Introduction
Bladder cancer is the most common urinary malignancy
in China, with an annual incidence rate and estimated mortality
rate of 80.5/100,000 and 32.9/100,000, respectively (1). Despite recent improvements in
treatment strategies, the overall survival rates of patients with
an advanced stage of the disease remains poor (2). Tumor progression and metastasis are
the main causes of bladder cancer-associated mortality, yet the
exact mechanisms underlying these processes have not been fully
elucidated. Hence, it is imperative to determine the key mechanisms
implicated in bladder cancer development and progression, in order
to identify potential therapeutic targets to improve patient
prognosis.
The pentose phosphate pathway (PPP), one of the
alternative routes for glucose metabolism, provides products for
biosynthesis and antioxidant defense in cells (3). PPP has been attracting increased
attention due to its ability to facilitate tumor progression or
chemotherapy resistance by satisfying the considerable biosynthetic
demands of rapidly growing cancer cells, in addition to their
resistance and survival under stress conditions (3). Glucose-6-phosphate dehydrogenase
(G6PD) is a rate-limiting enzyme of the PPP, and it is well known
to promote intracellular anabolic reactions and redox homeostasis
(4). Recently, multiple studies
have demonstrated that elevated G6PD levels promote cancer
progression in numerous tumor types, including melanoma, leukemia
and colon cancer (5-10). Considering the function of G6PD in
the critical processes of cancer cells, it is imperative to
identify the mechanisms underlying the function of G6PD in bladder
cancer in order to develop potent and selective G6PD inhibitors
(10).
The aim of the present study was to investigate
whether the high expression of G6PD in bladder cancer is associated
with tumor aggressiveness and poor clinical prognosis and to
determine whether targeting G6PD may be of value as a therapeutic
option for bladder cancer, particularly in advanced cases.
Materials and methods
Online database
Information on G6PD mRNA expression in bladder
cancer and normal tissues were acquired from the Oncomine database
(https://www.oncomine.org) using the following
searching terms: G6PD and bladder cancer (11). Dyrskjøt et al (12) bladder and Lee et al
(13) bladder were 2 independent
studies with bladder cancer samples recorded in the Oncomine
database. The information between G6PD expression and clinical
significance were downloaded from The Cancer Genome Atlas (TCGA)
database (https://cancergenome.nih.gov/) using the following
searching terms: Project, TCGA-Bladder Urothelial Carcinoma;
Primary site, bladder; expiration date, January 2018.
Cell culture
Human bladder cancer cell lines 5637 (thought to
have the same molecular features as high-risk superficial bladder
cancer) (14), T24 (thought to
have the same molecular features as muscle invasive bladder cancer
with grade III pathological grading) (15), TCCSUP (poorly differentiated and
high-risk muscle invasive bladder cancer with grade IV pathological
grading) (14), normal
uroepithelial cell line SV-HUC-1 (16) and the engineered 293T cell line
were purchased from the Cell Bank of the Chinese Academy of
Sciences (Shanghai, China), and short tandem repeat DNA profiling
analysis were performed by the supplier to validate all cell lines.
5637, T24 and TCCSUP cells were cultured in RPMI-1640 medium,
SV-HUC-1 cells were cultured in Minimum Essential medium and 293T
cells were cultured in DMEM. All the aforementioned mediums without
any antibiotics (Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) were pre-supplemented with 10% fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc.), and all cell lines were incubated
under standard conditions (37°C and 5% CO2).
Plasmid construction, lentiviral
packaging and transfection
The G6PD-overexpression plasmid was acquired from
Vigene Biosciences, Inc. (Rockville, MD, USA). Validated sequences
of short hairpin RNA (shRNA) against G6PD (shG6PD) were screened
from Sigma-Aldrich online (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany). Scramble sequences were designed with the corresponding
shG6PD sequences using the siRNA Wizard v3.1 online software
(https://www.invivogen. com/sirnawizard/)
declaring the absence of preclusive mRNA and miRNA seed sequence
matches. Oligonucleotides of G6PD shRNA and control shRNA were then
synthesized (Tsingke, Hangzhou, China) and inserted into the GIPZ
lenti-viral vector. The sequences used were as follows: shG6PD
forward,
5′-CCGGCAACAGATACAAGAACGTGAACTCGAGTTCACGTTCTTGTATCTGTTGTTTTTG-3′
and reverse,
5′-AATTCAAAAACAACAGATACAAGAACGTGAACTCGAGTTCACGTTCTTGTATCTGTTG-3′;
Scramble forward,
5′-CCGGGCAAAGCAAACGTGACATAAACTCGAGTTTATGTCACGTTTGCTTTGCTTTTTG-3′
and reverse,
5′-AATTCAAAAAGCAAAGCAAACGTGACATAAACTCGAGTTATGTCACGTTTGCTTTGC-3′.
Then the vectors were co-transfected with pSPAX2 and pMD2G
(purchased from Addgene, Inc., Cambridge, MA, USA) plasmids (4:3:1
for vectors, pSPAX2 and pMD2G, respectively) into the 293T cell
line cultured in 60 mm plates (at 60% cell density) using a calcium
phosphate precipitation method (17). The supernatant, which contained
lentivirus, was harvested 48 or 72 h post-transfection. Subsequent
to virus packaging, T24 and TCCSUP cells cultured in 60 mm plates
(at 30% cell density) were infected for 48 or 72 h with polybrene
(5 µl/ml; Sigma-Aldrich; Merck KGaA) and the medium was
changed 6–12 h later. Successfully transfected G6PD-shRNA cell
lines were screened with 0.5 mg/ml puromycin (Sigma-Aldrich; Merck
KGaA) and the transfection efficiency was validated using green
fluorescent protein (488 nm) expression and western blotting.
