Introduction
Colon cancer, an aggressive malignancy causes
substantial morbidity and mortality (1,2). The
current treatment for patients with colon cancer relies on
chemotherapy, despite its severe side-effects. Antineoplastic drugs
from natural resources (e.g., camptothecin and vincristine) are
extensively utilized in the treatment of colon cancer (3,4).
Oridonin (ORI), a vital diterpenoid from the Chinese herbal
medicine, Rabdosia rubescens (5) can effectively be utilized in the
treatment of breast cancer, colon cancer, osteosarcoma and prostate
cancer (5–8). However, the mechanisms underlying the
effects of ORI remain unclear.
Bone morphogenetic proteins (BMPs), a subgroup of
the transforming growth factor (TGF)-β superfamily are associated
with multiple physiological functions, including the regulation of
cell differentiation, proliferation and apoptosis (9–11).
BMP2, BMP4 and BMP7 can inhibit the proliferation of colon cancer
cells (9,12). BMP2 and BMP4 can even trigger the
differentiation of colon cancer stem cells (13). BMP7 can mediate the anticancer
activity of ORI by activating p38 mitogen-activated protein kinase
(MAPK) and thereby enhancing the function of phosphatase and tensin
homolog (PTEN) (7,14). However, the detailed underlying
mechanisms remain unclear.
p53, a well-known tumor suppressor and a critical
mediator of the cellular stress response, is considered as a valid
therapeutic target (15–18). The functional loss or mutation of
p53 is regarded as the primary cause of cancer. MAPK is another
crucial cell-growth regulator in the pathogenesis of cancer
(19). Aberrant p38 MAPK signals
have been noted in solid tumors, such as breast cancer and colon
cancer (20). p38 MAPK regulates
the p53 signal (21). Thus, it is
possible that BMP7 also regulates the activity of p53 in colon
cancer cells.
In the present study, in vitro, and in
vivo assays were performed to investigate the potential of p53
to mediate the anti-proliferative activity of ORI against colon
cancer cells and to elucidate the possible mechanistic association
between BMP7 and p53.
Materials and methods
Reagents and cell culture
ORI (procured from Hao-Xuan Bio-Tech Co., Ltd.,
Xi'an, China) was dissolved in DMSO at the concentration of 10
mmol/l and stored at −20°C. For the in vivo experiments, an
ORI suspension was prepared with a 0.4% carboxymethylcellulose
sodium (CMC-Na) solution. All primary antibodies used in this study
were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). The inhibitors of p53 [pifithrin-β hydrobromide (PFT) cat.
no. S2929] and p38 (SB203580; cat. no. S1076) were purchased from
Shanghai Selleck Chemicals Co., Ltd. (Shanghai, China). All the
cell lines used (HCT116, SW620, SW480 LoVo and FHC) were obtained
from the American Type Culture Collection (ATCC, Manassas, VA,
USA). The cells were cultured in Dulbecco's modified Eagle's medium
with 10% fetal bovine serum, 100 U/ml of penicillin and 100
μg/ml of streptomycin solution at 37°C under a 5%
CO2 environment.
Construction of BMP7 and p53 recombinant
adenoviruses
The recombinant adenoviruses for BMP7 and p53 were
constructed using the AdEasy system (22), tagged with green fluorescence
protein (GFP) and designated as AdBMP7 or Adp53. The GFP-expressing
recombinant adenovirus was used only as a vector control (AdGFP).
Samples were collected at 24 or 48 h following transfection.
Cell viability assay
Cell viability and proliferation were determined
using a cell counting kit-8 (CCK-8). Briefly, the SW620 cells were
seeded onto 96-well plates at 3×103 cells/well and
treated with various concentrations of ORI solutions (0, 5, 10, 15,
20 and 25 μM), corresponding reagents or DMSO. After 24, 48
or 72 h, 10 μl of CCK-8 were added to each well followed by
incubation at 37°C for 4 h. The optical absorbance was determined
at 450 nm using a microplate reader (ELx800; BioTek Instruments,
Inc., Winooski, VT, USA). Each assay was conducted in
triplicate.
Flow cytometric analysis of the cell
cycle and apoptosis
The SW620 cells were seeded into 6-well plates and
treated for 48 h as per protocol (0, 10, 15 and 20 μM). For
analyzing cellular apoptosis, the cells were collected and washed
with PBS (4°C) and incubated with Annexin V EGFP and propidium
iodide (PI; Keygenbio, Nanjing, China). The cells were then sorted
following fluorescence activation. For cell cycle analysis, the
cells were collected and washed with PBS (4°C), fixed with chilled
(4°C) 70% ethanol, washed with 50 and 30% ethanol, and PBS. The
cells were then stained with 1 ml of PI (20 mg/ml) containing RNase
(1 mg/ml) for 30 min and analyzed by flow cytometry (BD Influx; BD
Biosciences, Franklin Lakes, NJ, USA). Each assay was carried out
in triplicate.
