Introduction
In recent years, lung cancer has been ranked among
the primary malignant tumors associated with the highest morbidity
and mortality worldwide (1–3).
Non-small-cell lung cancer (NSCLC) is the most common type of this
disease, accounting for ~80% of all lung malignancies (4,5).
Treatment regimens vary and may include radiotherapy, chemotherapy,
molecular targeted therapies and surgery, alone or in combination.
However, the 5-year survival rate among NSCLC patients remains poor
(6,7). Recent technological advances have
allowed researchers to identify genetic factors that play essential
roles in the pathophysiology of NSCLC, providing novel approaches
for the development of treatments for this disease (8,9).
Therefore, elucidation of the molecular mechanisms underlying NSCLC
and identification of new genetic targets for its treatment should
be urgently conducted.
Aquaporins (AQPs), a family of small (30
kDa/monomer) transmembrane water channel proteins, were first
described in the late 1950s in red blood cells and later in renal
epithelia, and have been identified as key potential targets for
novel antitumor therapies (10–12).
AQP5, one of the most important members of this protein family, is
expressed in virtually all normal tissues and contributes to
cellular regulation of water homeostasis (13,14).
Malignant tumor cells have been reported to take advantage of this
process to facilitate their proliferation and development (15,16).
In addition, AQP5 gene silencing has been reported to inhibit such
proliferation, suggesting that altered AQP5 expression is crucial
in tumor progression (17,18). Numerous studies have demonstrated
that AQP5 is upregulated in NSCLC, and that AQP5-overexpressing
tumor cells are associated with increased tumor growth rates and
progression owing to heightened proliferation (19–21).
However, the association between the AQP5 gene and NSCLC is yet to
be fully elucidated.
The aims of the present study were to establish
whether AQP5 serves an important role in NSCLC and how AQP5 gene
silencing may influence cell proliferation and apoptosis in this
malignancy. Furthermore, the underlying molecular mechanisms were
examined to provide a new direction for the treatment of NSCLC.
Materials and methods
Cell culture
The cell lines A549, H358, HCC827 and H1299 were
obtained from the Cell Bank of the Type Culture Collection of the
Chinese Academy of Sciences (Shanghai, China). The MRC-5 cell line
was also purchased from Guangzhou Jinnio Biotechnology Co., Ltd.
(Guangzhou, China). All cells were cultured in Dulbecco’s modified
Eagle’s medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc.) in a humidified atmosphere
containing 5% CO2 at 37°C. Cells in the exponential
phase of growth were used in subsequent experiments.
Generation of an AQP5-silenced lung
cancer cell line
The AQP5 short hairpin RNA (shRNA) expression
construct, namely AQP5-pGCH1/Neo, and the non-targeting control
construct, namely NC-pGCH1/Neo, were designed and synthesized by
Sangon Biotech Co., Ltd. (Shanghai, China). The sequences of the
AQP5 and NC shRNA molecules were
5′-CCGGCCATCATCAAAGGCACGTATGCTCGAGCATACGTGCCTTTGATGATGGTTTTTG-3′
and
5′-GATCCCACTACCGTTGTTATAGGTGTTCAAGAGACACCTATAACAACGGTAGTTTTTTTCCAAA-3′,
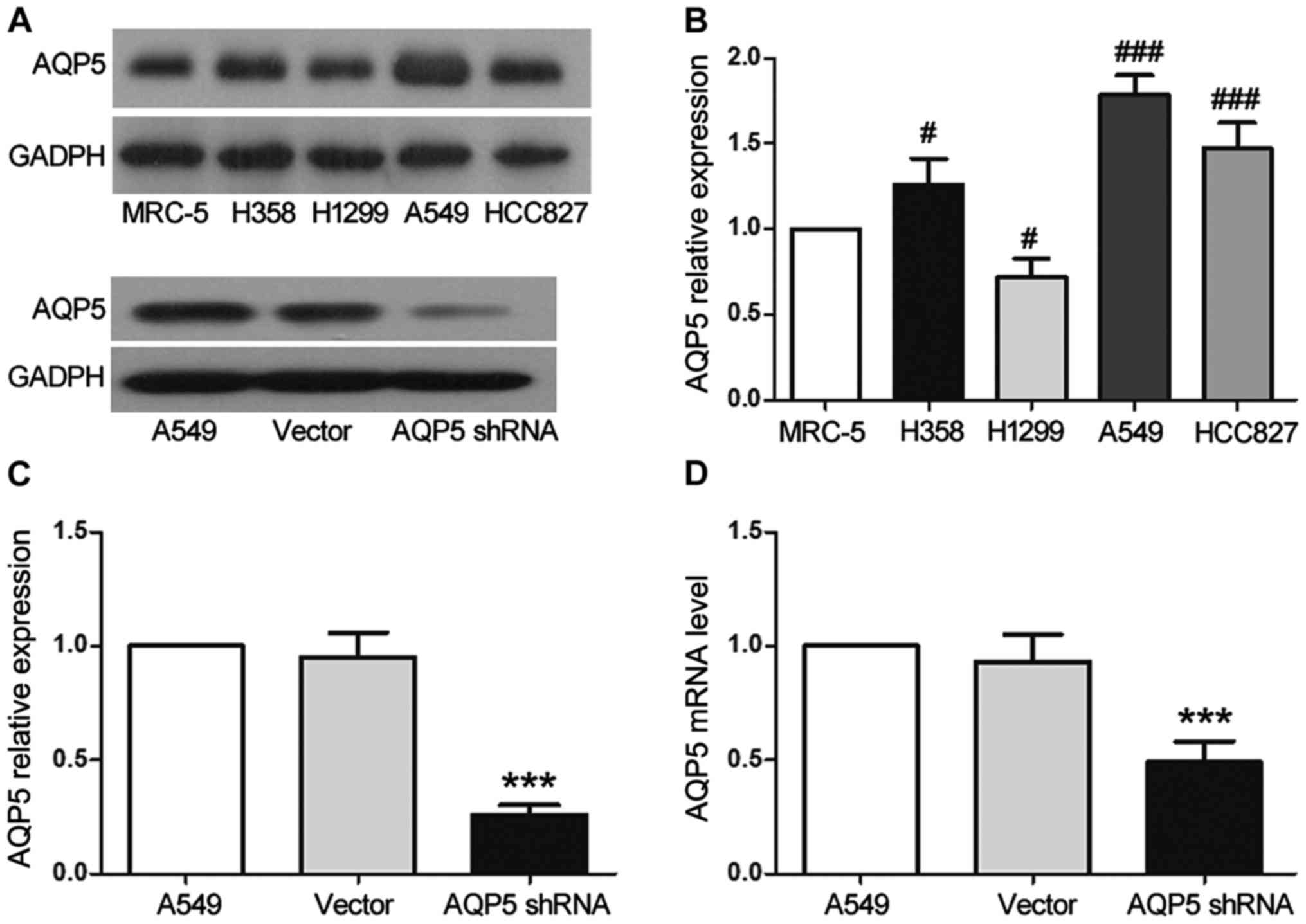
respectively. A549 cells, in which AQP5 was found to be highly
expressed (Fig. 1A and B), were
transfected with AQP5-pGCH1/Neo or NC-pGCH1/Neo using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer’s protocol. At 24 h
after transfection, the cells were exposed to 200 μg/ml G418
