Introduction
In the last decade, the incidence and mortality
rates of colorectal cancer (CRC) have decreased to a certain extent
owing to improved screening, diagnostic and therapeutic technical
expertise for cancer treatment. Despite these positive
developments, CRC remains one of the most common cancer types and
the primary cause for cancer-associated mortality worldwide
(1). For the majority of patients
with recurrent or metastatic CRC, chemotherapy is a palliative but
relatively effective treatment (2). At present, irinotecan (CPT-11) in the
presence or absence of other chemotherapeutic agents, including
5-fluorouracil and capecitabine, is used to reduce the symptoms in
patients with advanced CRC (3).
However, the 5-year survival rate of patients with metastatic or
recurrent CRC is <10% (4).
Chemoresistance is one of the major obstacles to treating this
malignancy (5); thus, the
identification of agents that are able to attenuate chemoresistance
in cancer cells is required.
Curcumin, a dietary polyphenolic compound extracted
from Curcuma longa, has been well-researched, and possesses
anti-inflammatory, anticancer, apoptotic and anti-metastatic
activities (6-10). It regulates several processes,
including cell proliferation, genes regulating apoptosis and growth
factors (11). Its low toxicity,
low cost and highly efficient anticancer functions further
demonstrate the potential of curcumin in preventing or treating
cancer (12). Accumulating
evidence has suggested that curcumin targets cancer stem cells
(CSCs) in numerous types of human cancer. For example, Zhu et
al (13) reported that lung
CSC is suppressed by curcumin through inhibition of the
wnt/β-catenin and sonic hedgehog signaling pathways. Additionally,
Mukherjee et al (14)
demonstrated that curcumin inhibits breast CSC migration by
amplifying the E-cadherin/β-catenin negative feedback loop. CSCs,
which comprise a minor subset of cells within the tumor, are the
primary contributor to the development of resistance to
chemotherapeutic agents, and subsequent cancer metastasis and
recurrence (15,16). It has been reported that the
majority of chemotherapeutic agents presently in use lack the
capacity to eliminate CSCs (17).
However, the associations between curcumin, and colon CSCs and
chemoresistance in CRC remain unclear. Furthermore, it has been
reported that the resistance of CSCs to chemotherapeutic agents is
a result of active DNA repair mechanisms, high expression of ABC
transporters and resistance to apoptosis (18). In addition to the aforementioned
mechanisms, autophagy is activated in CSCs in response to various
anticancer therapies as a protective mechanism, thus providing
chemoresistance (19). Visibly,
there are a variety of mechanisms associated with the
chemoresistance of CSCs. Therefore, the mechanisms of curcumin
targeting CSCs in CRC chemoresistance require further
investigation.
Based on the aforementioned observations,
CPT-11-resistant cells and sphere-forming cells were developed in
the present study to explore the effects of curcumin on
chemoresistance. Following the induction of resistance to CPT-11 in
LoVo cells, the changes in the expression levels of
drug-resistant-associated proteins and CSC identification markers
were detected. Next, the extent of chemoresistance and the
expression levels of CSC markers in CPT-11-resistant cells were
evaluated following treatment with curcumin. Furthermore, the
effect of curcumin on tumor sphere formation in colon CSCs, as well
as the expression levels of CSC identification markers in
sphere-forming cells were investigated. Finally, whether curcumin
targets CSCs via induction of apoptosis was explored.
Materials and methods
Cell lines and cell cultures
Human colon cancer LoVo cells were purchased from
the Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China). The cells were cultured in RPMI-1640
supplemented with 10% fetal bovine serum (FBS) (both from Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) and 1%
penicillin/streptomycin (Beijing Solarbio Science & Technology,
Co., Ltd., Beijing, China) in a humidified 37°C incubator with 5%
CO2. CPT-11 (Jiangsu Hengrui Medicine Co., Ltd.,
Lianyungang, China)-resistant cells, referred to as LoVo/CPT-11
cells, were established by repetitive treatment of the LoVo cells
with increasing concentrations of CPT-11 over a 10–12-month period,
based on the methods described in our previous studies (20).
Antibodies and reagents
Primary rabbit polyclonal antibodies against cluster
of differentiation (CD)133 (cat. no. 18470-1-AP), CD44 (cat. no.
15675-1-AP), epithelial cell adhesion molecule (EpCAM; cat. no.
21050-1-AP), CD24 (cat. no. 18330-1-AP) and β-actin (cat. no.
20536-1-AP) were purchased from ProteinTech Group, Inc. (Chicago,
IL, USA). Rabbit poly-clonal antibodies against ATP binding
cassette subfamily B member 1 (ABCB1; cat. no. ab155421), cleaved
caspase-3 (cat. no. ab13847), cleaved caspase-9 (cat. no. ab2324),
cleaved caspase-8 (cat. no. ab25901), BCL2 associated X apoptosis
regulator (Bax; cat. no. ab53154), apoptosis regulator Bcl-2
(Bcl-2; cat. no. ab196495), and the horseradish
peroxidase-conjugated goat anti-rabbit IgG secondary antibodies
(cat. no. ab7090), were purchased from Abcam (Cambridge, UK).
Curcumin was purchased from Merck KGaA (Sigma-Aldrich, Darmstadt,
Germany).
Growth inhibition assay
A Cell Counting kit-8 (CCK-8) assay (Dojindo
Molecular Technologies, Inc., Kumamoto, Japan) was used to assess
the inhibition of cell growth in response to CPT-11 (0, 5, 10, 20,
40, 80, 120, 160 and 200 µM) and curcumin (0, 2.5, 5, 10,
15, 20, 25, 30, 35 and 40 µM). Briefly, LoVo and LoVo/CPT-11
cells (1.0×104 cells/well) were seeded onto 96-well
plates in 100 µl culture medium (RPMI-1640, 10% FBS and 1%
penicillin/streptomycin)/well. Drugs diluted with the culture
medium were added to each well after 24 h of plating and then
co-incubated for 24 h. At the end of the treatment, the culture
medium was replaced with fresh culture medium containing 10% CCK-8
reagent and the reaction was allowed to proceed for 2 h. Absorbance
was measured at the wavelength of 450 nm. All assays were performed
as three replicates and the results were indicated as a percentage
compared with the corresponding control group.
