Introduction
Ovarian cancer is the fifth leading cause of
cancer-associated mortality in females, with 152,000 mortalities
globally according to The World Ovarian Cancer Coalition Atlas 2018
(1). Despite significant advances
in surgical treatment and chemotherapy for ovarian cancer, 50-70%
of patients relapse within a year (2). To improve poor outcomes recently,
targeted therapy has become an attractive option for ovarian cancer
treatment (3). Additionally,
sphingosine 1 phosphate (4),
phosphoinositide 3-kinase/protein kinase B/mechanistic target of
rapamycin (5,6) and receptor tyrosine kinase like
orphan receptor 1 (7) signaling
have been reported to be molecular targets for ovarian cancer
therapy.
Particularly, it is well established that signal
transducer and activator of transcription 3 (STAT3) is involved in
cancer progression, differentiation, proliferation, survival,
apoptosis, angiogenesis and the immune system (8,9).
Additionally, accumulating evidence revealed that
selective blockers of T-type Ca2+ channels have
anticancer potential, as T-type Ca2+ channels are
overexpressed in ovarian, lung and pancreatic cancer types
(10,11). In a recent study, eight compounds,
including KYS05090S, exhibited significant cytotoxicity in SKOV3
cells, which is more potent than mibefradil regardless of T-type
channel blocking activity (12).
Novel fluoro-substituted 3,4-dihydroquinazoline derivative
KYS05090S was reported to induce autophagy and apoptosis by
generating reactive oxygen species, and inhibition of T-type
Ca2+ channels and glucose uptake in human lung
adenocarcinoma A549 cells (13);
however, the underlying mechanism of KYS05090S in ovarian cancer is
not fully understood.
Thus, in the present study, the apoptotic mechanism
of KYS05090S in association with Janus kinase 2 (JAK2) and
downstream STAT3 signaling was investigated in ovarian cancer
cells.
Materials and methods
Chemical and reagents
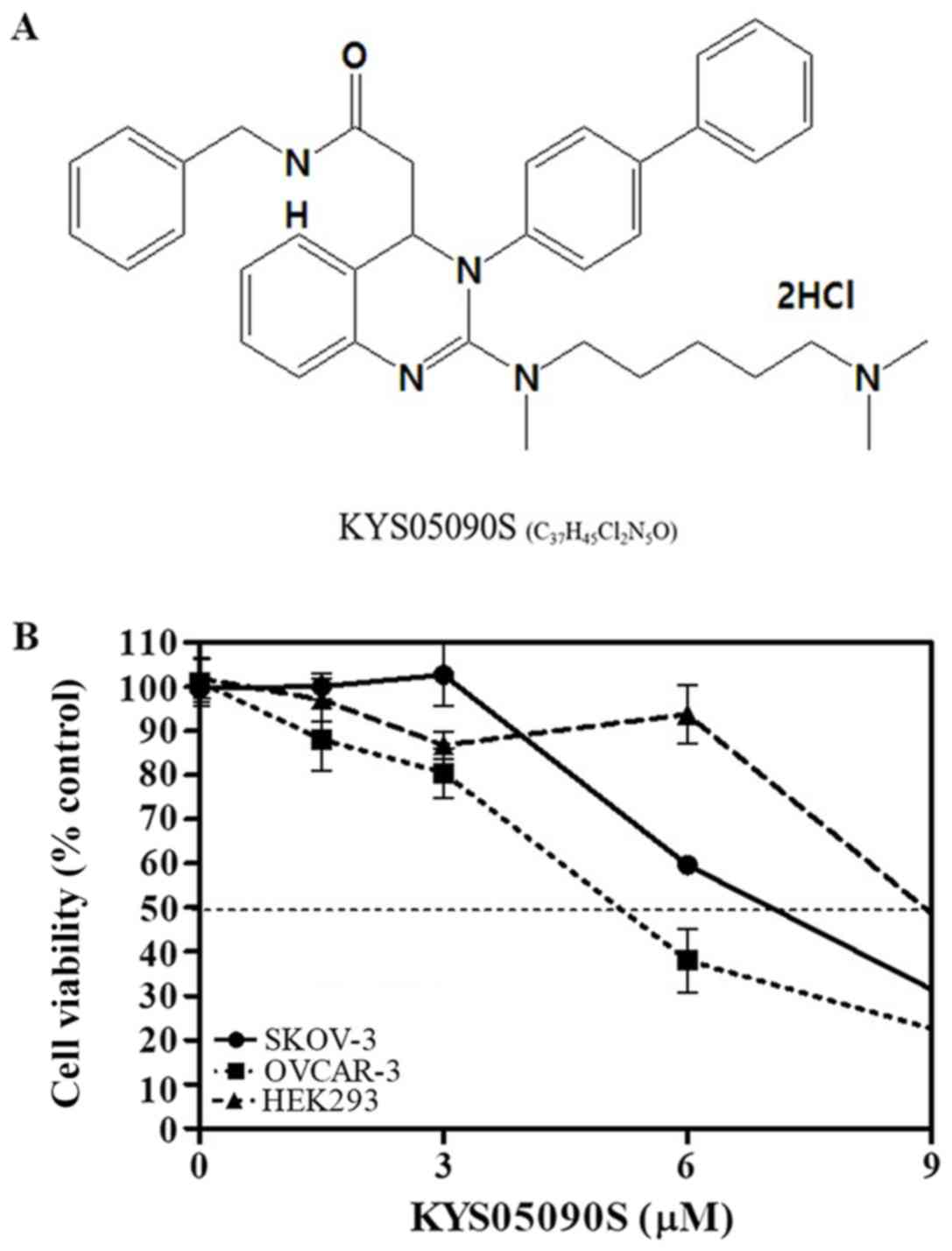
KYS05090S (molecular weight, 626.69 g/mol; molecular
formula, C37H45Cl2N5O) (10,14)
was synthesized and supplied by Professor Jae Yeol Lee (Research
Institute for Basic Sciences and Department of Chemistry, College
of Sciences, Kyung Hee University, Seoul, Korea; Fig. 1A). Interleukin-6 (IL-6) was
purchased from R&D Systems, Inc. (Minneapolis, MN, USA).
