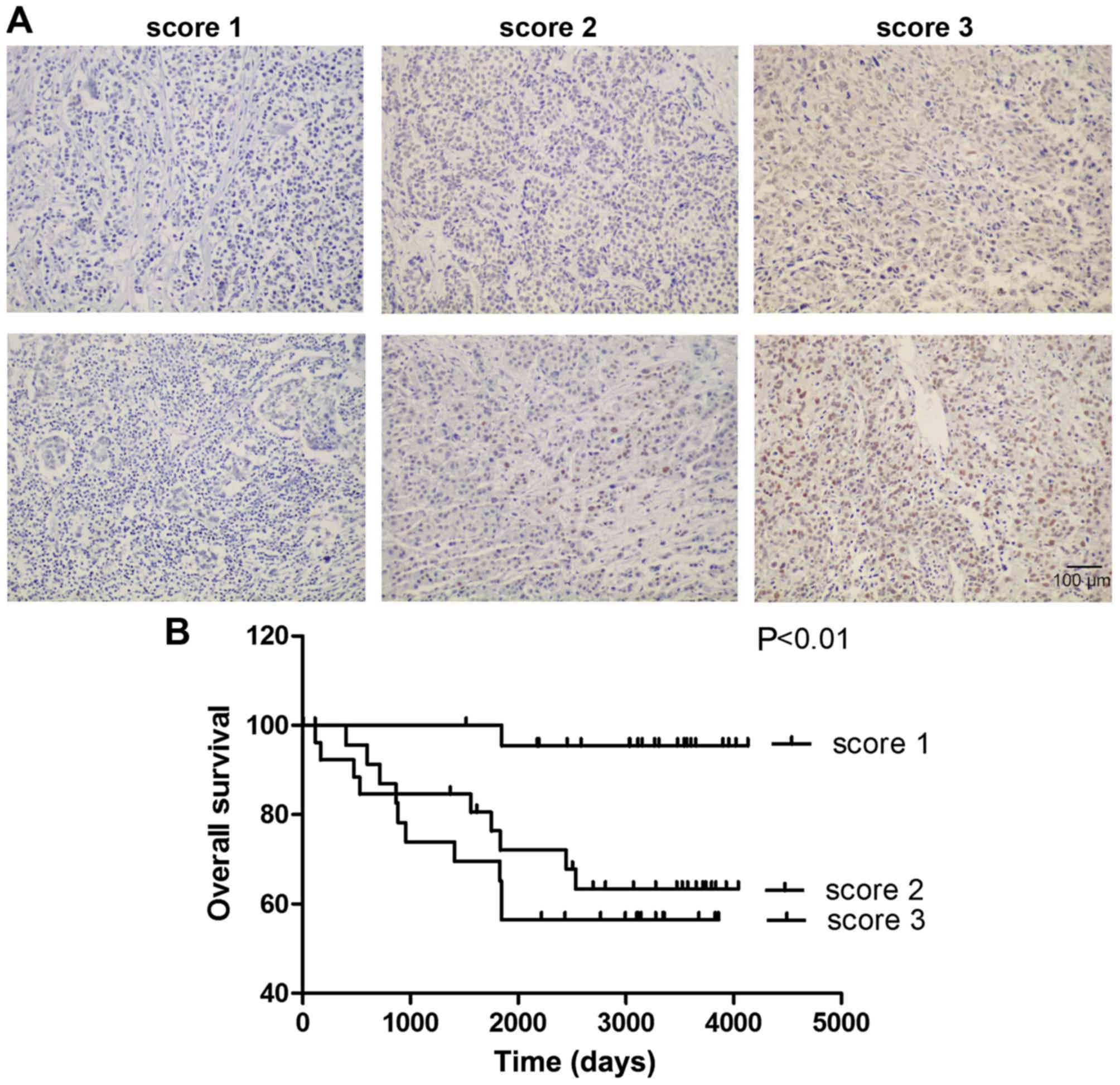
In order to estimate the expression status of
PFKFB3 in BC, the expression level of PFKFB3 was
analyzed by immunohistochemistry in tissues from 74 patients with
BC. The nuclear expression was analyzed by a proportion score of
positive tumor cells and was categorized into scores of 1 (<5%),
2 (5–25%), or 3 (>25%) for PFKFB3 (Fig. 1A). The patients with BC were then
separated according to the PFKFB3 expression level (median
split) and contrasted with different clinicopathological
characteristics [age, tumor size, estrogen receptor (ER) status,
progesterone receptor (PR) status, HER-2 status, TNM stage, distant
metastasis, recurrence and differentiation] (Table I). Recurrence was defined as the
return of cancer after a period of time during which it could not
be detected. The recurrence could be in a different place or in the
same location as the original tumor. Metastasis was defined as
cancer spread from the original site to a different site of the
body. The occurrence of distant metastasis was more frequent in
groups with a higher PFKFB3 expression (Chi-square test,
P=0.025; Table I). Furthermore,
Kaplan-Meier analysis revealed that a higher expression of
PFKFB3 was associated with a shorter OS time in patients
with BC (Fig. 1B, P<0.01,
log-rank test). In addition, online survival analysis revealed that
a higher PFKFB3 expression was associated with a poor OS
(Fig. 2A, HR=1.35, P=0.0063,
Kaplan-Meier) and RFS (Fig. 2B,
P=0.0034). There was no difference, however, between DMFS (Fig. 2C, P=0.33) or PPS (Fig. 2D, P=0.4) in patients with BC with
an altered PFKFB3 expression. These results suggest that
PFKFB3 expression is upregulated in BC tissues and is
associated with a poor patient prognosis.