Tumor sphere formation assay
LoVo/CPT-11 cells were cultured in serum-free
Dulbecco's modified Eagle's medium/F12 (Gibco; Thermo Fisher
Scientific, Inc.) with 20 ng/ml epidermal growth factor, 10 ng/ml
basic fibroblast growth factor (all from PeproTech, Inc., Rocky
Hill, NJ, USA), 2% B27 supplement (Thermo Fisher Scientific, Inc.),
and 5 µg/ml insulin (Sigma-Aldrich; Merck KGaA). Cells were
then plated into ultralow-attachment 6-well plates (Corning
Incorporated, Corning, NY, USA) at a density of 2.0×104
cells/well, and fresh medium was added every two days. Tumor sphere
formation was observed, and images were captured using a light
microscope (original magnification, ×100; Olympus BX51; Olympus
Corporation, Tokyo, Japan).
Detection of CD133-positive cells by flow
cytometry
The percentages of CD133-positive cells in LoVo,
LoVo/CPT-11 and sphere-forming cells were measured using a flow
cytometry assay. Briefly, cells were separately centrifuged (4°C, 5
min, 100 × g) and washed with ice-cold PBS. Subsequently,
1×106 cells were incubated with 5 µl of
phycoerythrin-conjugated mouse anti-human monoclonal antibody CD133
(1:50; cat. no. 12-1339-41; Affymetrix; Thermo Fisher Scientific,
Inc.) in the dark at 4°C for 1 h. Next, the cells were washed with
ice-cold PBS and each sample was measured using a flow cytometer.
The results were analyzed with FlowJo 7.6.1 software (FlowJo LLC,
Ashland, OR, USA).
Effects of curcumin on cells
To analyze the effect of curcumin on the
chemoresistance of LoVo/CPT-11 cells, different concentrations of
curcumin (0, 2.5 and 5 µM) were used to treat LoVo/CPT-11
cells in combination with CPT-11, then the results were analyzed
via a growth inhibition assay. The half maximal inhibitory
concentration (IC50) of CPT-11 in cells treated with
curcumin was calculated. Furthermore, cells were treated with
different concentrations of curcumin (0, 2.5 and 5 µM) and
CPT-11 (0, 10, 20, 40 and 100 µM) individually for 24 h, and
the expression levels of CSC identification markers were further
explored using reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) and western blot analysis. For colon cancer
CSCs, sphere-forming cells were treated with various concentrations
of curcumin (0, 2.5, 5, 10 and 20 µM) and CPT-11 (0, 10, 20,
40 and 100 µM) separately or in combination. For the
combined experiment, sphere-forming cells were separated into four
groups: Control group, curcumin (5 µM) group, CPT-11 (100
µM) group and combined group (5 µM curcumin and 100
µM CPT-11). After 3 days of treatment, the tumor spheres
with a sphere diameter >50 µm were imaged and counted the
sphere-forming efficiency (SFE) was normalized to the control group
and calculated as a percentage. After 3 days of various treatments,
the expression of CSC identification markers and
apoptosis-associated proteins were detected.
Apoptosis assessment by flow
cytometry
Apoptosis distribution was detected using a
fluorescein isothiocyanate Annexin V Apoptosis Detection kit (BD
Biosciences, Franklin Lakes, NJ, USA) according to the
manufacturer's protocol. In brief, sphere-forming cells were
treated with curcumin and CPT-11 individually and in combination
for 24 h, then cells were collected and washed with cold PBS twice.
Subsequently, the cells were resuspended in 100 µl binding
buffer, and incubated with Annexin V (5 µl) and PI (1
µl) at 4°C in the dark for 15 min. Finally, the samples were
detected using a flow cytometer within 1 h. Results were analyzed
using WinMDI v2.9 software (The Scripps Research Institute, San
Diego, CA, USA).
RT-qPCR assay
Total cellular mRNA from LoVo, LoVo/CPT-11 and
sphere-forming cells were extracted using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Subsequently, 500 ng of purified mRNA was
reverse-transcribed into cDNA following the manufacturer's protocol
(Beijing Transgen Biotech Co., Ltd., Beijing, China). The qPCR
amplification was performed in triplicate using a Light
Cycler® 480 II (Roche Applied Science, Penzberg,
Germany). The reaction system (20 µl) contained the cDNA
template, forward and reverse primers, ddH2O, and 2X
SYBR Green qPCR Super Mix (Beijing Transgen Biotech Co., Ltd.). The
PCR steps included incubations for 10 min at 95°C, followed by 40
cycles with each cycle consisting of 15 sec at 95°C and 1 min at
60°C. Expression levels of each target gene were standardized using
β-actin as an internal control, and the result was calculated using
the 2−ΔΔCq method (21). The primers of CD133, CD44, EpCAM,
CD24, ABCB1 and β-actin (Sangon Biotech Co., Ltd., Shanghai, China)
were used as follows: CD133 forward, 5′-TTCTTGAC CGACTGAGACCCA-3′
and reverse, 5′-TCATGTTCTCCAA CGCCTCTT-3′; CD44 forward,
5′-CTGCCGCTTTGCAGGT GTA-3′ and reverse,
5′-CATTGTGGGCAAGGTGCTATT-3′; EpCAM forward,
5′-AATCGTCAATGCCAGTGTACTT-3′ and reverse,
5′-TCTCATCGCAGTCAGGATCATAA-3′; CD24 forward,
5′-CTCCTACCCACGCAGATTTATTC-3′ and reverse,
5′-AGAGTGAGACCACGAAGAGAC-3′; ABCB1 forward,
5′-TTGCTGCTTACATTCAGGTTTCA-3′ and reverse,
5′-AGCCTATCTCCTGTCGCATTA-3′; β-actin forward,
5′-CATGTACGTTGCTATCCAGGC-3′ and reverse, 5′-CTCC
TTAATGTCACGCACGAT-3′.
Western blot assay
Cells were collected using a plastic scraper, washed
with cold PBS and then solubilized in ice-cold protein extract
solution RIPA containing protease inhibitors (Beyotime Institute of
Biotechnology, Shanghai, China). The supernatant was used for
western blot analysis following clarification. Protein
concentration was determined using a BCA kit (Beyotime Institute of
Biotechnology) and standardized between the samples. Equal amounts
(30 µg) of protein were then separated by SDS-PAGE using
8–12% polyacrylamide gel and transferred onto polyvinylidene
difluoride membranes (EMD Millipore, Billerica, MA, USA). The
membranes were blocked with Tris-buffered saline Tween-20 (TBST)
containing 5% skim milk for 1 h at room temperature. The membranes
were then incubated in primary antibodies including anti-CD44,
anti-CD133, anti-EpCAM, anti-CD24 (all 1:500), anti-ABCB1,
anti-β-actin (both 1:3,000), anti-cleaved caspase-3, anti-cleaved
caspase-9, anti-cleaved caspase-8, anti-Bax, anti-Bcl-2 (all
1:1,000) at 4°C overnight. Following washing with TBST, the
membranes were incubated with the appropriate horseradish
peroxidase-conjugated secondary antibodies (1:5,000) for 1 h at
room temperature. The bands were visualized using a Western Bright
ECL HRP substrate kit (EMD Millipore) in a Kodak Image station
(Kodak, Rochester, NY, USA). The expression levels of the proteins
were analyzed using ImagingJ 1.48 software (National Institutes of
Health, Bethesda, MD, USA). Specific β-actin protein was used as
the loading control to normalize the sample amounts.