Cell counting kit-8 (CCK-8) assay and
colony formation assay
The proliferation of different groups of cells were
compared using a CCK-8 kit (Dojindo Molecular Technologies, Inc.,
Kumamoto Japan) according to the manufacturer's protocol. 5637, T24
and TCCSUP cell lines with or without transfection were firstly
prepared at a density of 1,000 cells/plate in 96-well plates under
standard conditions (37°C and 5% CO2). Then, premixed
medium (as aforementioned) with a 10% concentration of CCK-8
reagent was added into each well and placed in standard conditions
(37°C and 5% CO2) for 1 h prior to measurement at an
optical density of 450 nm. The preparations of cells exposed to 10
µM 6-aminonicotinamide (6-AN; Sigma-Aldrich; Merck KGaA), 0,
5, 10, 20, 40 or 80 µg/ml cisplatin (Sigma-Aldrich; Merck
KGaA), 5 or 10 µM SC79 (MedChemExpress, Monmouth Junction,
NJ, USA) or controlled DMSO for 24 or 48 h following the same
procedure. As for colony formation assay, cells were seeded at a
density of 500 cells/plate in 6-well plates and cultured for 8–10
days under standard conditions followed by 15 min fixation at room
temperature and 15 min staining (0.5% crystal violet) at room
temperature of the colonies which were performed prior to
comparison.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.)
was used for cells total RNA extraction according to manufacturer's
protocol. Takara PrimeScript™ RT and SYBR EX Taq™ kits (Takara Bio,
Inc., Otsu, Japan) were used according to manufacturer's protocol.
The specify thermocycling conditions used were as follows: Step 1,
95.0°C for 30 sec; step 2, 40 cycles of 95°C for 5 sec and 60°C for
30 sec; and step 3, melt curve analysis at 65°C to 95°C, increasing
in 0.5°C increments for 5 sec. The primers were designed as
follows: G6PD forward, 5′-ACCGCATCGACCACTACCT-3′ and reverse
5′-TGGGGCCGAAGATCCTGTT-3′; β-actin forward,
5′-GCAAGCAGGAGTATGACGAG-3′ and reverse, 5′-CAAATAAAGCCATGCCAATC-3′.
Control groups were used to confirm the absence of the pollution of
agents or primer dimers, and melt-curve analysis was used to
identify the specificity of amplification. All genes were
normalized to β-actin expression, and the gene mRNA relative
expressions were calculated using the ΔΔCq method (18) using SPSS version 22.0 software (IBM
Corp., Armonk, NY, USA).
Western blotting
5637, T24 and TCCSUP cell lines were firstly washed
using PBS twice prior to being lysed using RIPA Lysis buffer with
1% cocktail protease inhibitor (Thermo Fisher Scientific, Inc.) for
4 h at 4°C and purified by centrifugation (4°C, 15,000 × g, 15
min). Subsequent to concentration measurement using a BCA protein
assay (Pierce; Thermo Fisher Scientific, Inc.), 15 µg/10
µl protein samples were loaded in 12% Tris-acetate gels
(Invitrogen; Thermo Fisher Scientific, Inc.) and then separated by
electrophoresis. Next, the proteins were transferred onto a
polyvinylidene fluoride membrane. Then, the membrane was blocked
with 5% non-fat milk in Tris-buffered saline containing 1% Tween-20
(TBST) for 1 h at room temperature and further incubated with
primary antibodies for 12 h at 4°C. Subsequent to washing with TBST
three times, the membrane was incubated with secondary antibodies
for 1 h at room temperature. The primary antibodies used in this
experiment were: Rabbit polyclonal antibody G6PD (1:10,000; cat no.
ab993; Abcam, Cambridge, UK); Rabbit monoclonal antibodies
phosphorylated-protein kinase B (AKT; Ser473; 1:1,000; cat no.
4060; CST Biological Reagents Co., Ltd., Shanghai, China), AKT
(1:1,000; cat no. 4685; CST Biological Reagents Co., Ltd.), P21
(1:2,000; cat no. ab109520; Abcam), P27 (1:2,000; cat no. ab32034;
Abcam), cleaved caspase-3 (1:1,000; cat no. 9664; CST Biological
Reagents Co., Ltd.), cleaved caspase-7 (1:1,000; cat no. 8438; CST
Biological Reagents Co., Ltd.), cleaved caspase-9 (1:1,000; cat no.
7237; CST Biological Reagents Co., Ltd.) and Mouse monoclonal
antibody β-actin (1:2,000; cat no. ab6276; Abcam). The secondary
antibodies used in this experiment were: Goat anti
Rabbit-horseradish peroxidase (HRP; 1:5,000; cat no. PDR007; Fdbio
Science, Hangzhou, China) and Goat anti Mouse-HRP (1:5,000; cat no.
PDM007; Fdbio Science). Immunodetection was performed by EZ-ECL
chemiluminescence detection kit (Biological Industries, Kibbutz
Beit Haemek, Israel). Protein bands were analyzed using Image-Pro
Plus software 6.0 (Media Cybernetics, Inc., Rockville, MD, USA),
and β-actin was selected as an internal reference.
Flow cytometry analysis of cell
apoptosis
Prepared T24 and TCCSUP cell lines (60% cell density
in 6 mm dishes) with or without the shRNA-mediated G6PD knockdown
were harvested with non-EDTA trypsin (Biological Industries),
washed twice with cold PBS and then incubated using BD Annexin
V-APC/7-aminoactinomycin D Apoptosis Detection Kit (BD Biosciences,
San Jose CA, USA) according to the manufacturer's protocol. All
aforementioned cells were harvested and stained with Annexin V (10
µl/200 µl) and 7-aminoactinomycin D (10 µl/200
µl) for 15 min in the dark at room temperature, and then
apoptosis was analyzed using BD FACSCanto™ II (BD Biosciences), and
data was then analyzed by FlowJo 7.6 software (FlowJo LLC, Ashland,
OR, USA).