Suppression of PFKFB3 inhibits BC cell
growth, migration and invasion and induces cell cycle arrest
In order to investigate the biological roles of
PFKFB3 in the progression of BC, we constructed lentiviral
vectors expressing shRNA targeting PFKFB3, and then infected
the MDA-MB-231 and NDA-MB-468 cells with this shRNA lentivirus
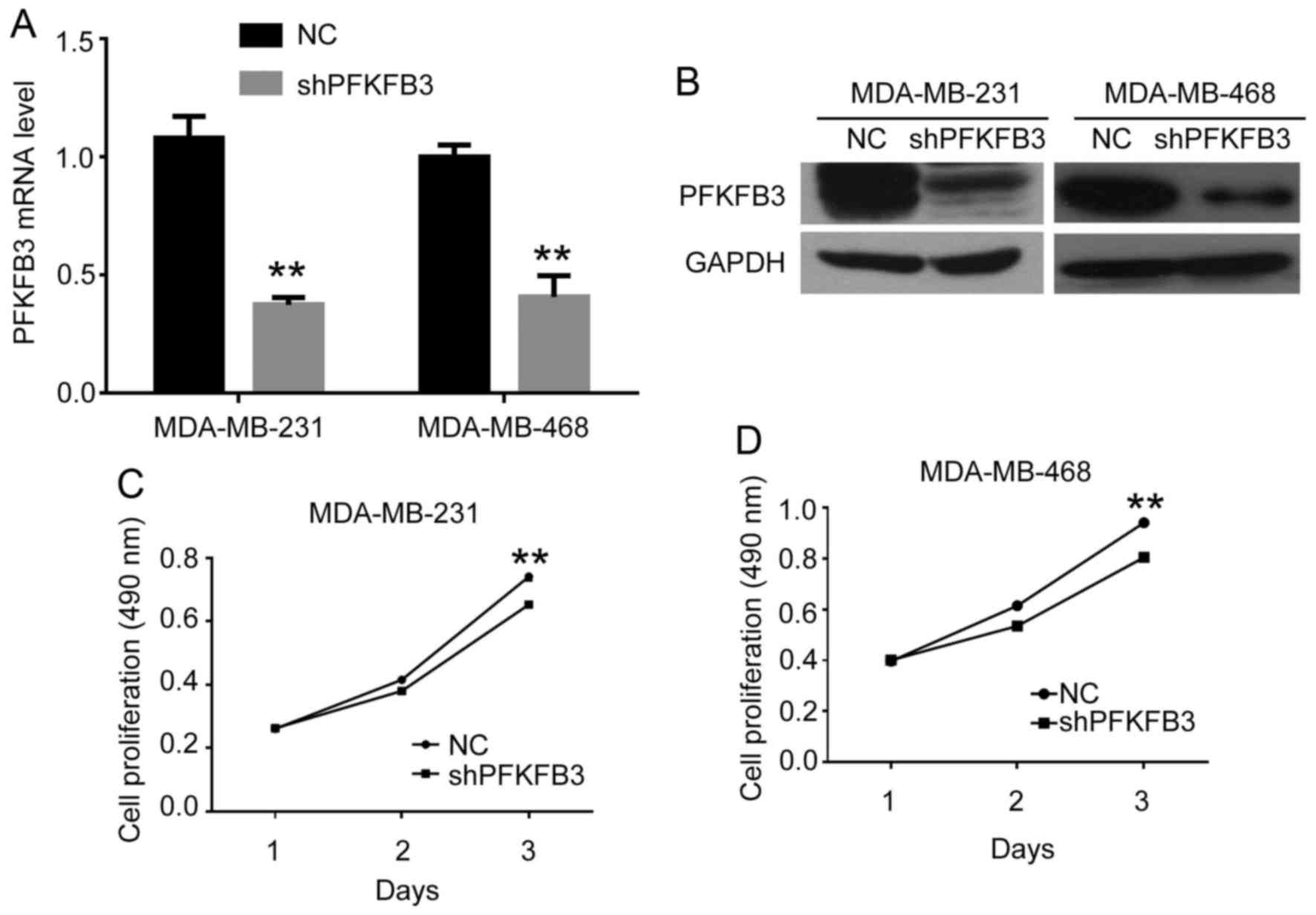
(shPFKFB3). The expression of PFKFB3 in the cells following
transfection with shPFKFB3 was confirmed by qPCR and western
blot analysis in BC cells (Fig. 3A and
B). We found that the mRNA and protein expression levels of
PFKFB3 were significantly inhibited by transfection with
shRNA. We then performed an MTT assay to assess the effects of
PFKFB3 on BC cell proliferation. As shown in Fig. 3C and D, cell proliferation was
significantly suppressed in both the MDA-MB-231 and MDA-MB-468