(Invitrogen; Thermo Fisher Scientific, Inc.) for over a week in
order to select clones stably expressing the constructs. Positive
clones were selected for further identification.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cultured cells with
TRIzol reagent (Tiangen Biotech Co., Ltd., Beijing, China)
according to the manufacturer’s protocol, and cDNA was then
synthesized by reverse transcription. The total PCR reaction volume
was 20 μl and consisted of 1 μl of cDNA, 0.5
μl of each primer, 10 μl of SYBR-Green master mix,
and 8 μl of ddH2O. The PCR reaction program was
as follows: 95°C for 10 min; 40 cycles of 95°C for 10 sec, 60°C for
20 sec, 72°C for 30 sec; and 4°C for 5 min. The primers used to
amplify AQP5 and β-actin cDNA were as follows: AQP5 sense,
5′-CTATGAG TCCGAGGAGGATT-3′, and antisense, 5′-GCTTCGCTGTC
ATCTGTT-3′; β-actin sense, 5′-CCTGTACGCCAACACAG TGC-3′, and
antisense, 5′-ATACTCCTGCTTGCTGATCC-3′. the relative mRNA levels in
each sample were calculated using the 2−ΔΔCq method
(22), using β-actin as a
reference. qPCR was performed using SYBR-Green Master Mix (Tiangen
Biotech Co., Ltd.) in an Exicycler™ 96 quantitative fluorescence
analyzer (Bioneer Corporation, Daejeon, Korea).
Western blotting
Cells were lysed (Beyotime Institute of
Biotechnology, Haimen, China), and the concentration of protein in
lysates was determined by bicinchoninic acid assays (Beyotime).
Total proteins (40 μg) from each sample were separated by
sodium dodecyl sulfate-polyacrylamide gel electrophoresis (5%
acrylamide and 10% separating gel) and then electroblotted onto a
polyvinylidene fluoride membrane (EMD Millipore, Bedford, MA, USA).
Subsequent to blocking with evaporated milk 37°C, the membrane was
incubated at 4°C overnight with primary antibodies against the
following: AQP5, cleaved caspase-3, B-cell lymphoma 2 (Bcl-2),
Bcl-2-associated X protein (Bax), extracellular signal-regulated
kinase (ERK), phosphorylated (p)-ERK, c-Fos, or p-cAMP response
element-binding protein (p-CREB) (all diluted 1:1,000; Abcam,
Cambridge, MA, USA). Next, the membranes were exposed to a
horseradish peroxidase-labeled secondary antibody (1:5,000; Abcam)
at room temperature for 45 min. Proteins were visualized using an
enhanced chemiluminescence reagent (Beyotime), and band intensities
were analyzed with Gel-Pro Analyzer software version 4 (Media
Cybernetics, Inc., Rockville, MD, USA) for comparison of the gray
densities.
Colony formation assay
Cells were seeded onto 35-mm dishes at a density of
200 cells per dish and incubated for 14 days at 37°C in a
humidified atmosphere containing 5% CO2. The resulting
colonies were rinsed with PBS, at room temperature and fixed with
paraformaldehyde for 20 min, and then stained with Giemsa solution
(Nanjing Jiancheng Bioengineering Institute, Nanjing, China) for
5–8 min. The number of colonies consisting of at least 50 cells was
then counted under a microscope. Colony formation efficiency was
calculated as follows: (Number of colonies / number of inoculated
cells) × 100%.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
Cells were seeded into 96-well microplates at a
density of 2×103 cells/well and with each well
containing 200 μl DMEM supplemented with 10% FBS. The cells
were allowed to adhere prior to the addition of MTT (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) to each well at a final
concentration of 0.2 mg/ml at various time-points (12, 24, 48, 72
and 96 h). Subsequent to incubation at 37°C for 4 h, the optical
density at 490 nm was measured using an ELx800 microplate reader
(BioTek Instruments, Inc., Winooski, VT, USA).