RNA extraction, reverse
transcription-quantitative PCR (RT-qPCR) assay
Sub-confluent SW620 cells were plated in T25 flasks
and treated with various concentrations of ORI (0, 5, 10, 15, 20
and 25 μM) or corresponding reagents. Total RNA was
extracted from the cells using TRIzol reagent (Invitrogen/Thermo
Fisher Scientific, Waltham, MA, USA) and subjected to reverse
transcription to generate cDNA using a PrimeScript RT Reagent kit
(Takara, Kosatsu, Japan). The cDNA was then used as a template for
the qPCR assay with 2X SYBR-Green qPCR Master Mix (Bimake, Houston,
TX, USA). The PCR thermocycling conditions were as follows: 95°C
for 1 min for pre-denaturation, then 30 cycles of 92°C for 2 min
for denaturation, 55°C for 45 sec for annealing and 72°C for 45 sec
for elongation, finally 72°C for 5 min for re-elongation. The
primer sequences used in this study were as follows: (5′–3′): GAPDH
forward, CAACGAATTTGGCTACAGCA and reverse, AGGGGAGATTCAGTGTGGTG;
p53 forward, GTCGGTGGGTTGGTAGTTTCTA and reverse,
AAAAAGAAATTGACCCTGAGCA; BMP7 forward, GGCAGGACTGGATCATCG and
reverse, AAGTGGACCAGCGTCTGC. RT-qPCR results were analyzed by
2−ΔΔCq method described by Livak and Schmittgen
(23). Each assay was carried out
in triplicate.
Western blot analysis
Sub-confluent SW620 cells were seeded in 6-well
plates and then treated with various concentrations of ORI (10, 15
and 20 μM) or corresponding regents. At the scheduled time
point (24 or 48 h), cells were lysed with 300 μl lysis
buffer (10% SD; Glycerinum; Tris-Hcl, 1 M, pH 6.8; protease
inhibitor cocktail, EDTA-Free, 100X in DMSO; phosphatase inhibitor
cocktail, 2 tubes, 100X; H2O) in each well, and the
lysate was boiled for 10 min. The protein concentration was
determined using the BCA Protein Assay kit, and a total of 40
μg protein was loaded per lane and subjected to
electrophoresis by 10% SDS-PAGE separation and transferred onto
polyvinylidene fluoride membranes. The membranes were then blotted
with corresponding primary antibodies against GAPDH (sc-32233-R,
1:3,000), Bad (sc-8044-M, 1:3,000), Bcl-2 (sc-7382-M, 1:3,000), p53
(sc-55476-M, 1:3,000), phospho-p53 (sc-17105-G, 1:3,000), BMP7
(sc-9305-G, 1:3,000), Smad1/5/8 (sc-6031-R, 1:3,000),
phospho-Smad1/5/8 (sc-12353-G, 1:3,000), p38 (sc-535-R, 1:3,000)
and phospho-p38 (sc-7973-M, 1:3,000) for 12 h at 4°C. The membranes
were then immunoblotted with HRP-conjugated secondary antibodies
(goat anti-rabbit lgG, SA00001-2, 1:3,000; goat anti-mouse lgG,
SA00001-1, 1:3,000; rabbit anti-goat IgG, SA00004-4, 1:3,000)
successively, all antibodies used in this study were purchased from
Santa Cruz Biotechnology, Inc.. The target proteins were developed
with SuperSignal West Femto Substrate (#34095; Thermo Scientifc,
Rockford, IL, USA). Each assay was done in triplicate.
Cell immunofluorescence staining
assay
The cells were seeded in 48-well plates and treated
as per protocol (0, 10 and 20 μM). At the scheduled time
point (48 h), the cells were fixed with methanol (4°C) for 15 min,
washed with PBS (4°C), permeabilized with 0.5% Triton X-100, and
then blocked with 5% BSA at room temperature for 1 h and incubated
with the primary antibody for the protein-target (p53, sc-55476-M,
1:100; BMP7, sc-9305-G; 1:100; Santa Cruz Biotechnology, Inc.). The
corresponding IgGs (negative control) were incubated with
FITC-conjugated with corresponding secondary antibodies (goat
anti-mouse lgG, SA00001-1, 1:100; rabbit anti-goat IgG, SA00004-4,
1:100; Santa Cruz Biotechnology, Inc.) for 30 min. Finally, the
cells were stained with DAPI (1 μg/ml, Solarbio, Beijing,
China). Images were recorded under an inverted microscope (Nikon
ECLIPSE Ti-S; Nikon Corporation, Tokyo, Japan). Each assay was
carried out in triplicate.