cells transfected with shPFKFB3 compared to the NC group at
3 days (P<0.01). The change in cell cycle distribution was
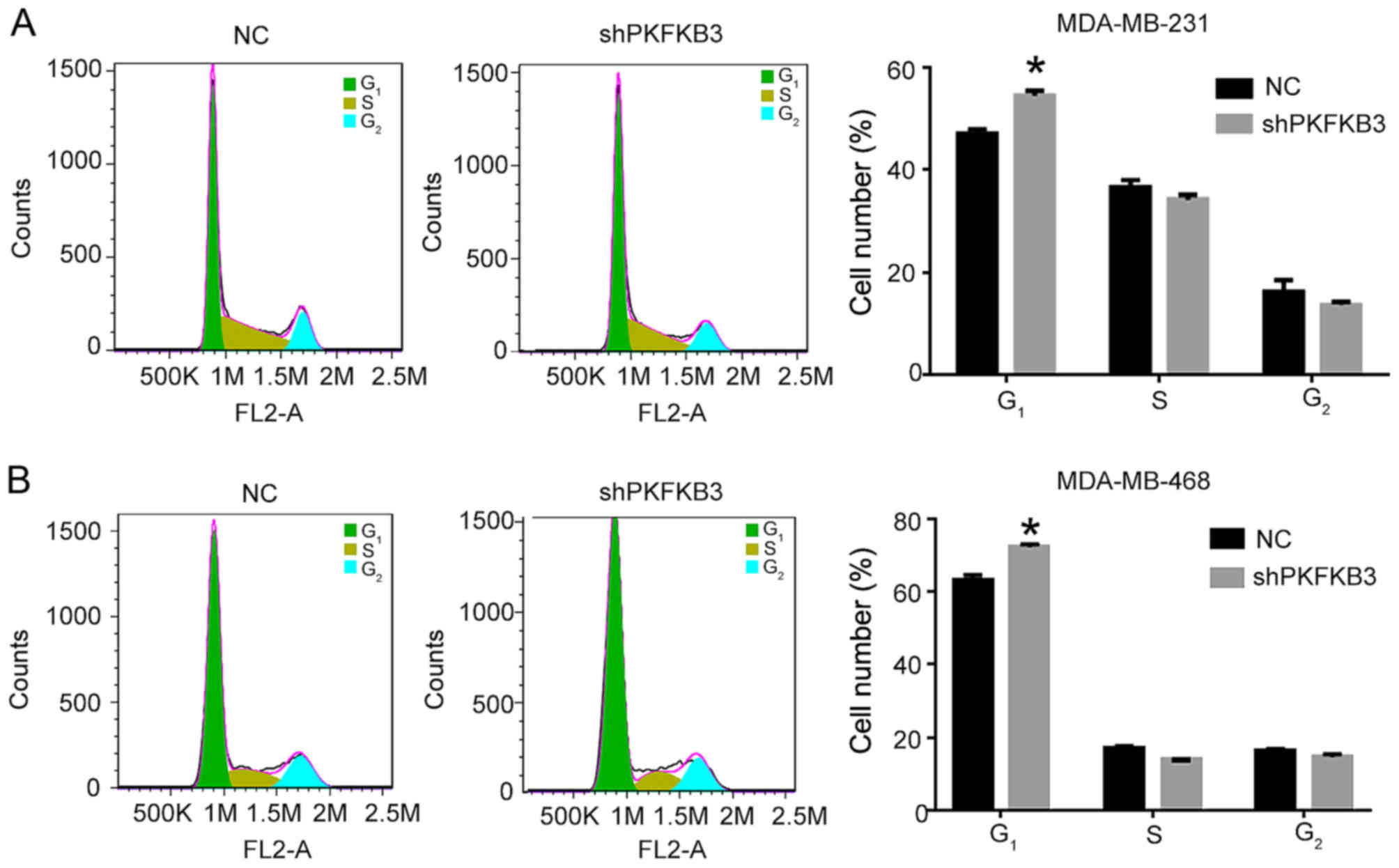
observed by flow cytometry in the cells in which PFKFB3 was
knocked down. We found that the cell cycle was arrested in the
G1 phase in the MDA-MB-231 and MDA-MB-468 cells
transfected with the shPFKFB3 lentivirus (P<0.05;
Fig. 4). The effect of PFKFB3
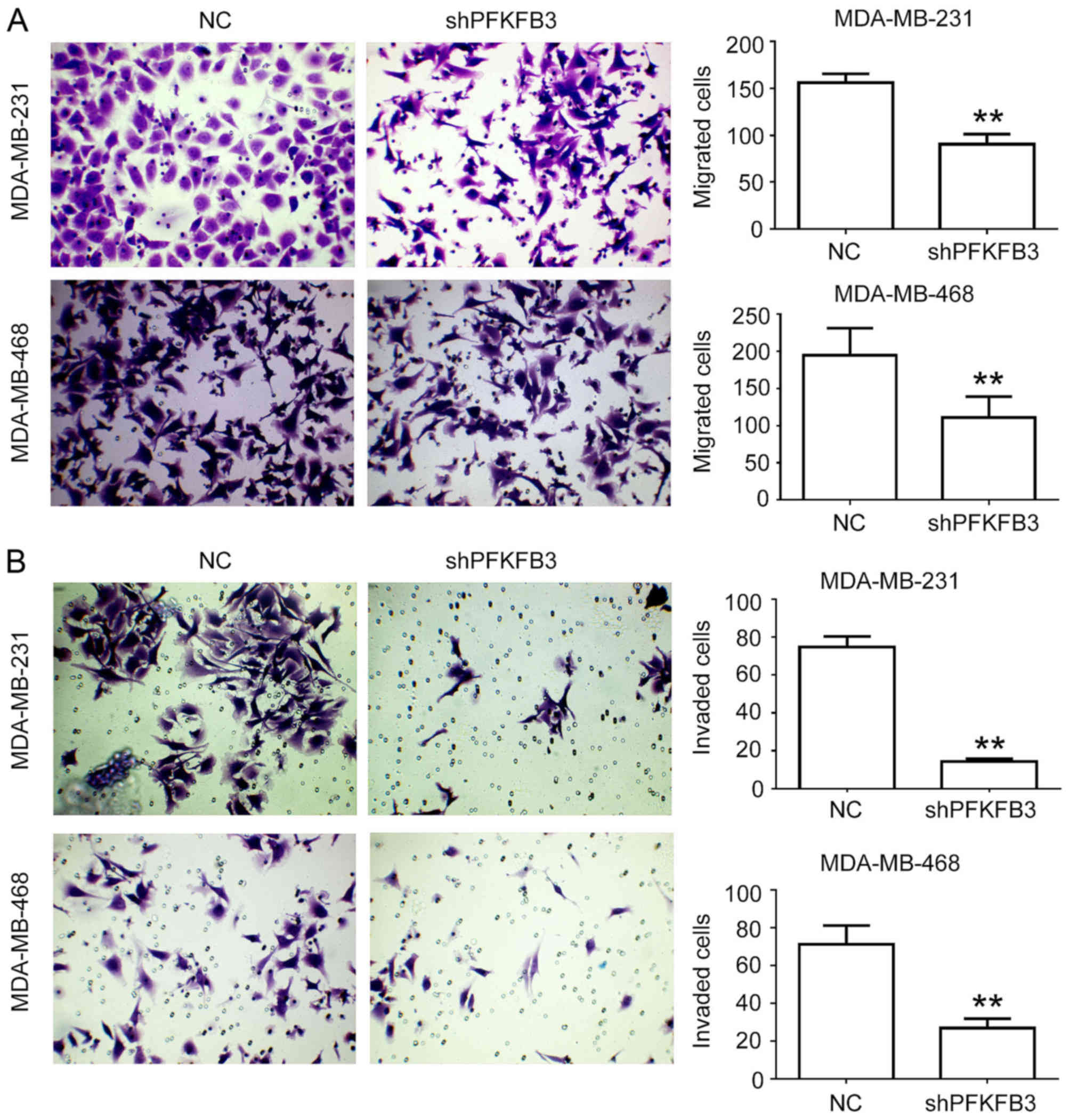
knockdown on cell migration and invasion was evaluated in
vitro by a Transwell assay, revealing that the knockdown of
PFKFB3 significantly reduced cell migration (P<0.01;
Fig. 5A) and invasion (P<0.01;
Fig. 5B). These results suggest
that PFKFB3 suppression inhibits cell proliferation,
migration and invasion, and induces cell cycle arrest in BC
cells.