Intracellular reactive oxygen species
(ROS) detection
T24, TCCSUP and 5637 cell lines with or without
shRNA-mediated G6PD knockdown or G6PD overexpression or in the
presence or absence of H2O2 were prepared in
9 wells in 96-well plates. Then, each group of cells was stimulated
with or without 50 µM H2O2 for 30 min
at 37°C prior to being co-incubated with 20 µM
diochloro-dihydro-fluorescein diacetate (Invitrogen; Thermo Fisher
Scientific, Inc.) for 30–60 min at 37°C. Then, 3 wells of each
group of cells (selected as the cell counting group) were used for
cell counting. The remaining wells (selected as the measuring
group) were used to detect the ROS levels. Fluorescence was read at
485 nm/520 nm by a fluorescent enzyme meter, Varioskan™ Flash
(Thermo Fisher Scientific, Inc.). Levels of cellular ROS were
normalized to the total number of cells.
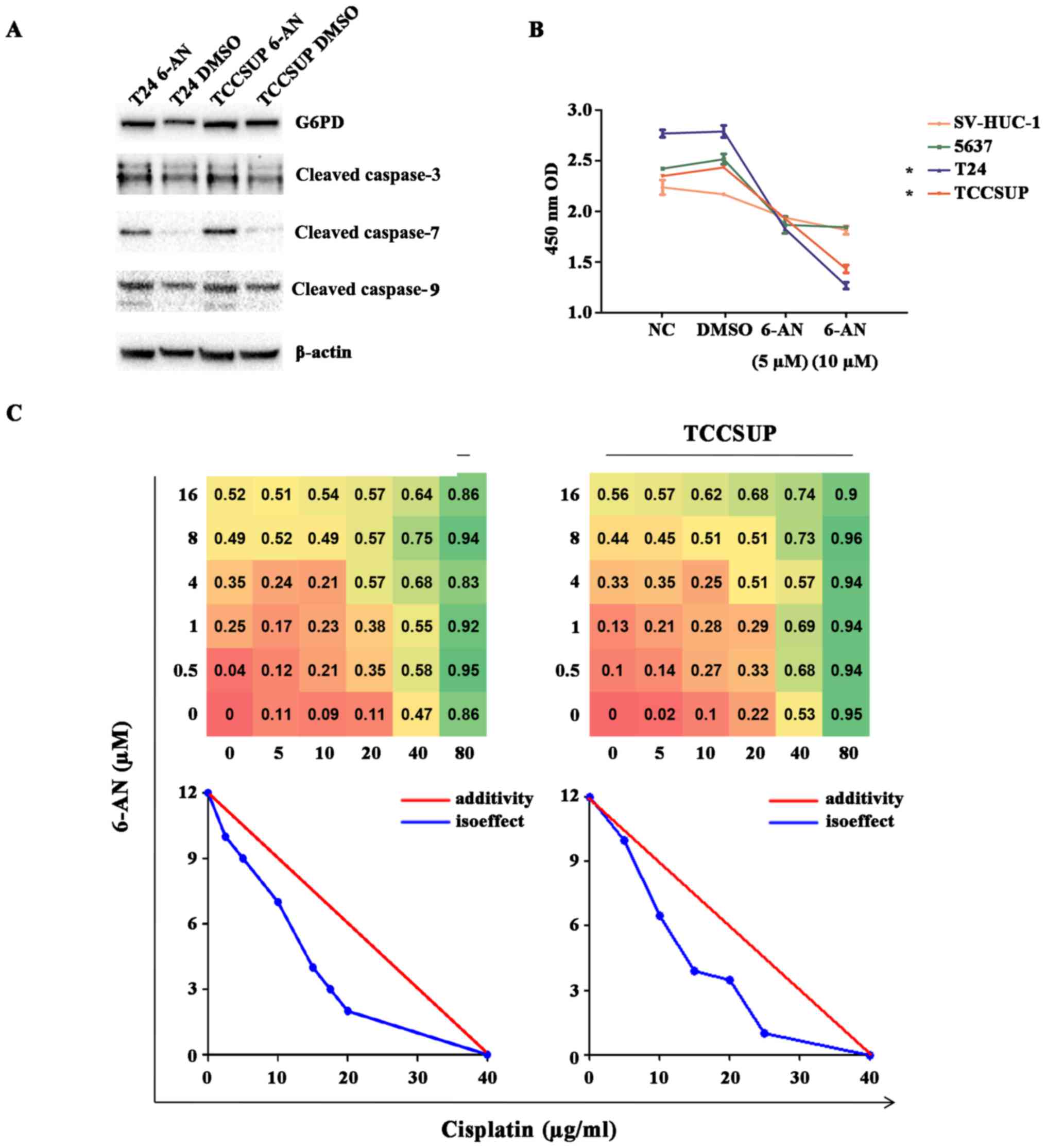
Isobolographic analysis
In order to determine the combination effects of
cisplatin and 6-AN, isobolographic analysis was performed. T24 and
TCCSUP cell lines were firstly prepared at a density of 5,000
cells/plate in 96-well plates under standard conditions (37°C and
5% CO2). Then cells were incubated with combination of
different concentrations of 6-AN (C1 = 0, 0.5, 1, 4, 8 or 16
µM) and cisplatin (C2 = 0, 5, 10, 20, 40 or 80
µg/ml). A total of 24 h later, premixed medium with a 10%
concentration of CCK-8 reagent was added into each well and placed
in standard conditions for 1 h prior to measurement at an optical
density of 450 nm. Once the background density was excluded, the
450 nm density (D) of each combination was used for cytotoxicity
calculation using the following formula: Cytotoxic effect (CE) =1-D
(C1, C2)/D (0, 0), and D(0, 0) was selected as the 0% cytotoxic
effect. Isoboles are defined as isoeffect curves that reveal the
concentration of the combination of two drugs which results in a
similar CE to a previous study (19,20).