Xenograft tumor model for human colon
cancer and histological evaluation
All animal experiments followed the guidelines of
the Institutional Animal Care and Use Committee of Chongqing
Medical University (Chongqing, China). Athymic nude mice (female,
weighing 14–15 g, 4–6 weeks old, 5/group, 4 groups in total) were
ordered and kept at the animal center of Chongqing Medical
University (26–28°C, 40–60% relative humidity), and fed by the
staff. The SW620 cells were cultured and treated with ORI and/or
AdBMP7. The cells were then harvested, resuspended in PBS (4°C) and
injected subcutaneously (1×106/injection) into the right
flanks of athymic nude mice. The mice were administered
intragastrically with an ORI suspension (50 or 100 mg/kg) or the
same volume of CMC-Na once daily for 3 weeks. After 3 weeks, all
nude mice were sacrificed to retrieve all the tumor samples. The
weights and diameters of the tumor samples were measured with
digital calipers, and the volume of a tumor was calculated using
the following formula: Tumor volume (cm3) = longer
diameter × shorter diameter2/2. The samples were then
fixed in 10% formalin and embedded in paraffin. Serial sections of
the embedded samples were stained with hematoxylin and eosin
(H&E) or immunohistochemical stains (All stains were made by
School of Basic Medicine of Chongqing Medical University,
Chongqing, China). Each experiment was carried out 3 times.
Statistical analysis
All data are presented as the means ± standard
deviation (SD). The differences between groups were estimated by
one-way analysis of variance followed by a Dunn-Bonferroni test for
multiple comparisons and a value of P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of ORI on the growth of colon
cancer cells
In this study, we tried to elucidate the possible
molecular mechanisms behind the anticancer effects of ORI against
colon cancer. The anti-proliferative activity of ORI against the
SW620 cell line was time- and concentration-dependent and
significantly higher than the other cell lines (Fig. 1A), which would justify the
selection of the SW620 cells for use in the following experiments.
The results of the flow cytometric assay revealed that ORI arrested
the cells at predominately the G2 phase of the cell cycle (Fig. 1B). These results suggest that ORI
inhibits the proliferation of colon cancer cells.
Effects of ORI on the apoptosis of SW620
cells
The pro-apoptotic effects of ORI against SW620 cells
were also determined. The results of flow cytometric analysis
revealed the concentration-dependent pro-apoptotic potential of ORI
against the colon cancer cells (Fig.
2A). ORI also increased the expression levels of Bad, and
decreased those of Bcl-2 (Fig.
2B). These data suggest that ORI may be a potent inducer of the
apoptosis of colon cancer cells.
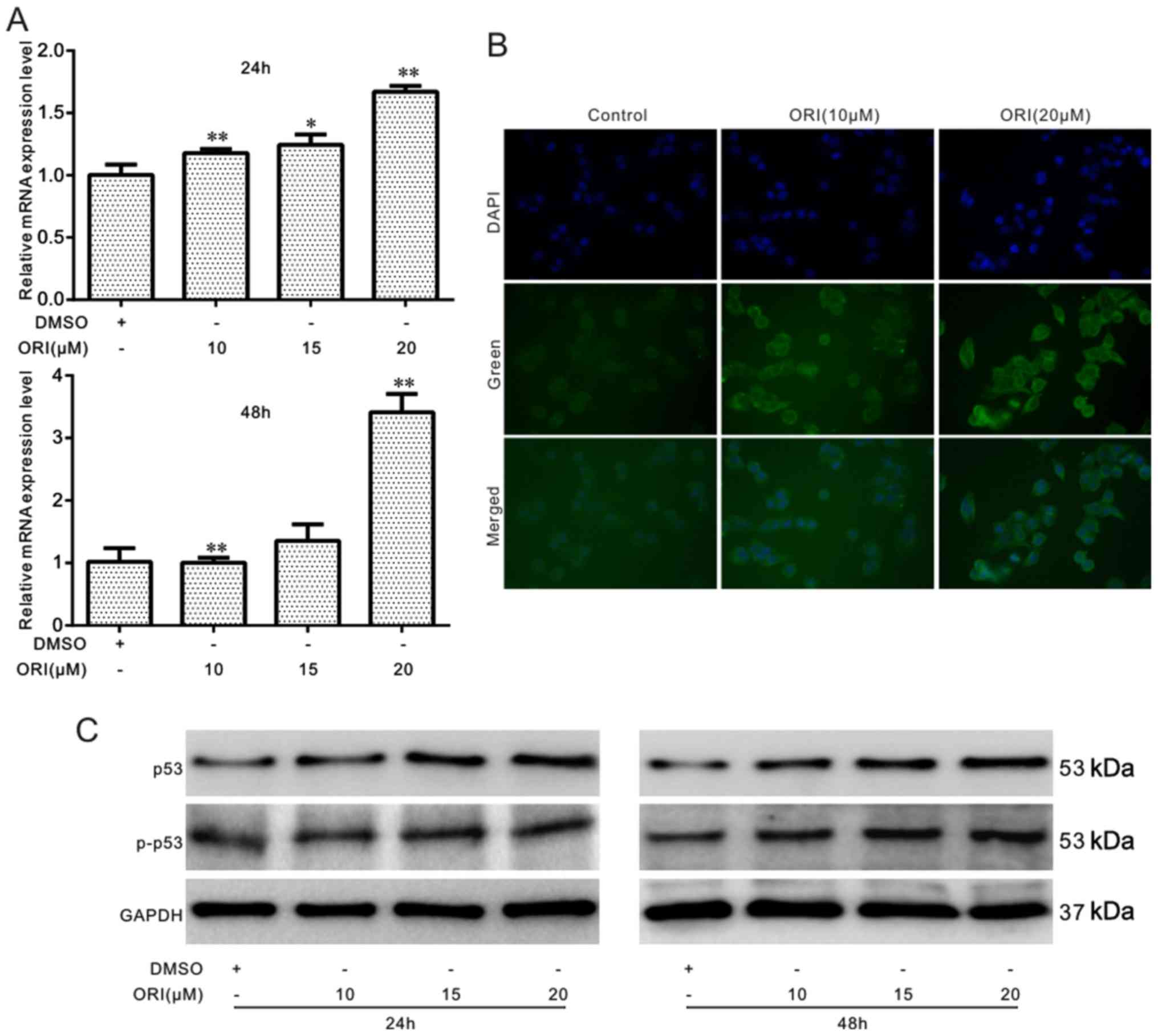
Effects of ORI on the level of p53 in
SW620 cells
The role of p53 in mediating the anticancer effects
of ORI was also investigated in colon cancer cells. The results of
the RT-qPCR assay revealed that ORI significantly upregulated the
expression of p53 (Fig. 3A). The
results of cell immunofluorescence staining and western blot assay