Ribonuclease A (RNase; cat. no. R6513) was purchased from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). DC Protein Assay
kit II (cat. no. 500-0113) for the protein quantification during
Western blotting was purchased from Bio-Rad Laboratories, Inc.
(Hercules, CA, USA). DMSO, propidium iodide (PI), bovine serum
albumin (BSA) and formaldehyde solution were obtained from
Sigma-Aldrich (Merck KGaA).
Cell culture
Ovarian cancer cell lines SKOV3 (cat. no.
ATCC® HTB-77™), OVCAR3 (cat. no. ATCC HTB-161™) and
HEK293 cells (cat. no. ATCC CRL-1573™) were obtained from the
American Type Culture Collection (Manassas, VA, USA). These cells
were cultured in Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% fetal bovine serum and 1% antibiotic
(Welgene, Inc., Gyeongsan, South Korea) at 37°C in a humid
environment containing 5% CO2.
Cell viability assay
To evaluate the cytotoxicity of KYS0590S, SKOV3 and
OVCAR3 ovarian cancer, and HEK293 cells were seeded onto 96-well
tissue culture plates (1×104 cells/well) and were
exposed to various concentrations (0, 1.5, 3, 6 and 12 μM)
of KYS0590S at 37°C for 24 h. Subsequently, MTT solution (1 mg/ml)
was added to each well for 1-2 h at 37°C in the dark and dimethyl
sulfoxide was then added to each well at room temperature for 10
min. Optical density (OD) was measured using a microplate reader
(Molecular Devices, LLC, Sunnyvale, CA, USA) at a wavelength of 570
nm. Cell viability was calculated as a percentage of viable cells
in the KYS0590S-treated group, compared with the untreated
control.
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) assay
DNA fragmentation was analyzed using an In Situ Cell
Death Detection kit (Roche Diagnostics, Basel, Switzerland). SKOV3
and OVCAR3 cells (4×104 cells/well) were plated onto 4
well Chamber slides (SPL Life Sciences, Pocheon, South Korea) and
exposed to KYS05090S (5 μM) at 37°C for 24 h. The cells were
fixed in 4% paraformaldehyde at room temperature for 1 h and
treated with TUNEL buffer containing fluorescein isothiocyanate
fluorescein-12-dUTP for 1 h at 37°C in the dark. The slides were
mounted with mounting medium containing DAPI (Vector Laboratories,
Inc., Burlingame, CA, USA) and visualized at ×200 magnification
using FLUOVIEW FV10i confocal microscopy (Olympus Corporation,
Tokyo, Japan) in three fields of view.