Suppression of PFKFB3 in BC cells
prevents angiogenic activity in HUVECs
HUVECs have been a standard for cell-based assays in
the field of in vitro angiogenesis research (30). Studies have shown that they can
retain the ability to form tridimensional tubules in the Matrigel
extract of the matrix-rich basement membrane (30–32).
We further determined whether PFKFB3 could stimulate
angiogenesis. Therefore, the MDA-MB-231 and MDA-MB-468 cells were
transfected with shPFKFB3 or NC for 12 h, the cell
supernatant was collected and the HUVECs in Matrigel were cultured
in the cell supernatant. Subsequently, tube formation was observed.
We found that the suppression of PFKFB3 significantly
decreased HUVEC tube length and the number of MDA-MB-231 and
NDA-MB-468 cells (Fig. 6). These
findings suggest that PFKFB3 is required for efficient tube
formation in BC.
PFKFB3 is involved in the expression of
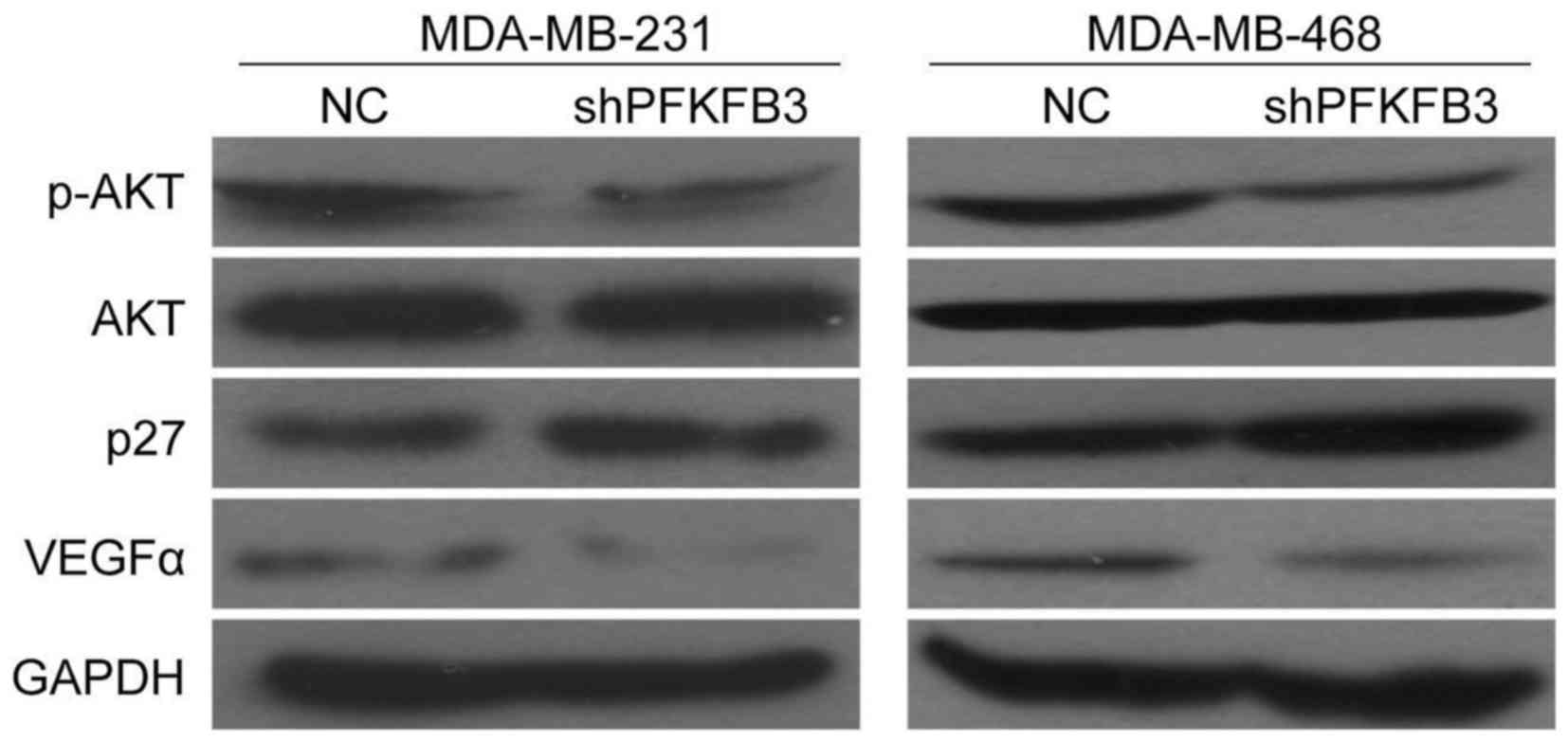
p-AKT, p27 and VEGFα in BC cells
As shown above, we demonstrated the effects of
PFKFB3 knockdown on the proliferation and cell cycle arrest
in BC cells. Therefore, we then wished to determine whether there
was an association between PFKFB3 expression and cell
proliferation-related genes (AKT), cell cycle-related genes (p27),
or angiogenesis-related genes (VEGF-α). The results revealed that
the suppression of PFKFB3 decreased p-AKT expression, but
not AKT expression, while it increased p27 expression in the
MDA-MB-231 and MDA-MB-468 cells (Fig.
7). Moreover, we also found that VEGFα expression was
downregulated in the BC cells transduced with shPFKFB3
compared with the NC group (Fig.
7). These findings provide further evidence that PFKFB3 affects
BC cell growth, migration and invasion abilities, and is involved
in the cell cycle and angiogenesis of BC cells in this study.