Statistical analysis
All data are expressed as the mean ± standard
deviation of three independent experiments. Statistical comparisons
were determined using unpaired t-tests or one-way analysis of
variance with Tukey's post hoc test. P<0.05 was considered to
indicate a statistically significant difference. All statistical
calculations were performed using SPSS software (version 20.0; IBM
Corp., Armonk, NY, USA), Graphs were prepared using GraphPad Prism
software (version 5.0; GraphPad Software, Inc., La Jolla, CA,
USA).
Results
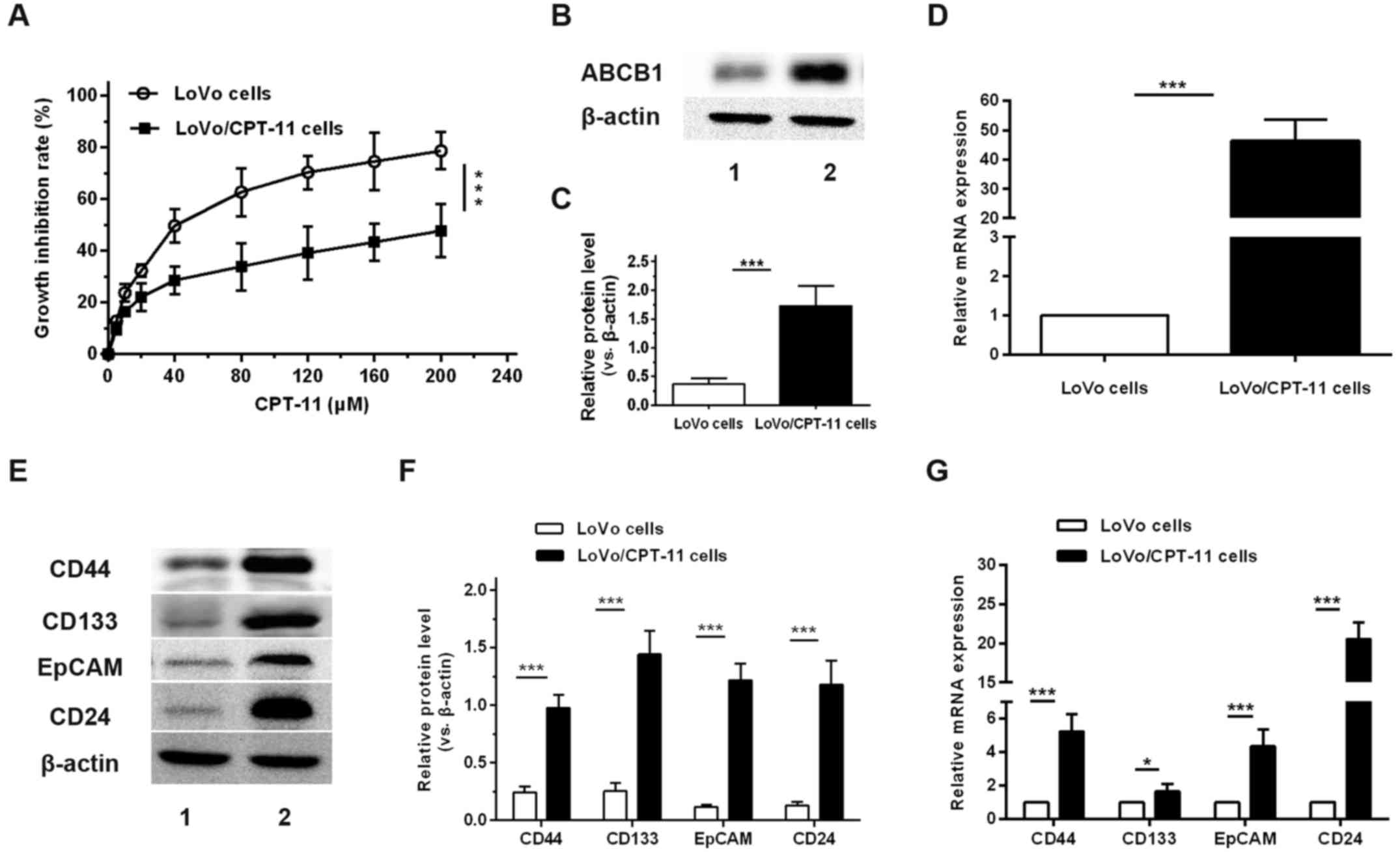
CPT-11-resistant cells express higher
levels of ABCB1 and CSC markers compared with parental cells
Via long-term culturing of original CPT-11-sensitive
LoVo cells by gradual adaptation to increasing CPT-11
concentrations, CPT-11-resistant LoVo cells were successfully
established. The resistance to CPT-11 in LoVo/CPT-11 cells was
examined by treating these cells with different concentrations of
CPT-11. The growth inhibitory rates were compared with those of
their parental cells using a CCK-8 assay. The results demonstrated
that the growth inhibitory rates of LoVo/CPT-11 cells treated with
CPT-11 were significantly decreased when compared with the parental
cells from concentration ≥5 µM (Fig. 1A). The IC50 of CPT-11 in
LoVo/CPT-11 cells were 268.72±2.43 µmol/l, remarkably higher
compared with that of parental cells (43.27±1.64 µmol/l).
The expression of ABCB1 in mRNA and protein levels between LoVo and
LoVo/CPT-11 cells was compared. The results revealed that the mRNA
and protein expression of ABCB1 was significantly increased in
LoVo/CPT-11 cells compared with parental cells (Fig. 1B–D). Based on these data,
LoVo/CPT-11 cells were effectively established. To further
determine whether the chemoresistance of CRC cells is associated
with CSC, the expression of CSC identification markers in
LoVo/CPT-11 cells were examined. As expected, the mRNA and protein
expression levels of CD44, CD133, EpCAM and CD24 were significantly
increased in LoVo/CPT-11 cells compared with the parental cells
(Fig. 1E–G).
Curcumin reduces chemoresistance to
CPT-11 and down-regulates the expression levels of CSC markers
Curcumin inhibited the growth of LoVo/CPT-11 cells
in a concentration-dependent manner. At 2.5 and 5 µM
curcumin concentrations, the growth inhibitory rate was 2.67±0.03
and 4.35±0.24%, respectively (Fig.