Sub-G1 accumulation by cell cycle
analysis
SKOV3 and OVCAR3 cells were treated with KYS05090S
(0, 3 and 5 μM) at 37°C for 24 h and washed with PBS three
times. Ethanol (70%) was used to fix the cells at 4°C overnight.
The cells were treated with RNase (1 mg/ml) at 37°C for 1 h,
stained with PI at 37°C for 1 h and then quantified using a FACS
Caliber flow cytometer and CellQuest software Version 5.2.1 (BD
Biosciences; Becton Dickinson and Company, Franklin Lakes, NJ,
USA).
Apoptosis detection by DAPI staining
SKOV3 and OVCAR3 cells were exposed to KYS05090S (3
and 5 μM) at 37°C for 24 h. The ovarian cells were fixed in
4% (v/v) methanol-free formaldehyde solution at room temperature
for 1 h. The slides were mounted, and nuclei were counterstained
with DAPI (10 μg/ml; Sigma-Aldrich; Merck KGaA) solution for
25 min at room temperature in the dark, and were visualized at ×40
and ×63 magnification by Zeiss LSM700 confocal microscopy.
Immunofluorescence assay
SKOV3 cells (5×104 cells/well) were
plated onto cell culture chamber slides (SPL Life Sciences)
following serum-starvation (DMEM) at 37°C for 3 h. The cells were
treated with KYS05090S (5 μM) at 37°C for 24 h and IL-6 (30
ng/ml) at 37°C for 15 min, and then fixed in 4% (v/v) methanol-free
formaldehyde solution (pH 7.4) at 4°C for 25 min. Following
permeabilization in 0.2% (v/v) Triton X-100, the cells were blocked
at room temperature for 1 h with 5% (v/v) BSA, 0.5% (v/v) Tween-20
in PBS and incubated with STAT3 antibody (cat. no. 12640; Cell
Signaling Technology, Inc., Danvers, MA, USA; 1:1,000) at 4°C
overnight. Anti-mouse IgG Alexa Fluor 488 (cat. no. A28175; Thermo
Fisher Scientific, Inc., Waltham, MA, USA; 1:1,000) was used as the
secondary antibody and incubated for 1 h at room temperature. The
slides were mounted using mounting medium (Vector Laboratories,
Inc.) and nuclei were counterstained with DAPI at room temperature
for 15 min. Subsequently, the slides were visualized under a
FLUOVIEW FV10i confocal microscope (Olympus Corporation).
Western blot analysis
SKOV3 and OVCAR3 cells were lysed in
Radioimmunoprecipitation assay buffer (50 mM Tris-HCl, 150 mM NaCl,
2 mM EDTA and 1% Triton X-100) containing protease inhibitors
(Roche Diagnostics GmbH, Mannheim, Germany), and phosphatase
inhibitors (Sigma-Aldrich; Merck KGaA). Protein samples (100
μg) were centrifuged at 15,000 × g and 4°C for 30 min and
the supernatant was distributed into 96-well plate, exposed to the
solutions of a DC protein assay kit at room temperature for 10 min
and then the absorbance was detected at 665 nm with Bio-Rad model
680 ELISA reader. The protein was separated by SDS-PAGE on an 8%
gel and an equal amount of protein was loaded on SDS-PAGE and
transferred to nitrocellulose membranes. Membranes were blocked by
5% skim-milk (diluted in TBST) solution at room temperature for 1
h. Membranes were incubated with primary antibodies to detect
cleaved-poly(ADP-ribose) polymerase (PARP; cat. no. 9542; 1:1,000),
cleaved-caspase-3 (cat. no. 9664; 1:1,000), cleaved-caspase-9 (cat.
no. 9505; 1:1,000), cyclin D1 (cat. no. 2978; 1:1,000), c-Myc (cat.
no. 5605; 1:1,000), phospho (p)-JAK2 (cat. no. 3776s; 1:1,000),
JAK2 (cat. no. 3230s; 1:1,000), p-STAT3 (cat. no. 9145; 1:1,000)
and STAT3 (cat. no. 12640s; 1:1,000; all Cell Signaling
Technologies, Inc.), B-cell lymphoma-2 (Bcl-2; cat. no. sc-492;
1:500; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and β-actin
(cat. no. A2228; 1:10,000; Sigma-Aldrich; Merck KGaA) diluted in
PBS-Tween-20 with 3% BSA overnight at 4°C, washed three times with
0.2% PBS-Tween-20, and incubated with horseradish
peroxidase-conjugated secondary anti-rabbit (cat. no. 7074;
1:5,000; Cell Signaling Technologies, Inc.) and anti-mouse (cat.
no. sc-516102; 1:10,000; Santa Cruz Biotechnology, Inc.) IgG
antibodies at 4°C overnight. The blot expression was visualized
with enhanced chemiluminescence (GE Healthcare Life Sciences,
Little Chalfont, UK). Additionally, SKOV3 cells were exposed to
IL-6 at 4°C for 15 min to activate the JAK2/STAT3 pathway.