Flow cytometry for cell cycle progression
and apoptosis analyses
For cell cycle analysis, cells were fixed in 70%
cold ethanol at 4°C for 3 h, washed in PBS, and then resuspended in
staining buffer containing 25 μl propidium iodide (PI) and
10 μl RNaseA (Abcam). The cell suspension was incubated for
45 min in the dark at 37°C, and then subjected to cell cycle
distribution analysis by flow cytometry using a FACSCalibur
instrument (BD Biosciences, Franklin Lakes, NJ, USA).
In addition, apoptotic cells were identified using
fluorescence-activated cell sorting and an apoptosis detection kit
(Beyotime Institute of Biotechnology, Nanjing, China), according to
the manufacturer’s protocol. Cells were harvested, centrifuged at
4°C, 503 x g, 5 min, and then washed with PBS prior to resuspending
in 400 μl binding buffer. Next, 5 μl Annexin
V-fluorescein isothiocyanate was added to the cell suspensions,
which were then incubated at 2–8°C for 15 min in the dark. PI (10
μl) was subsequently added, and the cell suspensions were
incubated at 28°C for 5 min in the dark. The cells were then
analyzed by flow cytometry within 1 h of this treatment.
In vivo experiments
A total of 18 BALB/c nude mice (4–6-week-old;
weight, 20 g) were purchased from Beijing Vital River Laboratory
Animal Technology Co., Ltd. (Beijing, China). The animals were
maintained under pathogen-free conditions at 22°C and 40–50%
humidity with a 12/12-h light/dark cycle, and had ad libitum access
to food and water. Their care and treatment and all animal
experiments were approved by the Experimental Animal Ethics
Committee of Jilin University (Changchun, China). Each mouse was
randomly assigned to one of following three groups (6 mice in each
group): A549 (untransfected cells), vector (non-targeting control
construct) or AQP5 shRNA groups. Cells (1×106) were
suspended in 0.2 ml normal saline and inoculated subcutaneously
into the right breast pads of the mice; and the A549 group with 0.2
ml saline only. Tumor volumes in mice were determined using the
following formula: Tumor volume = (a x b2)/2, where a
and b are the larger and smaller of the two dimensions,
respectively. Mice were sacrificed after 30 days, and tumor tissues
were removed and fixed in polyformaldehyde after imaging of the
tumors for further examination.
Detection of apoptosis using terminal
deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)
assay
Apoptosis in the xenograft tumor tissue was detected
using an In Situ Cell Death Detection kit (Roche Diagnostics
GmbH, Mannheim, Germany) according to the manufacturer’s protocol.
Briefly, paraffin sections (1 cm2) were treated with a
solution of H2O2 in order to inactivate
endogenous peroxidases, and incubated with 50 μl TUNEL
reaction solution at 37°C for 60 min. Subsequently, the sections
were washed with PBS and incubated with 50 μl Converter-POD
working solution at 37°C for 30 min. Following development using
3,3′-diaminobenzidine and staining of nuclei with hematoxylin, the
sections were observed under a microscope and images were
captured.
Statistical analysis
Data are presented as the mean ± standard deviation.
Comparisons between groups were performed using one-way analysis of
variance, and multiple comparisons were performed using the
Bonferroni post hoc test. GraphPad Prism version 5.0 software
(GraphPad Software, Inc., La Jolla, CA, USA) was used to analyze
data and generate graphs. Differences associated with P-values of
<0.05 were considered as statistically significant.
Results
AQP5 silencing by stable expression of
shRNA
In order to examine the function of AQP5 in lung
cancer cells, western blotting was used to measure its expression
in various human lung cancer cell lines and lung fibroblast cells.
Of the cell lines tested, AQP5 expression was observed in all the
experimental cell lines, with the highest expression observed in
the A549 cells compared with the MRC-5 cell line (Fig. 1A and B). Western blotting also
revealed that transfection of the A549 cells with AQP5 shRNA
resulted in the significant inhibition of AQP5 protein expression
to only 26% of that in the A549 (untransfected) group (Fig. 1A and C; P<0.001). These results
were further confirmed by RT-qPCR, which indicated that the
expression of AQP5 mRNA was also significantly inhibited in the
AQP5 shRNA group and was only 49% of that in the A549 group
(Fig. 1D; P<0.001).