analysis also revealed that ORI increased the level of p53 and
p-p53 in a time- and concentration-dependent manner (Fig. 3B and C). Thus, these results
suggest that ORI exerts anticancer effects on colon cancer cells
through the activation of the p53 signal.
Effects of p53 on the anti-proliferative
effects of ORI on SW620 cells
The effects of p53 on the anti-proliferative
activity of ORI were investigated in the colon cancer cells
transfected with p53 recombinant adenoviruses or treated with a
specific inhibitor (PFT, 3 μg/ml). The results of the CCK-8
assay indicated that PFT markedly attenuated the p53-induced
enhancement of the anti-proliferative effects of ORI against the
SW620 cells (Fig. 4A and B). The
results of flow cytometric analysis revealed that the combination
of ORI and p53 did not markedly affect the ORI-induced cell cycle
arrest; however, the p53 specific inhibitor substantially
attenuated the ORI-induced G2 phase arrest in the SW620 cells
(Fig. 4C). Therefore, these data
indicate that the anti-proliferative activity of ORI may be
partially mediated by the activation of the p53 signal.
Effects of ORI on BMP7 expression in
SW620 cells
We have already established the mediatory role of
p53 in the anti-proliferative effects of ORI against the SW620
cells. However, the molecular mechanisms behind these effects
(ORI-induced activation of the p53 signal) remain unknown.
According to our previous study, BMP7 mediated the anticancer
activity of ORI against HCT116 cells (14). In this study, the potential role of
ORI in upregulating BMP7 expression and the associations between
BMP7 and p53 wre investigated in colon cancer cells. The results of
RT-qPCR revealed that ORI upregulated the mRNA level of BMP7 in a
concentration-dependent manner in the SW620 cells (Fig. 5A). The results of western blot
analysis and cell immunofluorescence detection assays revealed that
ORI notably increased the level of BMP7 in the SW620 cells
(Fig. 5B and C). Furthermore, the
results revealed that either the mRNA or protein expression of BMP7
was detectable in the colon cancer cell lines and FHC cells (normal
colon epithelial cell line). Moreover, the endogenous expression of
BMP7 was higher in the colon cancer cell lines than that in the FHC
cells (Fig. 5D and E). All these
results indicate that BMP7 may also be involved in the anticancer
activity of ORI in SW620 cells.
Effects of BMP7 on the anticancer
activity of ORI in SW620 cells
The effects of BMP7 on the anticancer activity of
ORI were analyzed in SW620 cells. The results of the CCK-8 assay
revealed that exogenous BMP7 enhanced the anti-proliferative
effects of ORI; however, the BMP7-specific antibody significantly
decreased the anti-proliferative effects of ORI on the SW620 cells
(Fig. 6A and B). In addition, the
results of our in vivo experiments revealed that the weight
and size of the tumors in the mice who received a combination of
ORI and BMP7 were significantly smaller than those of the mice
treated with ORI alone (Fig.
6C–E). The results of H&E-staining also revealed that BMP7
enhanced ORI-induced karyopyknosis. Thus, these data indicate that
the anticancer effects of ORI may be partly mediated by the
upregulation of BMP7 in SW620 cells.