Suppression of PFKFB3 inhibits BC cell
growth in vivo
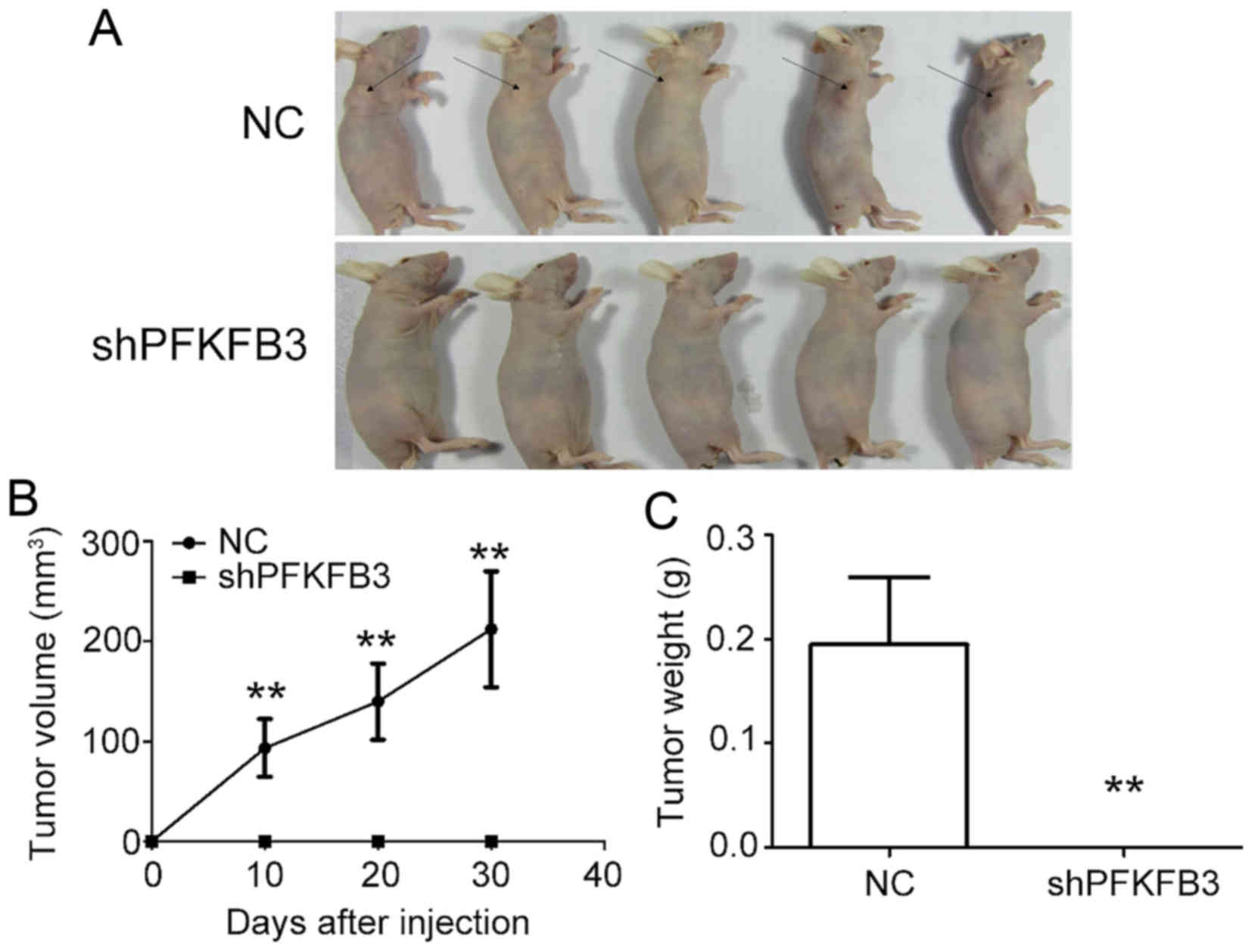
To examine the effect of PFKFB3 on BC cell
proliferation in vivo, we generated xenograft models by
implanting MDA-MB-231 cells transduced with shPFKFB3 or NC
into nude mice. No tumors formed in the mice injected with the BC
cells transfected with shPFKFB3 (Fig. 8A). The results also revealed a
marked decrease in the xenograft subcutaneous tumor volume
(Fig. 8B) and weight (Fig. 8C) in the PFKFB3 inhibition
group compared with the control group. Taken together, these data
confirm the inhibitory effect of PFKFB3 knockdown on BC cell
growth, both in vivo and in vitro.
Discussion
PFKFBs, a single homodimeric bifunctional
enzyme family, are the master control point in the malignant cell
glycolytic pathway (4,33). A number of studies have reported
that PFKFBs play an important role in the Warburg effect
(tumor cells exhibit a greater dependence on glycolysis for ATP
generation than their origin tissue) and cancer growth (4,34,35).
The over-expression of PFKFB3 and other PFKFB
isozymes has been observed in various tumor types, such as gastric,
pancreatic, lung, breast and colon cancers (9,33,36,37).
At present, several studies have indicated that
PFKFB3 increases proliferation and regulates glycolysis and
survival in response to mitophagy during mitotic arrest in
MDA-MB-231 cells (38,39). The silencing of PFKFB3 with
siRNAs can lead to the decreased proliferation of MDA-MB-231 and
MDA-MB-468 cells (40). Therefore,
in this study, we selected the MDA-MB-231 and MDA-MB-468 cells as
research targets. We examined the expression of PFKFB3 in BC
tissues and investigated the mechanisms responsible for the
inhibition of PFKFB3 expression in cell proliferation,
migration, cell cycle and angiogenesis. Our results revealed that
PFKFB3 was highly expressed in BC tissues and was associated
with a poor OS in patients with BC. We also found that the
silencing of PFKFB3 with shRNA significantly suppressed BC
cell proliferation, migration, invasion and angiogenic abilities,
and induced cell cycle arrest. In addition, we found that the
silencing of PFKFB3 caused a marked decrease in the rate of
xenograft subcutaneous tumor growth. Therefore, we proved that
PFKFB3 was overexpressed in BC tissues, and that the
suppression of PFKFB3 inhibited BC cell growth both in
vivo and in vitro. We confirmed that PFKFB3
inhibition reduced the phosphorylation of AKT and causes a marked
increase in p27 expression. Based on these results, we can
therefore conclude that PFKFB3 functions as a regulator of
cell progression in BC cells.