AQP5 downregulation inhibits A549 cell
proliferation and cell cycle progression
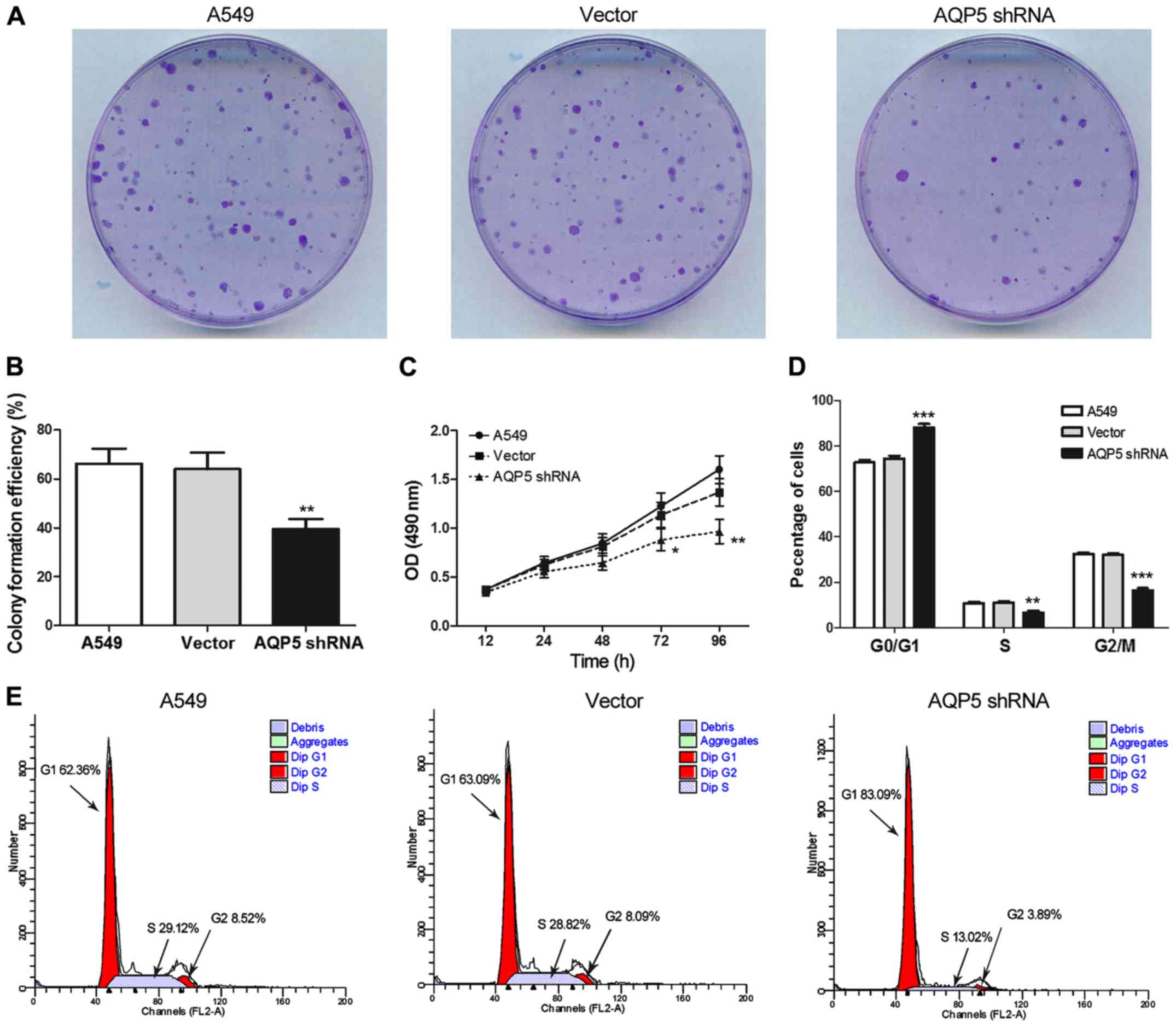
A colony formation assay was used to investigate the
possible role of AQP5 in A549 cell growth. The clonogenicity of
AQP5 shRNA cells was found to be significantly reduced compared
with that of the A549 and vector groups (Fig. 2A and B; P<0.01). Furthermore,
the MTT assay demonstrated that proliferation of AQP5 shRNA cells
at 72 h (P<0.05) and 96 h (P<0.01) of culture was markedly
lower compared with that of cells in the A549 group (Fig. 2C).
To further explore the effect of AQP5 silencing on
cell cycle progression, flow cytometry was used to evaluate the
distribution of cells in each phase of the cell cycle (Fig. 2D and E). In the AQP5 shRNA group,
numerous cells were in G0/G1 phase (P<0.001), whereas the number
of those in S (P<0.01) and G2/M (P<0.001) phases was
significantly reduced as compared with the A549 control group. No
evident difference in cell cycle distribution was noted between the
A549 and vector groups.
AQP5 downregulation induces A549 cell
apoptosis
Flow cytometry was employed to establish whether
AQP5 downregulation significantly affected A549 cell apoptosis. The
apoptotic ratio in the AQP5 shRNA group was observed to be
1.54-fold higher compared with that in the A549 group (Fig. 3A and B; P<0.001); thus,
apoptosis of A549 cells was significantly increased by AQP5
silencing. Western blotting was subsequently used to measure the
expression levels of the apoptosis suppressor Bcl-2 and the
downstream executioners of apoptosis, Bax and cleaved caspase-3.
The expression levels of cleaved caspase-3 and Bax in cells of the
AQP5 shRNA group was 2.68-fold (Fig.
3C and D; P<0.001) and 2.31-fold (Fig. 3C and E; P<0.001) higher,
respectively, when compared with those in cells of the A549 group.
In addition, the expression of Bcl-2 in the AQP5 shRNA group was
only 56% of that in the A549 group (Fig. 3C and F; P<0.001). These findings
further indicated that downregulation of AQP5 was able to induce
A549 cell apoptosis.