2A). To determine whether curcumin reduces LoVo/CPT-11 cell
chemoresistance, the cells were treated with CPT-11 in the presence
or absence of low concentrations of curcumin. The growth inhibitory
rates were significantly increased in the presence of 2.5 and 5
µM curcumin (Fig. 2B), with
the IC50 of CPT-11 reduced from 268.72±2.43 to
150.63±6.51 and 144.48±3.22 µM, respectively.
To further explore the possibility that curcumin
attenuated chemoresistance via reducing CSC-like characteristics,
LoVo/CPT-11 cells were treated with different concentrations of
curcumin and CPT-11 individually for 24 h. The results revealed
that, with the exception of CD133 mRNA expression, the protein
expression of CD133, and the mRNA and protein expression levels of
CD44, EpCAM and CD24 were inhibited by curcumin in a
concentration-dependent manner (Fig.
2C–E), whereas CPT-11 had no significant effect on the
expression of these markers (Fig.
2F–H).
Tumor sphere formation from
CPT-11-resistant cells in serum-free medium (SFM) culture
LoVo/CPT-11 cells were plated in SFM containing
several appropriate growth factors in order to form stable cell
spheroids. In order to verify the CSC-like characteristics of
sphere-forming cells, the percentages of CD133-positive cells were
detected. As demonstrated in Fig.
3, the percentage of CD133-positive cells was significantly
increased in LoVo/CPT-11 cells and sphere-forming cells,
particularly in sphere-forming cells compared with parental cells.
These results confirmed the characteristics of CSC in
sphere-forming cells and further verified that LoVo/CPT-11 cells
acquired CSC-like characteristics.
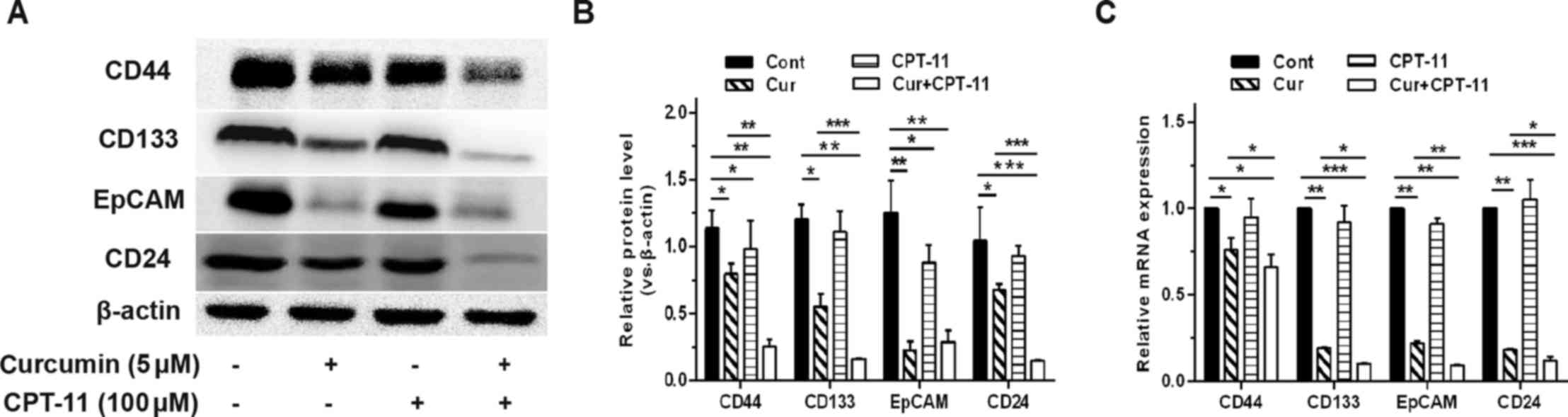
Curcumin diminishes the activity of colon
CSCs and the expression levels of CSC identification markers
To detect the effect of curcumin and CPT-11 on colon
CSCs, sphere-forming cells were treated with curcumin and CPT-11
separately or in combination for 3 days. As shown in Fig. 4, curcumin produced a
concentration-dependent reduction in the SFE with almost no sphere
formation following 10 µM curcumin treatment. In contrast,
no significant differences in the SFE were observed following
CPT-11 treatment alone. Furthermore, Fig. 4C and F demonstrate that the
inhibitory effect in the combined curcumin and CPT-11 group, was
significantly increased compared with that in the group treated
with 5 µM curcumin alone. Additionally, the mRNA and protein
expression levels of CSC identification markers (CD44, CD133, EpCAM
and CD24) were significantly inhibited following treatment with
curcumin alone or in combination with CPT-11 compared with
untreated cells (Fig. 5).
Curcumin induces apoptosis of colon
CSCs
To further to understand the effect of curcumin on
colon CSCs, the effects of curcumin on apoptosis in sphere-forming
cells were investigated. The results of flow cytometry analysis
indicated that cell apoptosis was induced in the presence of
curcumin in sphere-forming cells, and the apoptosis-inducing effect
of combined curcumin and CPT-11 was significantly increased
compared with that of curcumin alone (Fig. 6A and B). Additionally, western blot
analysis was used to measure the expression levels of proteins
associated with apoptosis. Fig. 6C and
D demonstrate that the expression level of anti-apoptotic
protein Bcl-2 was significantly decreased, and the levels of
pro-apoptotic proteins cleaved caspase-8, cleaved caspase-9,
cleaved caspase-3 and Bax were significantly increased in the
presence of curcumin compared with untreated control group.
Discussion
CRC is the fourth leading cause of cancer-associated
mortalities worldwide, and is associated with an unsatisfactory
prognosis and <10% rate of 5-year survival rate (4). Development of chemoresistance is
considered to be an important obstacle to the achievement of
satisfactory therapeutic effects for CPT-11 treatment. There is
increasing experimental evidence indicating that CSCs drive tumor
initiation, invasion and metastasis, and contributes to
chemoresistance, consequently promoting unrestricted tumor
progression (22-24). Understanding the mechanisms of
chemoresistance and identifying the agents that may reverse it are
of great importance for achieving effective therapeutic
strategies.