Several previous studies have demonstrated that
PFKFB3 is overexpressed in cancer tissues and cells. Han
et al (41) reported that
PFKFB3 was highly expressed in patients with gastric cancer
and that it promoted the proliferation and migration of gastric
cancer cells. PFKFB3 expression was also found to be
increased in pancreatic cancer cell lines (42) and BC cells (MCF-7RA and MCF-7RB)
(43). Of note, in this study,
survival analysis revealed that a high PFKFB3 expression
conferred a poor overall and recurrence-free survival in patients
with BC, indicating that PFKFB3 may be an essential
downstream target for cancer therapies. PFKFB3 inhibitors
have been proven to function as metabolic regulators, which can be
expected to suppress tumors both in vivo and in vitro
(44). In addition, in this study,
we found that PFKFB3 inhibition triggered cell cycle arrest
in BC cells in the G1 phase, suggesting that this
inhibition may prevent cancer cell proliferation. A previous study
demonstrated that PFKFB3 inhibition suppressed glycolysis
and induced G2 phase cell cycle arrest in HeLa cells
(22). Recent studies have also
found that the role of PFKFB3 in tumorigenesis was mainly
dependent on, not only its glycolysis regulatory function, but also
in regulating the cell cycle in the nucleus. For example, Yalcin
et al (23) reported that
PFKFB3 inhibition resulted in a G1 block and caused a
marked increase in p27 protein expression in HeLa cells. Our
results revealed that PFKFB3 inhibition decreased p-AKT
expression and increased p27 expression in BC cells. p27 is a
inhibitor of G1 cyclin/cyclin-dependent kinase (Cdk)
protein kinase activity, and plays an important role in the cancer
cell cycle (45). One study
indicated that p27 has an oncogenic potential to promote tumor
progression through the induction of metastasis (46). We demonstrated that PFKFB3
inhibition reduced AKT phosphorylation, causing a marked increase
in p27 expression. PFKFB3 is regulated by AKT and
phosphatase and tensin homolog (PTEN), which is required for the
survival and growth of multiple cancer types (47,48).
In addition, this study found that the suppression
of PFKFB3 weakened VEGFα expression and decreased angiogenic
activity in BC cells. The overexpression of different PFKFBs
has been reported to promote VEGF expression in gastric and
pancreatic cancer cells under hypoxic conditions (33). Studies have also shown that
vascularization is closely related to tumor development (49,50).
Abnormal vascularization promotes metastasis in malignant tumors,
and VEGF inhibitors have been used to treat tumors with a certain
therapeutic effect. According to the latest research, the
suppression of PFKFB3 in endothelial cells (ECs) can improve
tumor vessel maturation and perfusion, thereby inhibiting cancer
cell invasion, intravasation and metastasis (51). PFKFB3 inhibition reduces
VE-cadherin endocytosis, thereby tightening the vascular barrier in
ECs. Moreover, the suppression of PFKFB3 in perithelial
cells renders these cells more quiescent and adhesive through
glycolysis reduction (51). Our
data also indicated that PFKFB3 suppression in BC cells
significantly decreased HUVEC tube length and number. Since
PFKFB3 has powerful features of tumor cell metabolism, and
since there is conclusive evidence that the expression of
PFKFB3 is involved in a poor prognosis in BC, it may serve
as a potential target for the development of effective
antineoplastic therapies.
In conclusion, this study revealed that
PFKFB3 was over-expressed in BC tissues. The
lentivirus-mediated suppression of PFKFB3 inhibited BC cell
proliferation, migration and invasion, and induced cell cycle
arrest in vitro. Moreover, we also found that the
suppression of PFKFB3 inhibited vascularization in BC cells
by suppressing the VEGFα protein level and preventing HUVEC
angiogenic behavior. In addition, p-AKT expression decreased, while
the p27 level increased, in BC cells transduced with PFKFB3
lentivirus carrying shPFKFB3. Furthermore, the suppression of
PFKFB3 inhibited BC cell xenograft growth in nude mice.
Since no further evidence was found regarding the involvement of
the AKT-related pathway in the suppression of cell cycle arrest,
proliferation, migration and invasion induced by the inhibition of
PFKFB3, further studies are required to confirm our
findings. The association between PFKFB3 expression and
cancer types other than triple-negative BC remains unknown. Further
unequivocal evidence for the regulatory role of PFKFB3 in
cell biological functions and glycolysis in different types of
cancer is required.
Acknowledgments
This study was supported in part by the National
Natural Science Foundation of China (no. 81602661), the Natural
Science Foundation of Guangdong Province, China (no.
2016A030310164) and the Medical Scientific Research Foundation of
Guangdong Province, China (no. A2017023). The funders had no role
in the study design, data collection and analysis, decision to
publish, or preparation of the manuscript.
Notes
[1] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Prat A, Pineda E, Adamo B, Galván P,
Fernández A, Gaba L, Díez M, Viladot M, Arance A and Muñoz M:
Clinical implications of the intrinsic molecular subtypes of breast
cancer. Breast. 24(Suppl 2): S26–S35. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kocemba KA, Dulińska-Litewka J, Wojdyła KL
and Pękala PA: The role of 6-phosphofructo-2-kinase
(PFK-2)/fructose 2,6-bisphosphatase (FBPase-2) in metabolic
reprogramming of cancer cells. Postepy Hig Med Dosw. 70:938–950.
2016. View Article : Google Scholar
|
|
4
|
Yalcin A, Telang S, Clem B and Chesney J:
Regulation of glucose metabolism by
6-phosphofructo-2-kinase/fructose-2,6-bisphos-phatases in cancer.
Exp Mol Pathol. 86:174–179. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Okar DA, Manzano A, Navarro-Sabatè A,
Riera L, Bartrons R and Lange AJ: PFK-2/FBPase-2: Maker and breaker
of the essential biofactor fructose-2,6-bisphosphate. Trends
Biochem Sci. 26:30–35. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rider MH, Bertrand L, Vertommen D, Michels
PA, Rousseau GG and Hue L:
6-phosphofructo-2-kinase/fructose-2,6-bisphospha-tase: Head-to-head
with a bifunctional enzyme that controls glycolysis. Biochem J.
381:561–579. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim SG, Manes NP, El-Maghrabi MR and Lee
YH: Crystal structure of the hypoxia-inducible form of
6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (PFKFB3): A
possible new target for cancer therapy. J Biol Chem. 281:2939–2944.