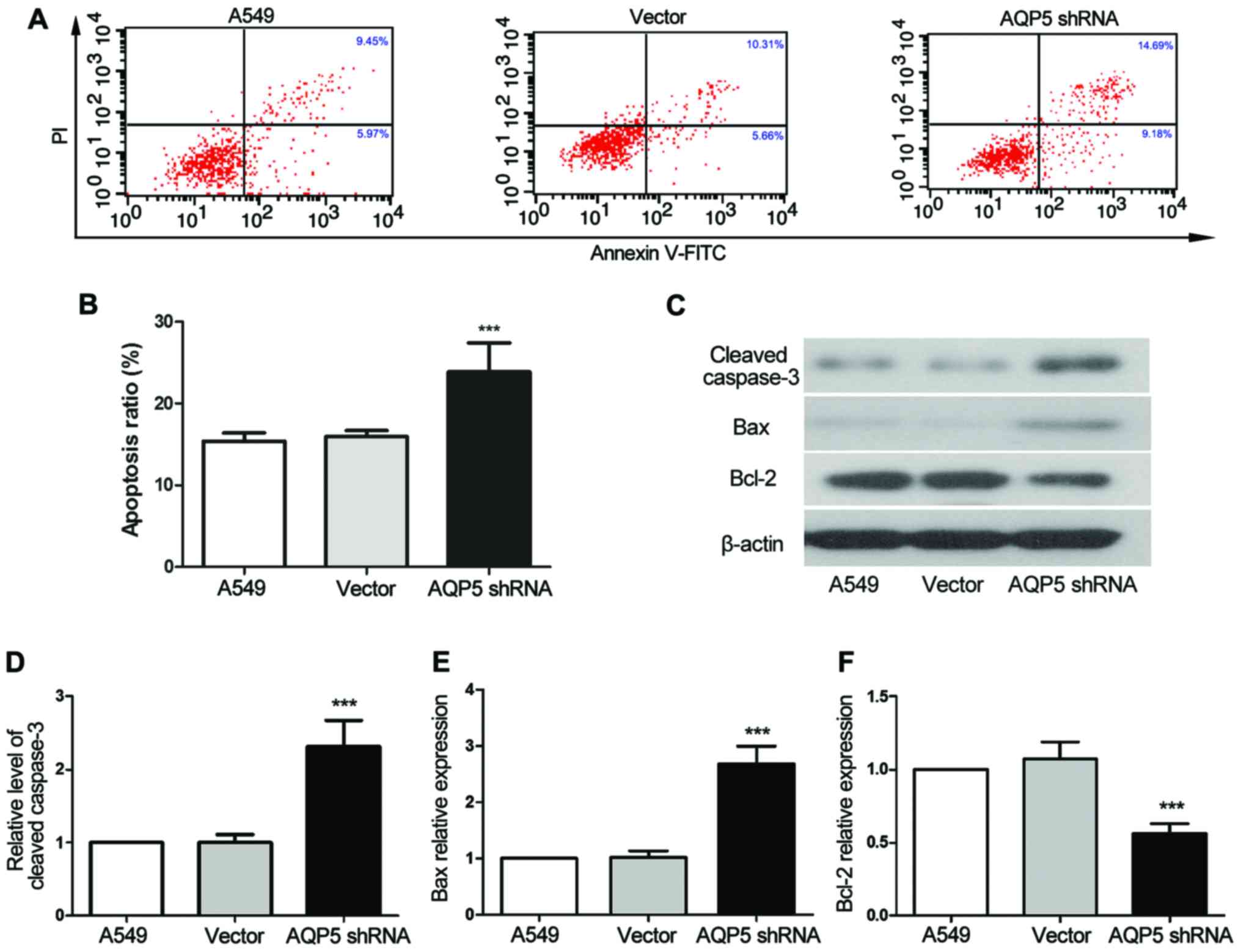 | Figure 3AQP5 downregulation induces apoptosis
of A549 cells. (A) Flow cytometry results and (B) apoptotic ratio
of cells are presented. The apoptosis of cultured cells was tested
by double-staining with FITC-conjugated Annexin V and PI, followed
by flow cytometry analysis. (C) Western blot analysis results, with
β-actin used as an internal control for grayscale analysis. (D)
Cleaved caspase-3, (E) Bax and (F) Bcl-2 relative protein levels in
AQP5-silenced cells. Data representative of three experiments are
shown, and data are presented as the mean ± standard deviation.
***P<0.001, vs. the A549 control group. AQP5,
aquaporin 5; shRNA, short hairpin RNA; FITC, fluorescein
isothiocyanate; Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X
protein. |
AQP5 downregulation inhibits ERK
signaling
The mechanism by which AQP5 silencing affects A549
cell behavior was investigated using western blotting. It was
observed that the protein levels of p-ERK, c-Fos and p-CREB in the
AQP5 shRNA group were 31% (P<0.001), 41% (P<0.001) and 53%
(P<0.001) of those in the A549 group, respectively (Fig. 4). These findings indicated that the
downregulation of AQP5 inhibited the activation of the ERK
signaling pathway by altering the phosphorylation levels of
important ERK pathway proteins, Fos and CREB.
 | Figure 4AQP5 downregulation inhibits the ERK
signaling pathway. (A) Western blot analysis was performed to
determine levels of ERK, p-ERK, c-Fos and p-CREB proteins in
AQP5-silenced cells. β-actin was used as an internal control for
grayscale analysis. (B) p-ERK, (C) c-Fos and (D) p-CREB protein
levels are displayed. Data representative of three experiments are
shown, and data are presented as the mean ± standard deviation.