The curves were obtained by ligaturing plots which represent the
concentration combination of two drugs resulting in a 50% CE. The
straight lines refer to the theoretical additivity line resulting
in a 50% CE, and all data were analyzed using SPSS 22.0 (IBM Corp.,
Armonk, NY, USA) software.
Statistical analysis
SPSS 22.0 (IBM Corp.) was used to analyze the data.
The normality of the data was initially determined using a
Kolmogorov-Smirnov test. Data was presented as the mean ± standard
deviation. The correlations between G6PD expression and
clinicopathological characteristics were analyzed using a Pearson's
χ2 test or a continuity correction χ2 test.
Overall survival and disease-free survival rates curves were
plotted using the Kaplan-Meier method, and data was analyzed by a
log-rank test. One-way analysis of variance test was used to
examine the differences between different groups.
Student-Newman-Keuls test was used as a post hoc test. P<0.05
was considered to indicate a statistically significant difference.
Data was derived from at least 3 repeated experiments.
Results
High G6PD expression is a poor prognostic
factor in bladder cancer
To investigate whether G6PD expression is associated
with bladder cancer progression and prognosis, the present study
compared the G6PD expression levels in bladder cancer using the
Oncomine database. G6PD mRNA expression levels were significantly
higher in bladder cancer tissues compared with that in adjacent
normal tissues (P<0.05; Fig. 1A and
B). Furthermore, the levels of G6PD expression notably
increased with increasing T stage (Fig. 1A and B). In addition, the results
of qPCR analysis and western blotting revealed that G6PD mRNA
levels were significantly upregulated in three tumor cell lines
compared with a normal urothelial cell line (P<0.05) and that
the protein levels were notably upregulated. Furthermore, T24 and
TCCSUP, which have a higher malignant potential compared with 5637,
exhibited higher G6PD expression levels compared with 5637
(Fig. 1C and D). Next, the
clinical significance of G6PD in 408 patients with muscle-invasive
bladder cancer (MIBC) from the TCGA database was investigated.
Analysis revealed a significant association of G6PD expression with
stage (P<0.05) and sex (P<0.001) (Table I). Kaplan-Meier survival analysis
of 402 patients with MIBC (as the data for 6 patients was
inaccessible) revealed that patients with high G6PD expression
levels had a worse overall survival rates (P=0.057, close to 0.05)
and a significantly worse disease-free survival rates (P=0.0013)
compared with those with lower G6PD expression levels (Fig. 1E and F).
 | Table IAssociation between G6PD expression
level and clinicopathological features of 408 patients with
muscle-invasive bladder cancer in The Cancer Genome Atlas
database. |
Table I
Association between G6PD expression
level and clinicopathological features of 408 patients with
muscle-invasive bladder cancer in The Cancer Genome Atlas
database.
| Characteristic | G6PD expression
levels
| P-value |
|---|
| Low | High |
|---|
| Sex | | | <0.001b |
| Male | 104 | 151 | |
| Female | 100 | 53 | |
| Age (years) | | | 0.265 |
| <65 | 75 | 86 | |
| ≥65 | 129 | 118 | |
| Tumor grade | | | |
| 0.207 | | | |
| Low | 15 | 9 | |
| High | 189 | 195 | |
| Stage | | | 0.034a |
| I or II | 76 | 56 | |
| III or IV | 128 | 148 | |
| Lymph node
metastasis | | | 0.070 |
| Absent | 148 | 56 | |
| Present | 131 | 73 | |
| Distant
metastasis | | | 0.221c |
| Absent | 201 | 3 | |
| Present | 196 | 8 | |
Knockdown of G6PD suppresses cell
proliferation and growth, while increasing intracellular ROS
levels
In order to determine the effect of upregulated G6PD
expression in bladder cancer cell lines highly expressing G6PD, T24
and TCCSUP cells were transfected with sh-G6PD lentivirus. The
infection efficiency was validated by western blotting and
fluorescence microscopy, and G6PD was demonstrated to be
significantly lower in transfected cell lines compared with their
respective scramble controls (P<0.05; Fig. 2A). The CCK8 cell proliferation
assay revealed that the knockdown of G6PD in the two cell lines
significantly reduced cell proliferation (P<0.05; Fig. 2B). Furthermore, the colony-forming
ability of the two cell lines was substantially suppressed in the
G6PD knockdown groups compared with the control groups (Fig. 2C). No significant difference was
observed between G6PD-overexpressing and control T24 or TCCSUP cell
lines, however the overexpression of G6PD was observed in the 5637
cell line, which exhibited lower G6PD expression prior to
transfection, in addition to enhanced cell proliferation and
colony-forming abilities compared with the control group (Fig. 2B and C). Additionally, the
knockdown of G6PD in the two cell lines resulted in significantly
higher ROS accumulation compared with the corresponding control
groups, which indicated a weaker ability to survive oxidative
stress (P<0.05; Fig. 2D)
(12,13).
 | Figure 2Knockdown of G6PD suppresses cell
proliferation and growth while increasing intracellular ROS levels.