In the present study, a CPT-11-resistant cell
subline was derived from the human colon cancer LoVo cell line,
which was confirmed to exhibit increased cell viability compared
with of the parental cell line in the presence of different
concentrations of CPT-11. A previous study demonstrated that ABC
transporters contribute to the chemoresistance by pumping
anticancer agents out of the cells (25). When compared with parental cells,
the expression level of ABCB1 (also known as P-glycoprotein) was
significantly increased in LoVo/CPT-11 cells. In addition, it has
been reported that the high expression of ABC transporters accounts
for the chemoresistance property of CSCs (26). Based on this observation, the
expression of specific cell surface or cytoplasmic biomarkers of
CSCs was further investigated. Since widely accepted biomarkers of
colon CSCs have not yet been defined, four potential and
frequently-used biomarkers, including CD44, CD133, CD24 and EpCAM
(also known as ESA) were chosen to identify colon CSCs (27–30).
As a transmembrane glycoprotein, CD44 possesses a particular cell
adhesion function, and consequently assists the matrix adhesion and
migration of CSCs (31). In the
prominin family of pentaspan transmembrane glycoproteins, CD133,
also called Prominin-1 or AC133, was the first member to be
verified, and now is widely used to identify or isolate CSCs from
various types of tumor, including that of the pancreas, colon and
liver (32). Zhang et al
(33) demonstrated that
CD133-positive colon cancer cells exhibited increased abilities to
form tumor spheres and initiate tumor development compared with
CD133-negative cells. Similarly, CD24 and EpCAM have been reported
to be candidates for identifying or sorting CSCs from colon cancer
cells (34). Even though CD44,
CD133, EpCAM and CD24 are commonly used to identify CSCs in CRC,
the specificity of each marker for the identification of CSCs has
not yet been defined. Thus, the use of a single biomarker to
identify CSCs may not be reliable, and a group of biomarkers is
preferable to identify CSCs, as was used in the current study. In
the present study, the data indicated that the expression of CD44,
CD133, CD24 and EpCAM at the mRNA and protein levels were
significantly higher in LoVo/CPT-11 cells compared with that of
parental cells. In addition, the proportion of CD133-positive cells
was significantly increased in CPT-11-resistant cells.
Consequently, LoVo/CPT-11 cells acquired CSC-like characteristics,
and the proportion of CSCs derived from LoVo/CPT-11 cells was
increased compared with that in LoVo cells. This observation
necessitates the exploration and development of anticancer agents
possessing CSC-targeting ability.
CPT-11 combined with 5-fluorouracil is one of the
standard chemotherapeutic options for metastatic CRC. Nevertheless,
the effectiveness of CPT-11 and other agents is restricted as they
ineffectively target CSCs, which contributes to the development of
chemoresistance (17). In the
current investigation, it was demonstrated that curcumin inhibited
the growth of LoVo/CPT-11 cells in a concentration-dependent
manner. Considering how the cytotoxic effect of curcumin may
interfere with the results, low concentrations of curcumin were
chosen to study its effect on the chemoresistance of LoVo/CPT-11
cells to CPT-11. The results revealed that curcumin increased the
effectiveness of CPT-11 in inhibiting the growth of LoVo/CPT-11
cells. The IC50 of CPT-11 in drug-resistant cells was
significantly reduced by treatment with low concentrations of
curcumin. Furthermore, the data indicated that curcumin
significantly downregulated the protein expression of the
CSC-associated biomarkers CD44, CD133, CD24 and EpCAM, with similar
results observed with mRNA expression. These results demonstrated
that curcumin may reduce chemoresistance to CPT-11 through the
targeting of CSCs. Thus, further investigations directly involving
CSCs were performed.
Several methods have been reported to isolate CSCs,
including the side population (SP) cell sorting method, magnetic
activated cells sorting (MACS), fluorescence activated cells
sorting (FACS) and SFM culture (35). Hoechst staining is toxic to cells,
and CSCs isolated via the SP cell sorting method may exhibit
decreased self-renewal, tumorigenicity and other CSC properties;
thus, the use of this technique is limited (36). FACS and MACS require knowledge of
specific surface markers of CSCs, and questions have been raised
regarding the specificity and reliability of markers for the
identification of CSCs as widely accepted specific surface markers
of CSCs have not yet been defined (37). Binding of an antibody to its
receptor during these two methods may result in cell activation,
influencing the biology of cells (38). In addition, cells are treated with
enzymes prior to collection for isolation, so the majority of
surface antigens may be damaged and perhaps this is one of the
major disadvantages of the use of surface markers to isolate cancer
stem cells. Certain factors, including the low viability of
isolated cells, high cost and the difficulty of using complex
equipment are other limitations of MACS or FACS use (36). Accumulating studies have
demonstrated that the formation of floating tumor spheres in SFM is
one of the major characteristics of CSCs, and only CSCs are able to
form tumor spheres in SFM (39,40).
The tumor sphere formation assay has been reported for culturing
mammospheres (41), neurospheres
(42) and colonospheres (15), and is considered to be a convenient
method for investigating CSCs (43). Considering the aforementioned
reasons, SFM was used to generate and culture tumor spheres from
LoVo/CPT-11 cells in anchorage-independent conditions. In
sphere-forming cells, the proportion of CD133-positive cells,
indicating the characteristics of CSCs (33,44),
increased over 63- and 12-fold when compared with parental cells
and CPT-11-resistant cells, respectively. Further investigation
revealed that curcumin significantly inhibited tumor sphere
formation, and decreased the expression of CD44, CD133, EpCAM and
CD24 at the mRNA and protein levels in the absence or presence of
CPT-11, while CPT-11 alone had almost no inhibitory effect.
Furthermore, the inhibitory effect of the combination of curcumin
and CPT-11 on sphere formation and expression of CSC markers was
significantly compared with that of curcumin alone; we hypothesized
that this may be the result of a synergistic effect. These results
were in accordance with the results in LoVo/CPT-11 cells and taken
together suggests that CSCs contribute to acquisition of
chemoresistance. Furthermore, these results suggest that curcumin
ameliorates chemoresistance via targeting and eliminating CSCs.
The association between the inhibitory effect of
curcumin and apoptosis was further explored. The results of the
flow cytometry assay suggested that curcumin significantly induced
apoptosis of CSCs, while CPT-11 had no significant effect on
induction of apoptosis. Furthermore, curcumin in combination with
CPT-11 demonstrated a stronger effect compared with curcumin alone.
Curcumin may not only be an 'effector' itself, but also serve as a
'catalyzer' for CPT-11 in inducing apoptosis, resulting in a
synergistic effect between curcumin and CPT-11. This may be
investigated in future studies. In order to achieve higher
credibility, western blot assays were used to verify the effect of
curcumin on the induction of apoptosis. As a family of cysteine
aspartic acid-specific proteases, caspases are essential components
of apoptotic pathways, which include the mitochondrial and death
receptor pathways (45). The
activation of distinct caspase cascades is known to be involved in
activating and cleaving certain proteins that are essential to the
physiological process of apoptosis (46). Caspase-8, the initiator of the
death receptor pathway, is activated by tumor necrosis factor,
which then activates the downstream effector protease. In addition,
caspase-8 participates in the mitochondrial apoptosis pathway, and
the release of cytochrome c activates caspase-9 and other
effector proteases. The two apoptosis pathways lead to the
activation and cleavage of downstream protease caspase-3, provoking
further activation events (47).