***P<0.001, vs. the A549 control group. AQP5,
aquaporin 5; shRNA, short hairpin RNA; ERK, extracellular
signal-regulated kinase; CREB, cAMP response element-binding
protein; p-, phosphorylated. |
AQP5 downregulation inhibits A549 cell
growth in vivo
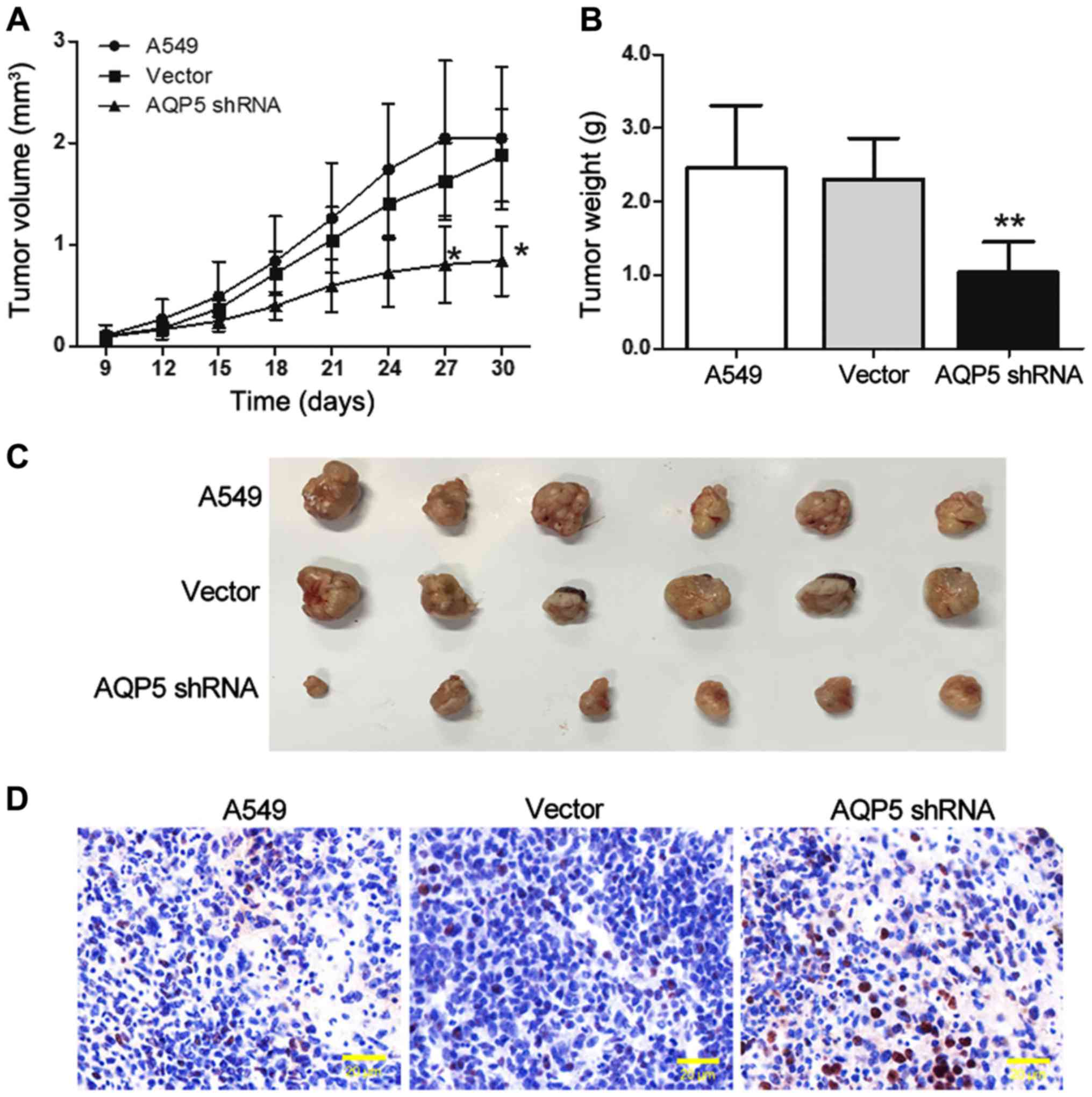
Based on the results of the in vitro
experiments, the present study next investigated the role of AQP5
in A549 cell growth in vivo. The results revealed that tumor
volumes and weights in the AQP5 shRNA group were significantly
lower in comparison with those in the A549 group (Fig. 5A–C). Furthermore, the TUNEL assay
revealed that the number of apoptotic cells was higher in the tumor
tissues of the AQP5 shRNA group as compared with those of the A549
groups (Fig. 5D). Thus,
downregulation of AQP5 expression may delay lung cancer
progression.
Discussion
Lung cancer remains one of the most common
malignancies worldwide, and morbidity and mortality associated with
this disease have been increasing (3). Despite advances in various treatment
options and systemic therapy, such as chemotherapy, in combination
with surgery and radiotherapy, the 5-year survival rate of lung
cancer patients remains poor (6).
AQP5 is one of the most important members of the AQP family, a
group of water-transporting transmembrane proteins. Previous
studies have demonstrated that due to the particular structure and
characteristics of AQP5, its overexpression can have oncogenic
effects (11,21,23),
including in cancer of the ovary (24), cervix (25) and colon (26), as well as in ovarian epithelium
(27) and gastric carcinoma cells
(28). However, in lung cancer, it
remains to be determined whether the silencing of AQP5 expression
affects lung cancer development and the underlying mechanisms
remain to be elucidated. In the present study, the effects of AQP5
silencing on the proliferation, cell cycle progression and
apoptosis of A549 lung cancer cells through the regulation of ERK
signaling, as well as on the promotion of apoptosis in lung cancer
xenograft tumors in nude mice, were investigated. It was observed
that silencing of AQP5 inhibited the proliferation and promoted the
apoptosis of A549 cells in vitro and in vivo,
suggesting that it may constitute a promising target in the
development of novel drugs for lung cancer treatment.
Proliferation, cell cycle progression and apoptosis
are the major mechanisms responsible for cell growth. Extension of
the G1/S phase transition can effectively inhibit cell
proliferation (29). The results
of the present study demonstrated that AQP5 was highly expressed in
A549 lung cancer cells and functioned as a negative regulator of
cell cycle progression. Silencing of AQP5 in A549 cells by RNA
interference resulted in cell cycle arrest, with an accumulation of
cells in G0/G1 phase and reduced numbers of cells in S and G2/M
phases observed. Apoptosis is a well-orchestrated cellular
mechanism that helps maintain the balance between cell
proliferation and death, the disruption of which is considered to
be an early and important event in cancer (30). Apoptosis is regulated by multiple
genes at the cellular level, including cleaved caspase-3, Bcl-2 and
Bax. Caspase-3 is an effector caspase that initiates cell
degradation in the final stages of apoptosis. In addition, Bax, a
pro-apoptotic protein, and Bcl-2, a survival-promoting protein, are
members of the Bcl-2 family that serve key roles in the regulation
of intrinsic apoptotic signaling (31–33).