(A) Transfection efficiency was validated by western blotting and
fluorescence microscopy, and shG6PD reduced the expression of G6PD
by at least 50%. *P<0.05 with comparisons shown by
lines. (B) Overexpression of G6PD promotes the proliferation of
5637 cell lines, whereas the inhibition of G6PD reduces the
proliferation rate of T24 and TCCSUP cell lines as determined by a
Cell Counting Kit-8 assay. *P<0.05 with comparisons
shown by lines. (C) Colony formation assays of T24, TCCSUP and 5637
cell lines. (D) Relative ROS levels in T24, TCCSUP and 5637 cell
lines with or without shRNA-mediated G6PD knockdown or G6PD
overexpression or in the presence or absence of
H2O2 (50 µg/ml), *P<0.05
with comparisons shown by lines. (E) Flow cytometric analysis
presenting the percentage of apoptosis distribution between
shG6PD-lentivirus- and scramble-lentivirus-transfected cells. Left,
percentage of apoptosis in different groups of cell lines; Middle,
enhancement of 2 types of apoptotic signals (Annexin V and 7AAD) in
different groups of cell lines; Right, percentage of early and late
apoptosis in different groups of cell lines. *P<0.05
with comparisons shown by lines. G6PD, glucose-6-phosphate
dehydrogenase; ROS, reactive oxygen species; sh/shRNA, short
hairpin RNA; 7AAD, 7-aminoactinomycin D; NC, negative control; OE,
overexpression. |
Knockdown of G6PD induces intracellular
apoptosis and suppresses the phosphorylated-AKT/AKT pathway
Multiple studies have reported that toxic ROS levels
tend to induce apoptosis or other adverse reactions in cells
(21-23). The present study compared the
apoptosis between G6PD-knockdown and control groups. Interestingly,
significantly increased apoptosis was demonstrated by flow
cytometry analysis in the G6PD-knockdown groups compared with the
control (P<0.05; Fig. 2E), in
addition to the significant upregulation of cleaved caspase-3, -7
and -9 levels in G6PD-knockdown cells compared with scramble
control cells (P<0.05; Fig.
3A). As reported in previous studies, the AKT pathway, which
serves a vital function in the proliferation and apoptosis of tumor
cells, was also revealed to sensitize cells to oxidative apoptosis
(24,25). In addition, the AKT signaling
pathway was also reported to promote the progression of bladder
cancer (26). Therefore the
present study investigated whether the knockdown of G6PD
additionally suppressed AKT signaling. The western blotting results
revealed a significant decrease of phosphorylated AKT (P<0.05),
but no significant change was observed of the total AKT in two
G6PD-knockdown cell lines (T24 and TCCSUP) compared with the
corresponding controls (Fig. 3B).
Furthermore, P27, a cell cycle regulator known to be inhibited by
AKT, was also revealed to be upregulated in G6PD-knockdown cell
lines (Fig. 3B). Interestingly, a
rescue assay with SC79, a specific AKT activator, successfully
restored the partial effects of G6PD knockdown (P<0.05; Fig. 4A). All this evidence suggests that
the suppression of AKT signaling in G6PD-knockdown bladder cancer
cells may exert a substantial tumor inhibitory effect.
 | Figure 3Knockdown of G6PD induced
intracellular apoptosis and suppressed the p-AKT/AKT pathway. (A)
Protein levels of G6PD, cleaved caspase-3, cleaved caspase-7,
cleaved caspase-9 and β-actin are presented in two groups of cell
lines. β-actin was used as a reference control.
*P<0.05 vs. the scramble control. (B) Protein levels
of G6PD, AKT, p-AKT, P21, P27 and β-actin are presented in three
groups of cell lines. β-actin was used as a reference control.
*P<0.05 vs. the scramble control. G6PD,
glucose-6-phosphate dehydrogenase; p-, phosphorylated; AKT, protein
kinase B; sh-, short hairpin RNA; NC, negative control; OE,
overexpression. |
Inhibition of G6PD activity with 6-AN
exerts antineoplastic effects and functions synergistically with
cisplatin
Subsequently, the effect of G6PD inhibition on T24
and TCCSUP cell lines using 6-AN, a competitive G6PD inhibitor, was
examined. The specific effect of 6-AN on G6PD was confirmed by the
fact that 6-AN treatment in the two cell lines resulted in similar
results with G6PD knockdown (Figs.
4B–D and 5A). The 6-AN
treatment in the two cell lines significantly reduced cell
proliferation determined using a CCK8 cell proliferation assay
(P<0.05; Fig. 4B) and resulted
in a significantly higher ROS accumulation compared with the
corresponding control groups (P<0.05; Fig. 2C). A rescue assay with SC79, a
specific AKT activator, successfully restored the partial effects
of the treatment of 6-AN (P<0.05; Fig. 4D). Additionally, the significant
upregulation of cleaved caspase-3, -7 and -9 levels in cells with
6-AN treatment compared with cells with DMSO treatment was observed
(P<0.05; Fig. 3A). Of note, it
was revealed that the T24 and TCCSUP cell lines (high G6PD
expression) displayed a higher sensitivity to 6-AN compared with
the SVHUC and 5637 cell lines (lower G6PD expression), suggesting
the specificity and rationale of targeting higher G6PD activity in
bladder cancer (*P<0.05; Fig. 5B). Interestingly, it was revealed
that the level of G6PD protein increased subsequent to 6-AN
treatment. 6-AN may be metabolized to 6-amino-NAD(P+), a
competitive inhibitor of NAD(P+)-requiring processes, particularly
G6PD. Additionally, it does not directly affect the expression of
G6PD protein itself (27). Thus,
the increase of G6PD may be explained as a compensatory increasing.
Finally, the present study investigated whether 6-AN has the
ability to enhance the antitumor effects of cisplatin, a classical
drug mostly used in bladder cancer chemotherapy. In the T24 and
TCCSUP cell lines, 6-AN and cisplatin functioned synergistically to
enhance cytotoxicity in the CCK8 assay, and a substantial dose
reduction with the combination of the two drugs by using
isoeffective drug concentrations resulting in 50% of the cytotoxic
effect was observed (Fig. 5C). All
these cumulative results indicate that inhibition of G6PD activity
by 6-AN may be a potential therapeutic method for bladder cancer,
particularly in cases with high G6PD expression, and that the
combination of cisplatin with 6-AN may optimize the clinical dose
of cisplatin or minimize the cisplatin-associated side effects.
Discussion
It has become apparent that cancer cells require
sugars to drive oncogenic processes (28,29).
For example, rapidly dividing cells require a constant supply of
building blocks to maintain their elevated biosynthetic activity.
In line with this increased metabolism are increased ROS levels
(23). Considered to be
by-products of oxygen consumption and cellular metabolism, ROS are
formed by the partial reduction of molecular oxygen (30,31).