Effects of ORI on p38 MAPK in SW620
cells
BMP7 exerts its physiological function through the
BMP/Smad pathway (canonical BMP/Smad pathway) or non-canonical
BMP/Smad pathway (24). The
ORI-induced upregulation of BMP7 may thus mediate the anticancer
activity of ORI in colon cancer cells. Therefore, in this study, we
hypothesized that ORI may affect the canonical or non-canonical
BMP/Smad signaling pathway. The results of western blot analysis
did not reveal any substantial effect of ORI on the levels of
Smad1/5/8 or phosphorylated Smad1/5/8 in the SW620 cells; however,
ORI markedly increased the level of phosphorylated p38 in the SW620
cells without any apparent effect on the level of total p38
(Fig. 7A). We also investigated
the effects of p38 on the p53 signal in SW620 cells. We employed a
p38 specific inhibitor (SB203580, 7 μg/ml) and found that
the inhibition of p38 markedly attenuated the promoting effects of
ORI on the levels of p53 and p-p53 (Fig. 7B). These data suggest that ORI
exerts its anticancer effects through p38 to trigger the p53 signal
in SW620 cells.
Effects of BMP7 on the activation of p38
and p53 in SW620 cells
In this study, we found that ORI can upregulate BMP7
and the p53 signal in SW620 cells and activate p38, but cannot
trigger the BMP/Smad signal in SW620 cells. Thus, we hypothesized
that ORI may affect the activation of the p53 signal by activating
p38. The results of western blot analysis indicated that BMP7
overexpression further increased the levels of p38 and p53 induced
by ORI (Fig. 8A). However, the use
of a specific antibody to BMP7 almost eliminated the promoting
effects of ORI on the activation of p38 and p53 (Fig. 8B). These data suggest that BMP7
mediates the anticancer effects of ORI by activating p38 and p53
successively in SW620 cells.
Discussion
Colorectal cancer is a severe malignancy affecting
the gastrointestinal system (25).
ORI has exhibited potent anticancer properties against many forms
of cancer (5,6), although the specific underlying
mechanisms remain unclear. In this study, the anticancer potential
of ORI against colon cancer was investigated, and the findings
demonstrated that BMP7 partially mediated the anticancer activity
of ORI through activating the p53 signal by increasing the
phosphorylation of p38 MAPK in SW620 cells.
The current therapies for colon cancer include
chemotherapy, surgery and targeted therapy (26). Although the treatment regimen has
been improved and optimized immensely over the past decades, the
prognosis of colon cancer remains inefficient, and novel drugs with
improved efficacy and safety profiles, along with novel drug
targets are required for the optimal therapy of the disease. Active
compounds or their derivates from traditional herbal medicine, such
as camptothecin and vincristine (3,4) have
provided new options for cancer treatment. ORI, a diterpenoid from
the traditional herbal medicine, Rasdosia rubescens,
possesses anti-microbial, anti-inflammatory and antioxidant
properties (27). Clinical studies
have demonstrated that ORI is effective in treating esophageal,
liver and breast cancer (5,6). ORI
has been proven to be an effective remedy for suppurative
tonsillitis, acute and chronic pharyngitis, and chronic bronchitis
(28). ORI also possesses
long-term or moderate-acting central nervous system depressant,
anti-hypertensive, and bidirectional regulating properties
(28). ORI can be used in
combination with other chemotherapeutic drugs, such as PN
(pingyangmycin + detoxification) or cisplatin for enhancing the
overall anticancer efficacy. ORI can inhibit the proliferation and
can induce the apoptosis of different cancer cells, such as
gastric, breast, pancreatic, prostate and colon cancer cells
(5,6,29,30).
Our previous studies also demonstrated the anticancer activity of
ORI against colon cancer (7,14).
The mechanisms underlying the effects of ORI may include the
inhibition of the Wnt/β-catenin and PI3K/Akt signal, the
downregulation of Bcl-2 and epidermal growth factor receptor, and
the upregulation of the p53 signal (6,31–33).
However, the detailed mechanism behind the anticancer effects of
ORI remain unclear.
The aberrant transduction of various signaling
pathways and mutations of certain essential factors, such as TGF-β,
Wnt/β-catenin, PI3K/Akt, p53 and PTEN are associated with colon
cancer (34). Different factors,
such as DNA damage, oxidative stress and p38 MAPK can regulate p53
(21). The results of this study
indicated that ORI substantially upregulated the p53 signal in
colon cancer, and the inhibition of p53 may decrease the
anti-proliferative effects of ORI. Therefore, the present study
suggests that p53 partially mediates the anticancer activity of
ORI; however, the exact mechanisms behind these phenomena remain
unclear.
BMPs belong to TGF-β superfamily, and play a crucial
role in bone development, fracture repair, and in the pathogenesis
of certain solid tumors by regulating cell differentiation,
proliferation and apoptosis (9–11).