The Bcl-2 family, including pro-apoptosis members such as Bax and
anti-apoptosis members such as Bcl-2, has been identified as
upstream regulators of the caspase cascade (48). In the present study, the results of
flow cytometry assay and western blot assay indicated that the
caspase apoptotic pathway is involved in curcumin-induced apoptosis
in LoVo/CPT-11 CSCs. Notably, the flow cytometry assay revealed
that CPT-11 did not induce apoptosis, but western blot analysis
revealed reduced expression levels of Bcl-2, and increased levels
of Bax and cleaved-caspase-3 following CPT-11 treatment. The
possible reasons may be as follows: Firstly, apoptosis is
characterized by a variety of functional and morphological changes
in the plasma membrane and nucleus, these changes result in a
change in the fluorescence intensity in cells. Flow cytometry
detects apoptosis via analyzing the difference of fluorescence
intensity among normal cells, apoptotic cells and dead cells, it
reflects apoptosis at a cellular level, while the expression of
apoptosis-associated proteins detected by western blot assay
reflects apoptosis at a molecular level (45,49).
Secondly, 7-ethyl-10-hydroxy-camptothecin (SN-38), the metabolic
product of CPT-11, induces DNA damage, and is the primary mechanism
for the pharmacological effect of CPT-11 (50). Studies have reported that CPT-11
induces apoptosis of colon cancer cells through the p53 signaling
pathway, and CPT-11 alone or in combination with other drugs may
induce apoptosis via altering the expression of Bcl-2 family,
caspase family and survival-associated genes in cancer cells
(50,51). CPT-11 or its metabolic product
SN-38 possibly intervenes in apoptosis-associated pathways,
influencing the expression of Bax, Bcl-2 and cleaved-caspase-3 in
LoVo/CPT-11 CSCs. However, on account of the active DNA repair
mechanisms, high expression of ABC transporters, and resistance to
apoptosis in CSCs, the function and morphology of plasma membrane
and nucleus may not change (18,52).
Several other cellular signaling pathways are known
to regulate cell apoptosis and proliferation, and we hypothesize
that the phosphatidylinositol 3-kinase (PI3K)/AKT serine/threonine
kinase (AKT), mitogen-activated protein kinases
(MAPK)/extracellular signal-regulated kinase (ERK) and signal
transducer and activator of transcription (STAT) signaling pathways
are associated with the effects of curcumin observed in the present
study. The PI3K/AKT signaling pathway is dysregulated in numerous
types of human cancer, and regulates the apoptotic response via
interaction with the key mediators of the apoptotic process
(53). Activation of this pathway
results in the phosphorylation of transcription factors, which
increases the expression of anti-apoptotic members and decreases
the levels of pro-apoptotic proteins in the Bcl-2 family,
subsequently inhibiting the release of cytochrome c from the
mitochondria, and activating the apoptotic caspase cascade
(47). The MAPK/ERK signaling
pathway is associated with the cell proliferation and apoptosis,
and cross-talk between the PI3K/AKT and MAPK/ERK pathways exists in
numerous cancer cells (54-56).
PI3K/AKT signaling pathway abrogation may lead to a compensatory
activation of the MAPK/ERK signaling pathway (57). The STAT pathway has a role in
relaying extracellular signals from the cytoplasm to the nucleus,
regulating the expression of several genes involved in cell cycle
progression and apoptosis, and curcumin analogues may reduce the
expression of the STAT downstream target gene and induce apoptosis
in colon cancer stem cells (58).
Thus, the natural compound curcumin possibly induces apoptosis and
suppresses proliferation in CSCs via attenuation of
apoptosis-associated signaling pathways, including the PI3K/AKT,
MAPK/ERK and STAT pathways, and it may be worth investigating in
future studies.
Taken together, these findings suggest that CSCs
serve an important role in the development of chemoresistance in
CRC cells. The results of the present study indicated that the
natural compound curcumin effectively attenuated chemoresistance of
CRC cells via targeting and inducing apoptosis in CSCs, hence
eliminating CSCs. The current study provides evidence for the
clinical therapeutic potential of curcumin as a supplement to
conventional chemotherapy in patients with CRC experiencing
resistance to conventional anticancer drugs.
Abbreviations:
|
CRC
|
colorectal cancer
|
|
CSC
|
cancer stem cell
|
|
CD133
|
cluster of differentiation 133
|
|
CD44
|
cluster of differentiation 44
|
|
CD24
|
cluster of differentiation 24
|
|
EpCAM
|
epithelial cell adhesion molecule
|
|
CPT-11
|
irinotecan
|
|
Cur
|
curcumin
|
Acknowledgments
The authors would like to thank the public
experimental platform (Research Center of Clinical Medicine of
Nanfang Hospital Affiliated to Southern Medical University,
Guangzhou, Guangdong, China) for providing experimental
facilities.
References
|
1
|
Armelao F and de Pretis G: Familial
colorectal cancer: A review. World J Gastroenterol. 20:9292–9298.
2014.PubMed/NCBI
|
|
2
|
James MI, Iwuji C, Irving G, Karmokar A,
Higgins JA, Griffin-Teal N, Thomas A, Greaves P, Cai H, Patel SR,
et al: Curcumin inhibits cancer stem cell phenotypes in ex vivo
models of colorectal liver metastases, and is clinically safe and
tolerable in combination with FOLFOX chemotherapy. Cancer Lett.