ROS homeostasis is crucial for cell survival and normal cell
signaling, in addition to protecting cells from damage. To some
extent, cancer cells must maintain cellular ROS at levels that
favor growth (32). Glutathione
(GSH), an important antioxidant for ROS detoxification, serves a
key role in maintaining ROS homeostasis in cells (32). The content of activated GSH were
revealed to be closely associated with the content of NADPH, the
product of G6PD (23). G6PD is the
rate-limiting enzyme in PPP; the oncogenic properties of G6PD have
been attracting increasing attention and its increased activity in
cancer cells was also recently demonstrated (33-35).
In the present study, it was revealed that high G6PD expression was
associated with a higher stage and poorer prognosis in patients
with bladder cancer. G6PD expression was upregulated in tumor
tissues compared with that in adjacent normal tissues, and the
level of G6PD expression increased with increasing T stage.
Analysis from the TCGA database revealed a significant association
of G6PD expression with stage and sex in patients with MIBC.
Patients with bladder cancer exhibited worse overall and
disease-free survival rates compared with those with lower G6PD
expression in the resected tumors.
Knockdown of G6PD in bladder cancer cell lines
resulted in an increase of intracellular ROS levels. Inhibition of
G6PD restricts the function of PPP, resulting in lower NADPH
production, an unstable GSH/oxidized GSH ratio and subsequent
intracellular ROS accumulation, ultimately resulting in the
disruption of the intracellular redox equilibrium (21-23).
Consistent with the results of these previous studies, the toxic
ROS levels induced apoptosis in bladder cancer cell lines, which
may explain why the inhibition of G6PD suppressed the cell
proliferation and colony formation ability of bladder cancer cell
lines in vitro.
The AKT signaling pathway is known to serve a key
role in the proliferation and apoptosis of tumor cells (24). An increasing number of studies
demonstrated that activated AKT suppresses apoptosis by the
phosphorylation of certain sites in Bcl2 associated agonist of cell
death, caspase-9, protease-activated receptor 4 or other
pro-apoptotic proteins (36,37).
AKT signaling was also reported to promote the progression of
bladder cancer (25) and activated
AKT signaling has been demonstrated to be positively correlated
with tumor progression and poor clinical prognosis in patients with
bladder cancer (38). The present
results demonstrated that the knockdown of G6PD suppressed AKT
signaling and increased the apoptosis of bladder cancer cells. This
may explain the weakening of survival ability in G6PD-knockdown
cancer cells. However, the detailed association between G6PD and
the AKT pathway and the mechanism underlying the mode of action of
AKT in bladder cancer requires further study.
6-AN, a competitive G6PD inhibitor (39), mostly used for radiosensitization
combined with 2-deoxy-D-glucose (40-42),
was also demonstrated to exert antineoplastic effects on cancer
cells, alone or combined with other agents (43,44).
Similar to other studies, the present results demonstrated that
G6PD inhibition with a fixed concentration of 6-AN significantly
reduced the proliferation of bladder cancer cells, particularly
those highly expressing G6PD, whereas it did not induce a
substantial decrease of cell survival in normal cells (P<0.05;
Fig. 5B) (45). Furthermore, the results
demonstrated that 6-AN functioned synergistically with cisplatin in
bladder cancer treatment. Cisplatin-based chemotherapy remains the
first-line chemotherapeutic treatment in patients with MIBC pre-
and postoperatively (46).
However, despite great success in tumor suppression, the
application of cisplatin is restricted by drug resistance and side
effects, the underlying mechanisms of which remain unclear
(47). However, the hypothesis
that the dysregulation of cell metabolism sustains drug resistance
has gained the support of an increasing number of researchers
(9,10,48).
Several agents are known to sensitize cancer cells to cisplatin,
one of which is 6-AN (49). This
effect is primarily due to the action of 6-amino-NAD(P+), which
results in intracellular cisplatin enrichment and the accumulation
of plasma tumor DNA adducts (50).
Pretreatment with 6-AN has been demonstrated to sensitize different
tumor cells to cisplatin cytotoxicity, even cisplatin-resistant
cells; this may explain the synergistic effects of 6-AN and
cisplatin on bladder cancer cells (49,50).
However, it has also been reported that 6-AN may cause
neurotoxicity or hematological toxicity under certain conditions
(43-46). Thus, further in vivo studies
are required to optimize the therapeutic window and dose of 6-AN in
cancer treatment in clinical practice.
Altogether, the results of the present study
demonstrate that high G6PD expression is associated with a higher
stage and poorer prognosis in patients with bladder cancer.
Inhibition of G6PD may suppress the growth of bladder cancer cells
via ROS accumulation and AKT pathway suppression. Thus, targeting
G6PD may be a potential therapeutic method for bladder cancer,
particularly in cases with high G6PD expression. Furthermore, 6-AN
used as a supplementary component in cisplatin-based chemotherapy
may optimize the therapeutic doses of cisplatin, thereby minimizing
the side effects (51).
Acknowledgments
The authors would like to thank The Key Laboratory
of Combined Multi-Organ Transplantation, Ministry of Public Health
(Hangzhou, China) for the use of the facilities and assistance from
the technicians.
Funding
The present study was funded by the Key Project of
the Science and Technology Program of Zhejiang Province (grant no.
2014C03028) and Science and Technology Program of Zhejiang Province
(grant no. LY15H050002).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XC and BJ designed the experiments, performed the
experiments, analyzed the data and wrote the paper. ZX, AC and GF
performed the experiments. ZZ, YW and HP provided the reagents and
helped with the experiments and the writing of the paper. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bellmunt J, Orsola A, Leow JJ, Wiegel T,
De Santis M and Horwich A; Group EGW; ESMO Guidelines Working
Group: Bladder cancer: ESMO Practice Guidelines for diagnosis,
treatment and follow-up. Ann Oncol. 25(Suppl 3): iii40–iii48. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Riganti C, Gazzano E, Polimeni M, Aldieri
E and Ghigo D: The pentose phosphate pathway: An antioxidant
defense and a crossroad in tumor cell fate. Free Radic Biol Med.