Currently, ~20 BMPs have been identified in human cells. The
functions of BMPs in the pathogenesis of cancer may depend on the
subtypes of BMPs and cancer, and even the microenvironment of the
malignancy (35). BMP7 is a
pleiotropic cytokine which transforms mesenchymal cells into bone
and cartilage. Owing to its osteogenic activity, BMP7 has been
approved by the FDA for the treatment of bone-related diseases,
such as spinal fusion and bone fracture healing (36,37).
BMP7 has also been implicated in tumors, such as prostate and
breast cancer (38,39). BMP7 can reduce bone metastasis and
invasive ability (40). In this
study, ORI substantially upregulated BMP7 expression in SW620
cells. The endogenous level of BMP7 was much higher in the colon
cancer cells than in the FHC cells. The overexpression of BMP7
enhanced the anti-proliferative activity of ORI, while
BMP7-specific antibody decreased the anti-proliferative effects of
ORI on SW620 cells. Our findings suggest that BMP7 may be a
suitable target of chemotherapeutic drugs in colon cancer. In this
study, we also investigated the influence of BMP7 on p53.
BMP7 carries out its physiological functions through
the canonical BMP/Smad pathway, or the non-canonical BMP/Smad
pathway, such as MAPK and PI3K/Akt signaling. In SW620 cells,
functional losses and the mutation of Smad4 are the primary causes
of malignancy (41,42). For this reason, non-canonical
BMP/Smad signaling is responsible for the anti-proliferative
effects of the drug. In this study, ORI exerted no obvious effects
on either the total level of Smad1/5/8 or the level of p-Smad1/5/8.
ORI increased the level of p-p38 in SW620 cells, though the level
of total p38 remained unaltered. These results indicate that BMP7
may mediate the anti-proliferative effects of ORI through the p38
MAPK signaling pathway.
The downregulation of p53 (a vital tumor suppressor)
is a primary causative factor for colon cancer. The mutation or
functional loss of p53 have been identified in >50% of human
tumor cells (43,44). Stress can activate the p35 signal,
such as p38 MAPK (21). BMP7 may
activate p53 through activating p38. The data of this study
suggested that the inhibition of p38 decreased the ORI-induced
activation of p53 in SW620 cells. Therefore, BMP7 may mediate the
anti-proliferative activity of ORI in colon cancer cells by
activating the p53 signal. Further experiments indicated that the
overexpression of BMP7 potentiated the ORI-induced activation of
p38 and p53 in SW620 cells. On the contrary, the immunosuppression
of BMP7 decreased the ORI-induced activation of p38 and p53. Hence,
the upregulation of BMP7 may trigger the ORI-induced activation of
p53 in colon cancer cells.
In conclusion, the results of this study suggest
that ORI possesses significant anti-proliferative activity against
colon cancer cell lines. The p53 signal may partially mediate the
anti-proliferative effects of ORI through the BMP7/p38 pathway.
Funding
This study was partially supported by a research
grant from the National Natural Science Foundation of China (Grants
nos. NSFC 81372120 and 81572226 to Bai-Cheng He) and the grants
from the Science and Technology Commission of Yuzhong, Chongqing,
China (no. 20150120).
Availability of data and materials
The datasets used or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BCH and WJS designed the experiments; RXL, YM and
XLH performed the experiments and prepared the figures; WYR
analyzed the data; YPL, HW, JHZ and KW helped to perform
experiments; BCH wrote the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
For experiments involving animals, all experimental
procedures were approved by the Institutional Animal Care and Use
Committee of Chongqing Medical University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Professor T.C. He
from the University of Chicago Medical Center (Chicago, IL, USA)
for providing the recombinant adenoviruses.
References
|
1
|
Applegate CC and Lane MA: Role of
retinoids in the prevention and treatment of colorectal cancer.
World J Gastrointest Oncol. 7:184–203. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fellner C: Promising drugs in clinical
development to treat advanced colorectal cancer. PT. 42:262–265.
2017.
|
|
3
|
Banjerdpongchai R, Chanwikruy Y,
Rattanapanone V and Sripanidkulchai B: Induction of apoptosis in
the human Leukemic U937 cell line by Kaempferia parviflora
Wall.ex.Baker extract and effects of paclitaxel and camptothecin.
Asian Pac J Cancer Prev. 10:1137–1140. 2009.PubMed/NCBI
|
|
4
|
Weaver BA: How Taxol/paclitaxel kills
cancer cells. Mol Biol Cell. 25:2677–2681. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Owona BA and Schluesener HJ: Molecular
insight in the multifunctional effects of oridonin. Drugs RD.
15:233–244. 2015. View Article : Google Scholar
|
|
6
|
Liu Y, Liu YZ, Zhang RX, Wang X, Meng ZJ,
Huang J, Wu K, Luo JY, Zuo GW, Chen L, et al: Oridonin inhibits the
proliferation of human osteosarcoma cells by suppressing
Wnt/β-catenin signaling. Int J Oncol. 45:795–803. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu QX, Yuan SX, Ren CM, Yu Y, Sun WJ, He
BC and Wu K: Oridonin upregulates PTEN through activating p38 MAPK
and inhibits proliferation in human colon cancer cells. Oncol Rep.