The current study revealed that silencing of AQP5 expression in
A549 cells effectively promoted non-viable apoptotic cell increase
in vitro and in vivo, downregulating the expression
of Bcl-2, and elevating the levels of Bax and cleaved
caspase-3.
The ERK1/2 pathway is a classic mitogen-activated
protein kinase signaling cascade that regulates cell proliferation
and growth (34,35). In the present study, it was
observed that silencing of AQP5 inhibited ERK1/2 signaling,
consistent with the observations of prior studies (17,36).
The downstream ERK signaling proteins c-Fos and CREB, which are
present in the eukaryotic nucleus, are phosphorylated upon the
activation of ERK signaling (37).
The current study results also demonstrated that AQP5 silencing
reduced the protein levels of c-Fos and p-CREB. These results
suggest that AQP5 downregulation may exert an inhibitory effect on
A549 lung cancer cell growth by reducing ERK1/2 signaling.
In conclusion, the present study demonstrated that
AQP5 silencing inhibited the proliferation and cell cycle
progression of A549 lung cancer cells, and that it inducted induce
cell apoptosis in vitro and in vivo. Furthermore,
this inhibitory effect may be brought about by restriction of
ERK1/2 signaling pathway activation. Nevertheless, as AQP5 is
widely expressed, further studies of the mechanisms responsible for
these effects should be conducted to assess its potential
application as a therapeutic target.
Acknowledgments
Not applicable.
Notes
[1]
Funding
This study was supported by the Jilin Province major
scientific and technological projects (grant no.
20160201001YY).
[2] Authors’
contributions
GZ was the overall advisor of the study; she
formulated the experiment plan and examined the accuracy of the
experimental results; LZ was involved in the performing of the
experiments; JL, HZ and ZD provided experimental and operational
support. All authors have read and approved the final
manuscript.
[3] Availability
of data and materials
Not applicable
[4] Ethics
approval and consent to participate
Animal care and treatment and all animal experiments
were approved by the Experimental Animal Ethics Committee of Jilin
University (Changchun, China).
[5] Consent for
publication
Not applicable.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jin C, Zhang G, Zhang Y, Hua P, Song G,
Sun M, Li X, Tong T, Li B and Zhang X: Isoalantolactone induces
intrinsic apoptosis through p53 signaling pathway in human lung
squamous carcinoma cells. PLoS One. 12:e01817312017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
He F, Du T, Jiang Q and Zhang Y:
Synergistic effect of Notch-3-specific inhibition and paclitaxel in
non-small cell lung cancer (NSCLC) cells via activation of the
intrinsic apoptosis pathway. Med Sci Monit. 23:3760–3769. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ridge CA, McErlean AM and Ginsberg MS:
Epidemiology of lung cancer. Semin Intervent Radiol. 30:93–98.
2013. View Article : Google Scholar :
|
|
6
|
Wang L, Li R, Che K, Liu Z, Xiang S, Li M
and Yu Y: Enhanced antitumor effect on intrapulmonary tumors of
docetaxel lung-targeted liposomes in a rabbit model of VX2
orthotopic lung cancer. Sci Rep. 7:100692017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Howington JA, Blum MG, Chang AC, Balekian
AA and Murthy SC: Treatment of stage I and II non-small cell lung
cancer: Diagnosis and management of lung cancer, 3rd ed: American
College of Chest Physicians evidence-based clinical practice
guidelines. Chest. 143:e278S–e313S. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Collisson EA, Campbell JD, Brooks AN,
Berger AH, Lee W, Chmielecki J, Beer DG, Cope L, Creighton CJ,
Danilova L, et al Cancer Genome Atlas Research Network:
Comprehensive molecular profiling of lung adenocarcinoma. Nature.
511:543–550. 2014. View Article : Google Scholar
|
|
9
|
Wang M, Lin T, Wang Y, Gao S, Yang Z, Hong
X and Chen G: CXCL12 suppresses cisplatin-induced apoptosis through
activation of JAK2/STAT3 signaling in human non-small-cell lung
cancer cells. Onco Targets Ther. 10:3215–3224. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Agre P and Kozono D: Aquaporin water
channels: Molecular mechanisms for human diseases. FEBS Lett.
555:72–78. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shen Q, Lin W, Luo H, Zhao C, Cheng H,
Jiang W and Zhu X: Differential expression of aquaporins in
cervical precursor lesions and invasive cervical cancer. Reprod
Sci. 23:1551–1558. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Agre P: The aquaporin water channels. Proc
Am Thorac Soc. 3:5–13. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Verkman AS: More than just water channels:
Unexpected cellular roles of aquaporins. J Cell Sci. 118:3225–3232.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chae YK, Woo J, Kim MJ, Kang SK, Kim MS,
Lee J, Lee SK, Gong G, Kim YH, Soria JC, et al: Expression of
aquaporin 5 (AQP5) promotes tumor invasion in human non small cell
lung cancer. PLoS One. 3:e21622008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jung HJ, Park JY, Jeon HS and Kwon TH:
Aquaporin-5: A marker protein for proliferation and migration of
human breast cancer cells. PLoS One. 6:e284922011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee SJ, Kang BW, Kim JG, Jung JH, Lee J,
Kim WW, Park HY, Jeong JH, Jeong JY, Park JY, et al: AQP5 variants
affect tumoral expression of AQP5 and survival in patients with
early breast cancer. Oncology. 92:153–160. 2017. View Article : Google Scholar
|
|
17
|
Yang J, Zhang JN, Chen WL, Wang GS, Mao Q,
Li SQ, Xiong WH, Lin YY, Ge JW, Li XX, et al: Effects of AQP5 gene
silencing on proliferation, migration and apoptosis of human glioma
cells through regulating EGFR/ERK/p38 MAPK signaling pathway.