364:135–141. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lam AK, Chan SS and Leung M: Synchronous
colorectal cancer: Clinical, pathological and molecular
implications. World J Gastroenterol. 20:6815–6820. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Edwards BK, Noone AM, Mariotto AB, Simard
EP, Boscoe FP, Henley SJ, Jemal A, Cho H, Anderson RN, Kohler BA,
et al: Annual Report to the Nation on the status of cancer,
1975–2010, featuring prevalence of comorbidity and impact on
survival among persons with lung, colorectal, breast, or prostate
cancer. Cancer. 120:1290–1314. 2014. View Article : Google Scholar
|
|
5
|
Toden S, Okugawa Y, Jascur T, Wodarz D,
Komarova NL, Buhrmann C, Shakibaei M, Boland CR and Goel A:
Curcumin mediates chemosensitization to 5-fluorouracil through
miRNA-induced suppression of epithelial-to-mesenchymal transition
in chemoresistant colorectal cancer. Carcinogenesis. 36:355–367.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fan X, Zhang C, Liu DB, Yan J and Liang
HP: The clinical applications of curcumin: Current state and the
future. Curr Pharm Des. 19:2011–2031. 2013.
|
|
7
|
Wang J, Zhu R, Sun D, Sun X, Geng Z, Liu H
and Wang SL: Intracellular uptake of curcumin-loaded solid lipid
nanoparticles exhibit anti-inflammatory activities superior to
those of curcumin through the NF-kB signaling pathway. J Biomed
Nanotechnol. 11:403–415. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jin H, Qiao F, Wang Y, Xu Y and Shang Y:
Curcumin inhibits cell proliferation and induces apoptosis of human
non-small cell lung cancer cells through the upregulation of
miR-192-5p and suppression of PI3K/Akt signaling pathway. Oncol
Rep. 34:2782–2789. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sobolewski C, Muller F, Cerella C, Dicato
M and Diederich M: Celecoxib prevents curcumin-induced apoptosis in
a hematopoietic cancer cell model. Mol Carcinog. 54:999–1013. 2015.
View Article : Google Scholar
|
|
10
|
Liao H, Wang Z, Deng Z, Ren H and Li X:
Curcumin inhibits lung cancer invasion and metastasis by
attenuating GLUT1/MT1-MMP/MMP2 pathway. Int J Clin Exp Med.
8:8948–8957. 2015.PubMed/NCBI
|
|
11
|
Shehzad A, Qureshi M, Anwar MN and Lee YS:
Multifunctional curcumin mediate multitherapeutic effects. J Food
Sci. 82:2006–2015. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yallapu MM, Jaggi M and Chauhan SC:
Curcumin nano-medicine: A road to cancer therapeutics. Curr Pharm
Des. 19:1994–2010. 2013.
|
|
13
|
Zhu JY, Yang X, Chen Y, Jiang Y, Wang SJ,
Li Y, Wang XQ, Meng Y, Zhu MM, Ma X, et al: Curcumin suppresses
lung cancer stem cells via inhibiting Wnt/β-catenin and Sonic
Hedgehog pathways. Phytother Res. 31:680–688. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mukherjee S, Mazumdar M, Chakraborty S,
Manna A, Saha S, Khan P, Bhattacharjee P, Guha D, Adhikary A,
Mukhjerjee S, et al: Curcumin inhibits breast cancer stem cell
migration by amplifying the E-cadherin/β-catenin negative feedback
loop. Stem Cell Res Ther. 5:1162014. View
Article : Google Scholar
|
|
15
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
Biffoni M, Todaro M, Peschle C and De Maria R: Identification and
expansion of human colon-cancer-initiating cells. Nature.
445:111–115. 2007. View Article : Google Scholar
|
|
16
|
Taniguchi H, Moriya C, Igarashi H, Saitoh
A, Yamamoto H, Adachi Y and Imai K: Cancer stem cells in human
gastrointestinal cancer. Cancer Sci. 107:1556–1562. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bitarte N, Bandres E, Boni V, Zarate R,
Rodriguez J, Gonzalez-Huarriz M, Lopez I, Javier Sola J, Alonso MM,
Fortes P, et al: MicroRNA-451 is involved in the self-renewal,
tumorigenicity, and chemoresistance of colorectal cancer stem
cells. Stem Cells. 29:1661–1671. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dean M, Fojo T and Bates S: Tumour stem
cells and drug resistance. Nat Rev Cancer. 5:275–284. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu S, Wang X, Chen J and Chen Y: Autophagy
of cancer stem cells is involved with chemoresistance of colon
cancer cells. Biochem Biophys Res Commun. 434:898–903. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang Y, Wang G, Zhu D, Huang Y, Luo Y, Su
P, Chen X and Wang Q: Epithelial-mesenchymal transition and cancer
stem cell-like phenotype induced by Twist1 contribute to acquired
resistance to irinotecan in colon cancer. Int J Oncol. 51:515–524.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
22
|
Botchkina G: Colon cancer stem cells -
from basic to clinical application. Cancer Lett. 338:127–140. 2013.
View Article : Google Scholar
|
|
23
|
Elshamy WM and Duhé RJ: Overview: Cellular
plasticity, cancer stem cells and metastasis. Cancer Lett. 341:2–8.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yoshida GJ and Saya H: Therapeutic
strategies targeting cancer stem cells. Cancer Sci. 107:5–11. 2016.
View Article : Google Scholar :
|
|
25
|
Stavrovskaya AA and Stromskaya TP:
Transport proteins of the ABC family and multidrug resistance of
tumor cells. Biochemistry (Mosc). 73:592–604. 2008. View Article : Google Scholar
|
|
26
|
Dandawate PR, Subramaniam D, Jensen RA and
Anant S: Targeting cancer stem cells and signaling pathways by
phytochemicals: Novel approach for breast cancer therapy. Semin
Cancer Biol. 40–41:192–208. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chang TC, Yeh CT, Adebayo BO, Lin YC, Deng
L, Rao YK, Huang CC, Lee WH, Wu AT, Hsiao M, et al:
4-Acetylantroquinonol B inhibits colorectal cancer tumorigenesis
and suppresses cancer stem-like phenotype. Toxicol Appl Pharmacol.
288:258–268. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dalerba P, Dylla SJ, Park IK, Liu R, Wang
X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM, et al:
Phenotypic characterization of human colorectal cancer stem cells.
Proc Natl Acad Sci USA. 104:10158–10163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sanders MA and Majumdar AP: Colon cancer
stem cells: Implications in carcinogenesis. Front Biosci.
16:1651–1662. 2011. View
Article : Google Scholar
|
|
30
|
Nautiyal J, Kanwar SS, Yu Y and Majumdar
AP: Combination of dasatinib and curcumin eliminates
chemo-resistant colon cancer cells. J Mol Signal. 6:72011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hong SP, Wen J, Bang S, Park S and Song
SY: CD44-positive cells are responsible for gemcitabine resistance
in pancreatic cancer cells. Int J Cancer. 125:2323–2331. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ma S: Biology and clinical implications of
CD133(+) liver cancer stem cells. Exp Cell Res. 319:126–132. 2013.
View Article : Google Scholar
|
|
33
|
Zhang SS, Han ZP, Jing YY, Tao SF, Li TJ,
Wang H, Wang Y, Li R, Yang Y, Zhao X, et al: CD133(+)CXCR4(+) colon
cancer cells exhibit metastatic potential and predict poor
prognosis of patients. BMC Med. 10:852012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cui S and Chang PY: Current understanding
concerning intestinal stem cells. World J Gastroenterol.