53:421–436. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tian WN, Braunstein LD, Pang J, Stuhlmeier
KM, Xi QC, Tian X and Stanton RC: Importance of glucose-6-phosphate
dehydrogenase activity for cell growth. J Biol Chem.
273:10609–10617. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hu T, Zhang C, Tang Q, Su Y, Li B, Chen L,
Zhang Z, Cai T and Zhu Y: Variant G6PD levels promote tumor cell
proliferation or apoptosis via the STAT3/5 pathway in the human
melanoma xenograft mouse model. BMC Cancer. 13:2512013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Batetta B, Pulisci D, Bonatesta RR, Sanna
F, Piras S, Mulas MF, Spano O, Putzolu M, Broccia G and Dessì S:
G6PD activity and gene expression in leukemic cells from
G6PD-deficient subjects. Cancer Lett. 140:53–58. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Van Driel BE, Valet GK, Lyon H, Hansen U,
Song JY and Van Noorden CJ: Prognostic estimation of survival of
colorectal cancer patients with the quantitative histochemical
assay of G6PDH activity and the multiparameter classification
program CLASSIF1. Cytometry. 38:176–183. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Polat MF, Taysi S, Gul M, Cikman O, Yilmaz
I, Bakan E and Erdogan F: Oxidant/antioxidant status in blood of
patients with malignant breast tumour and benign breast disease.
Cell Biochem Funct. 20:327–331. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Philipson KA, Elder MG and White JO: The
effects of medroxyprogesterone acetate on enzyme activities in
human endometrial carcinoma. J Steroid Biochem. 23A:1059–1064.
1985. View Article : Google Scholar
|
|
10
|
Zhang C, Zhang Z, Zhu Y and Qin S:
Glucose-6-phosphate dehydrogenase: A biomarker and potential
therapeutic target for cancer. Anticancer Agents Med Chem.
14:280–289. 2014. View Article : Google Scholar
|
|
11
|
Rhodes DR, Yu J, Shanker K, Deshpande N,
Varambally R, Ghosh D, Barrette T, Pandey A and Chinnaiyan AM:
ONCOMINE: A cancer microarray database and integrated data-mining
platform. Neoplasia. 6:1–6. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dyrskjøt L, Kruhøffer M, Thykjaer T,
Marcussen N, Jensen JL, Møller K and Ørntoft TF: Gene expression in
the urinary bladder: A common carcinoma in situ gene expression
signature exists disregarding histopathological classification.
Cancer Res. 64:4040–4048. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee JS, Leem SH, Lee SY, Kim SC, Park ES,
Kim SB, Kim SK, Kim YJ, Kim WJ and Chu IS: Expression signature of
E2F1 and its associated genes predict superficial to invasive
progression of bladder tumors. J Clin Oncol. 28:2660–2667. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fogh J: Cultivation, characterization, and
identification of human tumor cells with emphasis on kidney,
testis, and bladder tumors. Natl Cancer Inst Monogr. 49:5–9.
1978.
|
|
15
|
Bubeník J, Baresová M, Viklický V,
Jakoubková J, Sainerová H and Donner J: Established cell line of
urinary bladder carcinoma (T24) containing tumour-specific antigen.
Int J Cancer. 11:765–773. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Christian BJ, Loretz LJ, Oberley TD and
Reznikoff CA: Characterization of human uroepithelial cells
immortalized in vitro by simian virus 40. Cancer Res. 47:6066–6073.
1987.PubMed/NCBI
|
|
17
|
Graham FL and van der Eb AJ: A new
technique for the assay of infectivity of human adenovirus 5 DNA.
Virology. 52:456–467. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)). Method. 25:402–408. 2001. View Article : Google Scholar
|
|
19
|
Loewe S: The problem of synergism and
antagonism of combined drugs. Arzneimittelforschung. 3:285–290.
1953.PubMed/NCBI
|
|
20
|
Tallarida RJ: An overview of drug
combination analysis with isobolograms. J Pharmacol Exp Ther.
319:1–7. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ichijo H, Nishida E, Irie K, ten Dijke P,
Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K and Gotoh
Y: Induction of apoptosis by ASK1, a mammalian MAPKKK that
activates SAPK/JNK and p38 signaling pathways. Science. 275:90–94.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Moon DO, Kim MO, Choi YH, Hyun JW, Chang
WY and Kim GY: Butein induces G(2)/M phase arrest and apoptosis in
human hepatoma cancer cells through ROS generation. Cancer Lett.
288:204–213. 2010. View Article : Google Scholar
|
|
23
|
Moloney JN and Cotter TG: ROS signalling
in the biology of cancer. Semin Cell Dev Biol. 80:50–64. 2018.
View Article : Google Scholar
|
|
24
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nogueira V, Park Y, Chen CC, Xu PZ, Chen
ML, Tonic I, Unterman T and Hay N: Akt determines replicative
senescence and oxidative or oncogenic premature senescence and
sensitizes cells to oxidative apoptosis. Cancer Cell. 14:458–470.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Calderaro J, Rebouissou S, de Koning L,
Masmoudi A, Hérault A, Dubois T, Maille P, Soyeux P, Sibony M, de
la Taille A, et al: PI3K/AKT pathway activation in bladder
carcinogenesis. Int J Cancer. 134:1776–1784. 2014. View Article : Google Scholar
|
|
27
|
Street JC, Alfieri AA and Koutcher JA:
Quantitation of metabolic and radiobiological effects of
6-aminonicotinamide in RIF-1 tumor cells in vitro. Cancer Res.