Oncotarget. 8:38444–38455. 2017.PubMed/NCBI
|
|
18
|
Xin LB, Jiang XX, Ye XL, Wu RJ, Xu KH, Ma
JY and Lin J: AQP5 gene silencing inhibits proliferation and
migration of ectopic endometrial glandular epithelial cells in
endometriosis. Zhejiang Da Xue Xue Bao Yi Xue Ban. 44:285–292.
2015.In Chinese. PubMed/NCBI
|
|
19
|
Sugihara I, Yoshida M, Shigenobu T, Takagi
H, Maruyama K, Takeuchi N, Toda M, Inoue M and Nakada H: Different
progression of tumor xenografts between mucin-producing and
mucin-non-producing mammary adenocarcinoma-bearing mice. Cancer
Res. 66:6175–6182. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guo K and Jin F: NFAT5 promotes
proliferation and migration of lung adenocarcinoma cells in part
through regulating AQP5 expression. Biochem Biophys Res Commun.
465:644–649. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Direito I, Madeira A, Brito MA and Soveral
G: Aquaporin-5: From structure to function and dysfunction in
cancer. Cell Mol Life Sci. 73:1623–1640. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
23
|
Verkman AS, Hara-Chikuma M and
Papadopoulos MC: Aquaporins - new players in cancer biology. J Mol
Med (Berl). 86:523–529. 2008. View Article : Google Scholar
|
|
24
|
Yang JH, Shi YF, Cheng Q and Deng L:
Expression and localization of aquaporin-5 in the epithelial
ovarian tumors. Gynecol Oncol. 100:294–299. 2006. View Article : Google Scholar
|
|
25
|
Zhang T, Zhao C, Chen D and Zhou Z:
Overexpression of AQP5 in cervical cancer: Correlation with
clinicopathological features and prognosis. Med Oncol.
29:1998–2004. 2012. View Article : Google Scholar
|
|
26
|
Shi X, Wu S, Yang Y, Tang L, Wang Y, Dong
J, Lü B, Jiang G and Zhao W: AQP5 silencing suppresses p38 MAPK
signaling and improves drug resistance in colon cancer cells.
Tumour Biol. 35:7035–7045. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yan C, Zhu Y, Zhang X, Chen X, Zheng W and
Yang J: Downregulated aquaporin 5 inhibits proliferation and
migration of human epithelial ovarian cancer 3AO cells. J Ovarian
Res. 7:782014. View Article : Google Scholar
|
|
28
|
Huang YH, Zhou XY, Wang HM, Xu H, Chen J
and Lv NH: Aquaporin 5 promotes the proliferation and migration of
human gastric carcinoma cells. Tumour Biol. 34:1743–1751. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Simpson DJ, Fryer AA, Grossman AB, Wass
JA, Pfeifer M, Kros JM, Clayton RN and Farrell WE: Cyclin D1
(CCND1) genotype is associated with tumour grade in sporadic
pituitary adenomas. Carcinogenesis. 22:1801–1807. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Qi S, Song Y, Peng Y, Wang H, Long H, Yu
X, Li Z, Fang L, Wu A, Luo W, et al: ZEB2 mediates multiple
pathways regulating cell proliferation, migration, invasion, and
apoptosis in glioma. PLoS One. 7:e388422012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zarnescu O, Brehar FM, Chivu M and Ciurea
AV: Immunohistochemical localization of caspase-3, caspase-9 and
Bax in U87 glioblastoma xenografts. J Mol Histol. 39:561–569. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bhattacharjee M, Acharya S, Ghosh A,
Sarkar P, Chatterjee S, Kumar P and Chaudhuri S: Bax and Bid act in
synergy to bring about T11TS-mediated glioma apoptosis via the
release of mitochondrial cytochrome c and subsequent caspase
activation. Int Immunol. 20:1489–1505. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guo Z, Chen Q, Liu B, Tian D, Zhang S and
Li M: LRIG1 enhances chemosensitivity by modulating BCL-2
expression and receptor tyrosine kinase signaling in glioma cells.
Yonsei Med J. 55:1196–1205. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
McCubrey JA, Steelman LS, Chappell WH,
Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M,
Tafuri A, et al: Roles of the Raf/MEK/ERK pathway in cell growth,
malignant transformation and drug resistance. Biochim Biophys Acta.
1773:1263–1284. 2007. View Article : Google Scholar
|
|
35
|
Oh SE and Mouradian MM: Cytoprotective
mechanisms of DJ-1 against oxidative stress through modulating
ERK1/2 and ASK1 signal transduction. Redox Biol. 14:211–217. 2018.
View Article : Google Scholar
|
|
36
|
Zhang Z, Chen Z, Song Y, Zhang P, Hu J and
Bai C: Expression of aquaporin 5 increases proliferation and
metastasis potential of lung cancer. J Pathol. 221:210–220. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Niu C, Xiao F, Yuan K, Hu X, Lin W, Ma R,
Zhang X and Huang Z: Nardosinone suppresses RANKL-induced
osteoclastogenesis and attenuates lipopolysaccharide-induced
alveolar bone resorption. Front Pharmacol. 8:6262017. View Article : Google Scholar : PubMed/NCBI
|