35:3341–3348. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lu J, Chen X, Qu S, Yao B, Xu Y, Wu J, Jin
Y and Ma C: Oridonin induces G2/M cell cycle arrest and apoptosis
via the PI3K/Akt signaling pathway in hormone-independent prostate
cancer cells. Oncol Lett. 13:2838–2846. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bollum LK, Huse K, Oksvold MP, Bai B,
Hilden VI, Forfang L, Yoon SO, Wälchli S, Smeland EB and Myklebust
JH: BMP-7 induces apoptosis in human germinal center B cells and is
influenced by TGF-β receptor type I ALK5. PLoS One.
12:e01771882017. View Article : Google Scholar
|
|
10
|
Yoo HS, Kim GJ, Song DH, Chung KH, Lee KJ,
Kim DH and An JH: Calcium supplement derived from Gallus gallus
domesticus promotes BMP-2/RUNX2/SMAD5 and suppresses TRAP/RANK
expression through MAPK signaling activation. Nutrients.
9:E5042017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bernatik O, Radaszkiewicz T, Behal M, Dave
Z, Witte F, Mahl A, Cernohorsky NH, Krejci P, Stricker S and Bryja
V: A novel role for the BMP antagonist noggin in sensitizing cells
to non-canonical Wnt-5a/Ror2/disheveled pathway activation. Front
Cell Dev Biol. 5:472017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Y, Chen X, Qiao M, Zhang BQ, Wang N,
Zhang Z, Liao Z, Zeng L, Deng Y, Deng F, et al: Bone morphogenetic
protein 2 inhibits the proliferation and growth of human colorectal
cancer cells. Oncol Rep. 32:1013–1020. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Basu S, Haase G and Ben-Ze'ev A: Wnt
signaling in cancer stem cells and colon cancer metastasis. F1000
Res. 5:699–709. 2016. View Article : Google Scholar
|
|
14
|
Ren CM, Li Y, Chen QZ, Zeng YH, Shao Y, Wu
QX, Yuan SX, Yang JQ, Yu Y, Wu K, et al: Oridonin inhibits the
proliferation of human colon cancer cells by upregulating BMP7 to
activate p38 MAPK. Oncol Rep. 35:2691–2698. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Briest F and Grabowski P: The p53 network
as therapeutic target in gastroenteropancreatic neuroendocrine
neoplasms. Cancer Treat Rev. 41:423–430. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu L, Harutyunyan K, Jin W, Wu J, Yang T,
Chen Y, Joeng KS, Bae Y, Tao J, et al: RECQL4 regulates p53
function in vivo during skeletogenesis. J Bone Miner Res.
30:1077–1089. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rajeshkumar NV, Dutta P, Yabuuchi S, de
Wilde RF, Martinez GV, Le A, Kamphorst JJ, Rabinowitz JD, Jain SK,
Hidalgo M, et al: Therapeutic targeting of the warburg effect in
pancreatic cancer relies on an absence of p53 function. Cancer Res.
75:3355–3364. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Inobe T, Nozaki M and Nukina N: Artificial
regulation of p53 function by modulating its assembly. Biochem
Biophys Res Commun. 467:322–327. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gan F, Zhou Y, Hou L, Qian G, Chen X and
Huang K: Ochratoxin A induces nephrotoxicity and immunotoxicity
through different MAPK signaling pathways in PK15 cells and porcine
primary splenocytes. Chemosphere. 182:630–637. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Koul HK, Pal M and Koul S: Role of p38 MAP
Kinase Signal Transduction in Solid Tumors. Genes Cancer.
4:342–359. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim GT, Lee SH, Kim JI and Kim YM:
Quercetin regulates the sestrin 2-AMPK-p38 MAPK signaling pathway
and induces apoptosis by increasing the generation of intracellular
ROS in a p53-independent manner. Int J Mol Med. 33:863–869. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Luo J, Deng ZL, Luo X, Tang N, Song WX,
Chen J, Sharff KA, Luu HH, Haydon RC, Kinzler KW, et al: A protocol
for rapid generation of recombinant adenoviruses using the AdEasy
system. Nat Protoc. 2:1236–1247. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
24
|
Stayce EB, Barbara HJ, Antonio F, Jessica
G, Eunice DR, Betty LC, Sherry CH, Jimmy YC and John MC: Bone
morphogenetic protein signaling and growth suppression in colon
cancer. Am J Physiol Gastrointest Liver Physiol. 291:G135–G145.
2006. View Article : Google Scholar
|
|
25
|
Carvalho C and Glynne-Jones R: Challenges
behind proving efficacy of adjuvant chemotherapy after preoperative
chemora-diation for rectal cancer. Lancet Oncol. 18:e354–e363.