2006. View Article : Google Scholar
|
|
8
|
Clem BF, O'Neal J, Tapolsky G, Clem AL,
Imbert-Fernandez Y, Kerr DA II, Klarer AC, Redman R, Miller DM,
Trent JO, et al: Targeting 6-phosphofructo-2-kinase (PFKFB3) as a
therapeutic strategy against cancer. Mol Cancer Ther. 12:1461–1470.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Atsumi T, Chesney J, Metz C, Leng L,
Donnelly S, Makita Z, Mitchell R and Bucala R: High expression of
inducible 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase
(iPFK-2; PFKFB3) in human cancers. Cancer Res. 62:5881–5887.
2002.PubMed/NCBI
|
|
10
|
O'Neal J, Clem A, Reynolds L, Dougherty S,
Imbert-Fernandez Y, Telang S, Chesney J and Clem BF: Inhibition of
6-phosphofructo-2-kinase (PFKFB3) suppresses glucose metabolism and
the growth of HER2+ breast cancer. Breast Cancer Res
Treat. 160:29–40. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ge X, Lyu P, Cao Z, Li J, Guo G, Xia W and
Gu Y: Overexpression of miR-206 suppresses glycolysis,
proliferation and migration in breast cancer cells via PFKFB3
targeting. Biochem Biophys Res Commun. 463:1115–1121. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hu KY, Wang G, Liu PF, Cao YW, Wang YH,
Yang XC, Hu CX, Sun LJ and Niu HT: Targeting of MCT1 and PFKFB3
influences cell proliferation and apoptosis in bladder cancer by
altering the tumor microenvironment. Oncol Rep. 36:945–951. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu Y, An X, Guo X, Habtetsion TG, Wang Y,
Xu X, Kandala S, Li Q, Li H, Zhang C, et al: Endothelial PFKFB3
plays a critical role in angiogenesis. Arterioscler Thromb Vasc
Biol. 34:1231–1239. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Panigrahy D, Singer S, Shen LQ,
Butterfield CE, Freedman DA, Chen EJ, Moses MA, Kilroy S, Duensing
S, Fletcher C, et al: PPARgamma ligands inhibit primary tumor
growth and metastasis by inhibiting angiogenesis. J Clin Invest.
110:923–932. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim JW and Dang CV: Cancer's molecular
sweet tooth and the Warburg effect. Cancer Res. 66:8927–8930. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schoors S, De Bock K, Cantelmo AR,
Georgiadou M, Ghesquière B, Cauwenberghs S, Kuchnio A, Wong BW,
Quaegebeur A, Goveia J, et al: Partial and transient reduction of
glycolysis by PFKFB3 blockade reduces pathological angiogenesis.
Cell Metab. 19:37–48. 2014. View Article : Google Scholar
|
|
17
|
Ruiter GA, Zerp SF, Bartelink H, van
Blitterswijk WJ and Verheij M: Anti-cancer alkyl-lysophospholipids
inhibit the phosphatidylinositol 3-kinase-Akt/PKB survival pathway.
Anticancer Drugs. 14:167–173. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cardone MH, Roy N, Stennicke HR, Salvesen
GS, Franke TF, Stanbridge E, Frisch S and Reed JC: Regulation of
cell death protease caspase-9 by phosphorylation. Science.
282:1318–1321. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qu J, Lu D, Guo H, Miao W, Wu G and Zhou
M: PFKFB3 modulates glycolytic metabolism and alleviates
endoplasmic reticulum stress in human osteoarthritis cartilage.
Clin Exp Pharmacol Physiol. 43:312–318. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chu IM, Hengst L and Slingerland JM: The
Cdk inhibitor p27 in human cancer: Prognostic potential and
relevance to anticancer therapy. Nat Rev Cancer. 8:253–267. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Slingerland J and Pagano M: Regulation of
the cdk inhibitor p27 and its deregulation in cancer. J Cell
Physiol. 183:10–17. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Calvo MN, Bartrons R, Castaño E, Perales
JC, Navarro-Sabaté A and Manzano A: PFKFB3 gene silencing decreases
glycolysis, induces cell-cycle delay and inhibits
anchorage-independent growth in HeLa cells. FEBS Lett.
580:3308–3314. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yalcin A, Clem BF, Imbert-Fernandez Y,
Ozcan SC, Peker S, O'Neal J, Klarer AC, Clem AL, Telang S and
Chesney J: 6-Phosphofructo-2-kinase (PFKFB3) promotes cell cycle
progression and suppresses apoptosis via Cdk1-mediated
phosphorylation of p27. Cell Death Dis. 5:e13372014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wagner KD, Cherfils-Vicini J, Hosen N,
Hohenstein P, Gilson E, Hastie ND, Michiels JF and Wagner N: The
Wilms' tumour suppressor Wt1 is a major regulator of tumour
angiogenesis and progression. Nat Commun. 5:58522014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Carmeliet P and Jain RK: Molecular
mechanisms and clinical applications of angiogenesis. Nature.
473:298–307. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Steidl C, Lee T, Shah SP, Farinha P, Han
G, Nayar T, Delaney A, Jones SJ, Iqbal J, Weisenburger DD, et al:
Tumor-associated macrophages and survival in classic Hodgkin's
lymphoma. N Engl J Med. 362:875–885. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yan F, Tan XY, Geng Y, Ju HX, Gao YF and
Zhu MC: Inhibition effect of siRNA-downregulated UHRF1 on breast
cancer growth. Cancer Biother Radiopharm. 26:183–189. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
29
|
Matsui TA, Murata H, Sowa Y, Sakabe T,
Koto K, Horie N, Tsuji Y, Sakai T and Kubo T: A novel MEK1/2
inhibitor induces G1/S cell cycle arrest in human fibrosarcoma
cells. Oncol Rep. 24:329–333. 2010.PubMed/NCBI
|
|
30
|
Bouïs D, Hospers GA, Meijer C, Molema G
and Mulder NH: Endothelium in vitro: A review of human vascular
endothelial cell lines for blood vessel-related research.