57:3956–3962. 1997.PubMed/NCBI
|
|
28
|
Ward PS and Thompson CB: Metabolic
reprogramming: A cancer hallmark even warburg did not anticipate.
Cancer Cell. 21:297–308. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wittig R and Coy JF: The role of glucose
metabolism and glucose-associated signalling in cancer. Perspect
Medicin Chem. 1:64–82. 2008.PubMed/NCBI
|
|
30
|
Giorgio M, Trinei M, Migliaccio E and
Pelicci PG: Hydrogen peroxide: A metabolic by-product or a common
mediator of ageing signals? Nat Rev Mol Cell Biol. 8:722–728. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zorov DB, Juhaszova M and Sollott SJ:
Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS
release. Physiol Rev. 94:909–950. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: The metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kuo W, Lin J and Tang TK: Human
glucose-6-phosphate dehydrogenase (G6PD) gene transforms NIH 3T3
cells and induces tumors in nude mice. Int J Cancer. 85:857–864.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jiang P, Du W and Yang X: A critical role
of glucose-6-phosphate dehydrogenase in TAp73-mediated cell
proliferation. Cell Cycle. 12:3720–3726. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rao X, Duan X, Mao W, Li X, Li Z, Li Q,
Zheng Z, Xu H, Chen M, Wang PG, et al: O-GlcNAcylation of G6PD
promotes the pentose phosphate pathway and tumor growth. Nat
Commun. 6:84682015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Song G, Ouyang G and Bao S: The activation
of Akt/PKB signaling pathway and cell survival. J Cell Mol Med.
9:59–71. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Goswami A, Burikhanov R, de Thonel A,
Fujita N, Goswami M, Zhao Y, Eriksson JE, Tsuruo T and Rangnekar
VM: Binding and phosphorylation of par-4 by akt is essential for
cancer cell survival. Mol Cell. 20:33–44. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sun CH, Chang YH and Pan CC: Activation of
the PI3K/Akt/mTOR pathway correlates with tumour progression and
reduced survival in patients with urothelial carcinoma of the
urinary bladder. Histopathology. 58:1054–1063. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hothersall JS, Gordge M and Noronha-Dutra
AA: Inhibition of NADPH supply by 6-aminonicotinamide: Effect on
glutathione, nitric oxide and superoxide in J774 cells. FEBS Lett.
434:97–100. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sharma PK and Varshney R:
2-Deoxy-D-glucose and 6-aminonicotinamide-mediated Nrf2 down
regulation leads to radiosensitization of malignant cells via
abrogation of GSH-mediated defense. Free Radic Res. 46:1446–1457.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sharma PK, Bhardwaj R, Dwarakanath BS and
Varshney R: Metabolic oxidative stress induced by a combination of
2-DG and 6-AN enhances radiation damage selectively in malignant
cells via non-coordinated expression of antioxidant enzymes. Cancer
Lett. 295:154–166. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Varshney R, Gupta S and Dwarakanath BS:
Radiosensitization of murine Ehrlich ascites tumor by a combination
of 2-deoxy-D-glucose and 6-aminonicotinamide. Technol Cancer Res
Treat. 3:659–663. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Stolfi RL, Colofiore JR, Nord LD, Koutcher
JA and Martin DS: Biochemical modulation of tumor cell energy:
Regression of advanced spontaneous murine breast tumors with a
5-fluoro-uracil-containing drug combination. Cancer Res.
52:4074–4081. 1992.PubMed/NCBI
|
|
44
|
Koutcher JA, Alfieri AA, Stolfi RL, Devitt
ML, Colofiore JR, Nord LD and Martin DS: Potentiation of a three
drug chemotherapy regimen by radiation. Cancer Res. 53:3518–3523.
1993.PubMed/NCBI
|
|
45
|
Poulain L, Sujobert P, Zylbersztejn F,
Barreau S, Stuani L, Lambert M, Palama TL, Chesnais V, Birsen R,
Vergez F, et al: High mTORC1 activity drives glycolysis addiction
and sensitivity to G6PD inhibition in acute myeloid leukemia cells.
Leukemia. 31:2326–2335. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Alfred Witjes J, Lebret T, Compérat EM,
Cowan NC, De Santis M, Bruins HM, Hernández V, Espinós EL, Dunn J,
Rouanne M, et al: Updated 2016 EAU Guidelines on muscle-invasive
and metastatic Bladder Cancer. Eur Urol. 71:462–475. 2017.
View Article : Google Scholar
|
|
47
|
Köberle B, Tomicic MT, Usanova S and Kaina
B: Cisplatin resistance: Preclinical findings and clinical
implications. Biochim Biophys Acta. 1806:172–182. 2010.PubMed/NCBI
|
|
48
|
Liu H, Liu Y and Zhang JT: A new mechanism
of drug resistance in breast cancer cells: Fatty acid synthase
overexpression-mediated palmitate overproduction. Mol Cancer Ther.
7:263–270. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Budihardjo II, Walker DL, Svingen PA,
Buckwalter CA, Desnoyers S, Eckdahl S, Shah GM, Poirier GG, Reid
JM, Ames MM, et al: 6-Aminonicotinamide sensitizes human tumor cell
lines to cisplatin. Clin Cancer Res. 4:117–130. 1998.PubMed/NCBI
|
|
50
|
Catanzaro D, Gaude E, Orso G, Giordano C,
Guzzo G, Rasola A, Ragazzi E, Caparrotta L, Frezza C and Montopoli
M: Inhibition of glucose-6-phosphate dehydrogenase sensitizes
cisplatin-resistant cells to death. Oncotarget. 6:30102–30114.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhelev Z, Ivanova D, Bakalova R, Aoki I
and Higashi T: Inhibition of the pentose-phosphate pathway
selectively sensitizes leukemia lymphocytes to chemotherapeutics by
ROS-independent mechanism. Anticancer Res. 36:6011–6020. 2016.
View Article : Google Scholar : PubMed/NCBI
|