22:7099–7110. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Khan MI, Czarnecka AM, Helbrecht I,
Bartnik E, Lian F and Szczylik C: Current approaches in
identification and isolation of human renal cell carcinoma cancer
stem cells. Stem Cell Res Ther. 6:1782015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Abbaszadegan MR, Bagheri V, Razavi MS,
Momtazi AA, Sahebkar A and Gholamin M: Isolation, identification,
and characterization of cancer stem cells: A review. J Cell
Physiol. 232:2008–2018. 2017. View Article : Google Scholar
|
|
37
|
Cai J, Peng T, Wang J, Zhang J, Hu H, Tang
D, Chu C, Yang T and Liu H: Isolation, culture and identification
of choriocarcinoma stem-like cells from the human choriocarcinoma
cell-line JEG-3. Cell Physiol Biochem. 39:1421–1432. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Moghbeli M, Moghbeli F, Forghanifard MM
and Abbaszadegan MR: Cancer stem cell detection and isolation. Med
Oncol. 31:692014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shaheen S, Ahmed M, Lorenzi F and Nateri
AS: Spheroid-formation (colonosphere) assay for in iitro assessment
and expansion of stem cells in colon cancer. Stem Cell Rev.
12:492–499. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ning X, Du Y, Ben Q, Huang L, He X, Gong
Y, Gao J, Wu H, Man X, Jin J, et al: Bulk pancreatic cancer cells
can convert into cancer stem cells (CSCs) in vitro and 2 compounds
can target these CSCs. Cell Cycle. 15:403–412. 2016. View Article : Google Scholar
|
|
41
|
Kakarala M, Brenner DE, Korkaya H, Cheng
C, Tazi K, Ginestier C, Liu S, Dontu G and Wicha MS: Targeting
breast stem cells with the cancer preventive compounds curcumin and
piperine. Breast Cancer Res Treat. 122:777–785. 2010. View Article : Google Scholar
|
|
42
|
Singh SK, Hawkins C, Clarke ID, Squire JA,
Bayani J, Hide T, Henkelman RM, Cusimano MD and Dirks PB:
Identification of human brain tumour initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Pozzi V, Sartini D, Rocchetti R,
Santarelli A, Rubini C, Morganti S, Giuliante R, Calabrese S, Di
Ruscio G, Orlando F, et al: Identification and characterization of
cancer stem cells from head and neck squamous cell carcinoma cell
lines. Cell Physiol Biochem. 36:784–798. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Roy S, Lu K, Nayak MK, Bhuniya A, Ghosh T,
Kundu S, Ghosh S, Baral R, Dasgupta PS and Basu S: Activation of
D2 dopamine receptors in CD133+ve cancer stem cells in
non-small cell lung carcinoma inhibits proliferation, clonogenic
ability, and invasiveness of these cells. J Biol Chem. 292:435–445.
2017. View Article : Google Scholar
|
|
45
|
Taylor RC, Cullen SP and Martin SJ:
Apoptosis: Controlled demolition at the cellular level. Nat Rev Mol
Cell Biol. 9:231–241. 2008. View Article : Google Scholar
|
|
46
|
Gupta S, Kass GE, Szegezdi E and Joseph B:
The mitochondrial death pathway: A promising therapeutic target in
diseases. J Cell Mol Med. 13:1004–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang L, Huo X, Liao Y, Yang F, Gao L and
Cao L: Zeylenone, a naturally occurring cyclohexene oxide, inhibits
proliferation and induces apoptosis in cervical carcinoma cells via
PI3K/AKT/mTOR and MAPK/ERK pathways. Sci Rep. 7:16692017.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Martinou JC and Youle RJ: Mitochondria in
apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev
Cell. 21:92–101. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Adan A, Alizada G, Kiraz Y, Baran Y and
Nalbant A: Flow cytometry: Basic principles and applications. Crit
Rev Biotechnol. 37:163–176. 2017. View Article : Google Scholar
|
|
50
|
Keyvani-Ghamsari S, Rabbani-Chadegani A,
Sargolzaei J and Shahhoseini M: Effect of irinotecan on HMGB1, MMP9
expression, cell cycle, and cell growth in breast cancer (MCF-7)
cells. Tumour Biol. 39:1010428317698354. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Rudolf E, John S and Cervinka M:
Irinotecan induces senescence and apoptosis in colonic cells in
vitro. Toxicol Lett. 214:1–8. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ogden A, Rida PC, Reid MD, Kucuk O and
Aneja R: Die-hard survivors: Heterogeneity in apoptotic thresholds
may underlie chemoresistance. Expert Rev Anticancer Ther.
15:277–281. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Spangle JM, Roberts TM and Zhao JJ: The
emerging role of PI3K/AKT-mediated epigenetic regulation in cancer.
Biochim Biophys Acta. 1868:123–131. 2017.PubMed/NCBI
|
|
54
|
Daaboul HE, Daher CF, Bodman-Smith K,
Taleb RI, Shebaby WN, Boulos J, Dagher C, Mroueh MA and El-Sibai M:
Antitumor activity of β-2-himachalen-6-ol in colon cancer is
mediated through its inhibition of the PI3K and MAPK pathways. Chem
Biol Interact. 275:162–170. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yu ST, Zhong Q, Chen RH, Han P, Li SB,
Zhang H, Yuan L, Xia TL, Zeng MS and Huang XM: CRLF1 promotes
malignant phenotypes of papillary thyroid carcinoma by activating
the MAPK/ERK and PI3K/AKT pathways. Cell Death Dis. 9:3712018.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhou Q, Chen J, Feng J, Xu Y, Zheng W and
Wang J: SOSTDC1 inhibits follicular thyroid cancer cell
proliferation, migration, and EMT via suppressing PI3K/Akt and
MAPK/Erk signaling pathways. Mol Cell Biochem. 435:87–95. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Serra V, Scaltriti M, Prudkin L, Eichhorn
PJ, Ibrahim YH, Chandarlapaty S, Markman B, Rodriguez O, Guzman M,
Rodriguez S, et al: PI3K inhibition results in enhanced HER
signaling and acquired ERK dependency in HER2-overexpressing breast
cancer. Oncogene. 30:2547–2557. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Lin L, Liu Y, Li H, Li PK, Fuchs J,
Shibata H, Iwabuchi Y and Lin J: Targeting colon cancer stem cells
using a new curcumin analogue, GO-Y030. Br J Cancer. 105:212–220.
2011. View Article : Google Scholar : PubMed/NCBI
|