Angiogenesis. 4:91–102. 2001. View Article : Google Scholar
|
|
31
|
Lau WH, Pandey V, Kong X, Wang XN, Wu Z,
Zhu T and Lobie PE: Trefoil factor-3 (TFF3) stimulates de novo
angiogenesis in mammary carcinoma both directly and indirectly via
IL-8/CXCR2. PLoS One. 10:e01419472015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bagley RG, Walter-Yohrling J, Cao X, Weber
W, Simons B, Cook BP, Chartrand SD, Wang C, Madden SL and Teicher
BA: Endothelial precursor cells as a model of tumor endothelium:
Characterization and comparison with mature endothelial cells.
Cancer Res. 63:5866–5873. 2003.PubMed/NCBI
|
|
33
|
Minchenko OH, Tsuchihara K, Minchenko DO,
Bikfalvi A and Esumi H: Mechanisms of regulation of PFKFB
expression in pancreatic and gastric cancer cells. World J
Gastroenterol. 20:13705–13717. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chesney J:
6-phosphofructo-2-kinase/fructose-2,6-bisphospha-tase and tumor
cell glycolysis. Curr Opin Clin Nutr Metab Care. 9:535–539. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chesney J, Mitchell R, Benigni F, Bacher
M, Spiegel L, Al-Abed Y, Han JH, Metz C and Bucala R: An inducible
gene product for 6-phosphofructo-2-kinase with an AU-rich
instability element: Role in tumor cell glycolysis and the Warburg
effect. Proc Natl Acad Sci USA. 96:3047–3052. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Minchenko OH, Ogura T, Opentanova IL,
Minchenko DO, Ochiai A, Caro J, Komisarenko SV and Esumi H:
6-Phosphofructo-2-kinase/fructose-2,6-bisphosphatase gene family
overexpression in human lung tumor. Ukr Biokhim Zh (1999).
77:46–50. 2005.
|
|
37
|
Minchenko OH, Ochiai A, Opentanova IL,
Ogura T, Minchenko DO, Caro J, Komisarenko SV and Esumi H:
Overexpression of
6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-4 in the human
breast and colon malignant tumors. Biochimie. 87:1005–1010. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yalcin A, Clem BF, Simmons A, Lane A,
Nelson K, Clem AL, Brock E, Siow D, Wattenberg B, Telang S, et al:
Nuclear targeting of 6-phosphofructo-2-kinase (PFKFB3) increases
proliferation via cyclin-dependent kinases. J Biol Chem.
284:24223–24232. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Doménech E, Maestre C, Esteban-Martínez L,
Partida D, Pascual R, Fernández-Miranda G, Seco E, Campos-Olivas R,
Pérez M, Megias D, et al: AMPK and PFKFB3 mediate glycolysis and
survival in response to mitophagy during mitotic arrest. Nat Cell
Biol. 17:1304–1316. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Clem B, Telang S, Clem A, Goswami U and
Chesney J: Inhibition of 6-phosphofructo-2-kinase suppresses breast
tumor growth in vivo. Cancer Res. 69(2 Suppl): 30642009. View Article : Google Scholar
|
|
41
|
Han J, Meng Q, Xi Q, Wang H and Wu G:
PFKFB3 was overexpressed in gastric cancer patients and promoted
the proliferation and migration of gastric cancer cells. Cancer
Biomark. 18:249–256. 2017. View Article : Google Scholar
|
|
42
|
Bobarykina AY, Minchenko DO, Opentanova
IL, Moenner M, Caro J, Esumi H and Minchenko OH: Hypoxic regulation
of PFKFB-3 and PFKFB-4 gene expression in gastric and pancreatic
cancer cell lines and expression of PFKFB genes in gastric cancers.
Acta Biochim Pol. 53:789–799. 2006.PubMed/NCBI
|
|
43
|
Ge X, Cao Z, Gu Y, Wang F, Li J, Han M,
Xia W, Yu Z and Lyu P: PFKFB3 potentially contributes to paclitaxel
resistance in breast cancer cells through TLR4 activation by
stimulating lactate production. Cell Mol Biol (Noisy-le-Grand,
France). 62:119–125. 2016.
|
|
44
|
Lu L, Chen Y and Zhu Y: The molecular
basis of targeting PFKFB3 as a therapeutic strategy against cancer.
Oncotarget. 8:62793–62802. 2017.PubMed/NCBI
|
|
45
|
Møller MB: P27 in cell cycle control and
cancer. Leuk Lymphoma. 39:19–27. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhao D, Besser AH, Wander SA, Sun J, Zhou
W, Wang B, Ince T, Durante MA, Guo W, Mills G, et al: Cytoplasmic
p27 promotes epithelial-mesenchymal transition and tumor metastasis
via STAT3-mediated Twist1 upregulation. Oncogene. 34:5447–5459.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Simon-Molas H, Nieves Calvo-Vidal M,
Castaño E, Bartrons R and Manzano A: Akt mediates TIGAR induction
in HeLa cells following PFKFB3 inhibition. FEBS Lett. 17:pp.
59020162016, https://doi.org/10.1002/1873-3468.12338.
|
|
48
|
Rodríguez-García A, Samsó P, Fontova P,
Simon-Molas H, Manzano A, Castaño E, Rosa JL, Martinez-Outshoorn U,
Ventura F, Navarro-Sabaté À, et al: TGF-β1 targets Smad, p38 MAPK,
and PI3K/Akt signaling pathways to induce PFKFB3 gene expression
and glycolysis in glioblastoma cells. FEBS J. 284:3437–3454. 2017.
View Article : Google Scholar
|
|
49
|
Guo C, Buranych A, Sarkar D, Fisher PB and
Wang XY: The role of tumor-associated macrophages in tumor
vascularization. Vasc Cell. 5:202013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang LZ, Zhang CQ, Yan ZY, Yang QC, Jiang
Y and Zeng BF: Tumor-initiating cells and tumor vascularization.
Pediatr Blood Cancer. 56:335–340. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Cantelmo AR, Conradi LC, Brajic A, Goveia
J, Kalucka J, Pircher A, Chaturvedi P, Hol J, Thienpont B, Teuwen
LA, et al: Inhibition of the glycolytic activator PFKFB3 in
endothelium induces tumor vessel normalization, impairs metastasis,
and improves chemotherapy. Cancer Cell. 30:968–985. 2016.
View Article : Google Scholar : PubMed/NCBI
|