2017. View Article : Google Scholar
|
|
26
|
Link KH, Coy P, Roitman M, Link C,
Kornmann M and Staib L: Minimum volume discussion in the treatment
of colon and rectal cancer: A review of the current status and
relevance of surgeon and hospital volume regarding result quality
and the impact on health economics. Visc Med. 33:140–147. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li D, Han T, Liao J, Hu X, Xu S, Tian K,
Gu X, Cheng K, Li Z, Hua H, et al: Oridonin, a promising
ent-kaurane diterpenoid lead compound. Int J Mol Sci. 17:E13952016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu J, Wold EA, Ding Y, Shen Q and Zhou J:
Therapeutic potential of oridonin and its analogs: From anticancer
and antiinflammation to neuroprotection. Molecules. 23:E4742018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu J, Ding Y, Chen CH, Zhou Z, Ding C,
Chen H, Zhou J and Chen C: A new oridonin analog suppresses
triple-negative breast cancer cells and tumor growth via the
induction of death receptor 5. Cancer Lett. 380:393–402. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gui Z, Luo F, Yang Y, Shen C, Li S and Xu
J: Oridonin inhibition and miR 200b 3p/ZEB1 axis in human
pancreatic cancer. Int J Oncol. 50:111–120. 2017. View Article : Google Scholar
|
|
31
|
Zhang LD, Liu Z, Liu H, Ran DM, Guo JH,
Jiang B, Wu YL and Gao FH: Oridonin enhances the anticancer
activity of NVP-BEZ235 against neuroblastoma cells in vitro and in
vivo through autophagy. Int J Oncol. 49:657–665. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pi J, Jin H, Jiang J, Yang F, Cai H, Yang
P, Cai J and Chen ZW: Single molecule force spectroscopy for
in-situ probing oridonin inhibited ROS-mediated EGF-EGFR
interactions in living KYSE-150 cells. Pharmacol Res. 119:479–489.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yao Z, Xie F, Li M, Liang Z, Xu W, Yang J,
Liu C, Li H, Zhou H and Qu LH: Oridonin induces autophagy via
inhibition of glucose metabolism in p53-mutated colorectal cancer
cells. Cell Death Dis. 8:e26332017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Arvelo F, Sojo F and Cotte C: Biology of
colorectal cancer. Ecancermedicalscience. 9:5202015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lochab AK and Extavour CG: Bone
morphogenetic protein (BMP) signaling in animal reproductive system
development and function. Dev Biol. 427:258–269. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tsuji K, Cox K, Gamer L, Graf D,
Economides A and Rosen V: Conditional deletion of BMP7 from the
limb skeleton does not affect bone formation or fracture repair. J
Orthop Res. 28:384–389. 2010.
|
|
37
|
Wang Z, Fu C, Chen Y, Xu F, Wang Z, Qu Z
and Liu Y: FoxC2 enhances BMP7-mediated anabolism in nucleus
pulposus cells of the intervertebral disc. PLoS One.
11:e01477642016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lim M, Chuong CM and Roy-Burman P: PI3K,
Erk signaling in BMP7-induced epithelial-mesenchymal transition
(EMT) of PC-3 prostate cancer cells in 2- and 3-dimensional
cultures. Horm Cancer. 2:298–309. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rodriguez-Martinez A, Alarmo EL, Saarinen
L, Ketolainen J, Nousiainen K, Hautaniemi S and Kallioniemi A:
Analysis of BMP4 and BMP7 signaling in breast cancer cells unveils
time-dependent transcription patterns and highlights a common
synexpression group of genes. BMC Med Genomics. 4:802011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Buijs JT, Henriquez NV, van Overveld PG,
van der Horst G, ten Dijke P and van der Pluijm G: TGF-beta and
BMP7 interactions in tumour progression and bone metastasis. Clin
Exp Metastasis. 24:609–617. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yan P, Klingbiel D, Saridaki Z, Ceppa P,
Curto M, McKee TA, Roth A, Tejpar S, Delorenzi M, Bosman FT, et al:
Reduced expression of SMAD4 is associated with poor survival in
colon cancer. Clin Cancer Res. 22:3037–3047. 2006. View Article : Google Scholar
|
|
42
|
Zhang B, Halder SK, Kashikar ND, Cho YJ,
Datta A, Gorden DL and Datta PK: Antimetastatic role of Smad4
signaling in colorectal cancer. Gastroenterology. 138:969–980.
e961–963. 2010. View Article : Google Scholar
|
|
43
|
Vousden KH and Lane DP: p53 in health and
disease. Nat Rev Mol Cell Biol. 8:275–283. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nayak D, Kumar A, Chakraborty S, Rasool
RU, Amin H, Katoch A, Gopinath V, Mahajan V, Zilla MK, Rah B, et
al: Inhibition of Twist1-mediated invasion by Chk2 promotes
premature senescence in p53-defective cancer cells. Cell Death
Differ. 24:1275–1287. 2017. View Article : Google Scholar : PubMed/NCBI
|