Statistical analysis
Data are presented as the mean ± standard deviation.
All experiments were repeated at least three times. The
statistically significant differences between control and KYS05090S
treated groups were determined using one-way analysis of variance
and Tukey post hoc test. P<0.05 was considered to indicate a
statistically significant difference by using GraphPad Prism 5
(GraphPad Software, Inc., La Jolla, CA, USA).
Results
KYS05090S exerts cytotoxic effects in
SKOV3 and OVCAR3 ovarian cancer cells
The cytotoxicity of KYS05090S (Fig. 1A) was evaluated in SKOV3 and OVCAR3
ovarian cancer cells, and HEK293 cells. KYS05090S notably increased
cytotoxicity in a concentration-dependent manner in SKOV3 and
OVCAR3 cancer cells; whereas, it exhibited comparatively reduced
cytotoxicity in normal HEK293 cells (Fig. 1B).
KYS05090S increases the number of
apoptotic bodies, TUNEL positive cells and sub-G1 population in
SKOV3 and OVCAR3 cells
Subsequently, to determine the apoptotic effect of
KYS05090S, DAPI staining and a TUNEL assay were conducted in SKOV3
and OVCAR3 cells. Nuclei fragmentation, abnormal margins and
condensed chromosomes were observed in KYS05090S-treated SKOV3 and
OVCAR3 cells (Fig. 2A). To further
investigate the KYS05090S-induced apoptosis, cell cycle analysis
was performed, since the sub-G1 phase population exhibits DNA
fragmentation and loss due to apoptosis (15). As expected, KYS05090S
dose-dependently increased the sub-G1 cell population in SKOV3 and
OVCAR3 ovarian cancer cells (Fig.
2B-D). Similarly, a TUNEL assay demonstrated that KYS05090S
increased the number of TUNEL-positive SKOV3 and OVCAR3 cells,
compared with untreated control cells (Fig. 2E).
 | Figure 2KYS05090S increases the number of
apoptotic bodies, TUNEL-positive cells (FITC-fluorescence) and the
sub-G1 population in ovarian cancer cells. (A) Apoptotic bodies in
KYS05090S-treated SKOV3 and OVCAR3 cells by DAPI staining. Arrows
indicate apoptotic bodies. Scale bars, 10 or 15 μm. (B)
Sub-G1 population in KYS05090S-treated SKOV3 and OVCAR3 cells.
Quantification of cell cycle phase in (C) SKOV3 and (D) OVCAR3
cells. (E) TUNEL-positive cells in KYS05090S-treated SKOV3 and
OVCAR3 cells by TUNEL assay. Scale bar, 100 μm. TUNEL,
terminal deoxynucleotidyl transferase dUTP nick end labeling; M1,
sub-G1; M2, G1/G0; M3, S; M4, G2/M phase; FITC, fluorescein
isothiocyanate; Con, control. |
KYS05090S regulates pro- and
anti-apoptotic proteins in SKOV3 and OVCAR3 cells
To investigate the apoptotic mechanism of KYS05090S,
western blot analysis was performed to determine the expression of
pro- and anti-apoptotic proteins in SKOV3 and OVCAR3 cells. In the
present study, KYS05090S treatment increased the cleavage of PARP,
and activated caspase-9 and caspase-3 in the ovarian cancer cells,
compared with untreated control cells (Fig. 3). Consistently, KYS05090S decreased
the expression of anti-apoptotic proteins, including cyclin D1,
Bcl-2 and Bcl-extra large (Bcl-xl) in OVCAR3 and SKOV3 cells
(Fig. 4). Additionally, the level
of p-STAT3 was significantly (P<0.05) attenuated by KYS05090S
treatment in both ovarian cancer cell lines by KYS05090S treatment.
KYS05090S treatment also dose-dependently reduced the level of
p-JAK2 and c-Myc in SKOV3 cells (Fig.
4B).
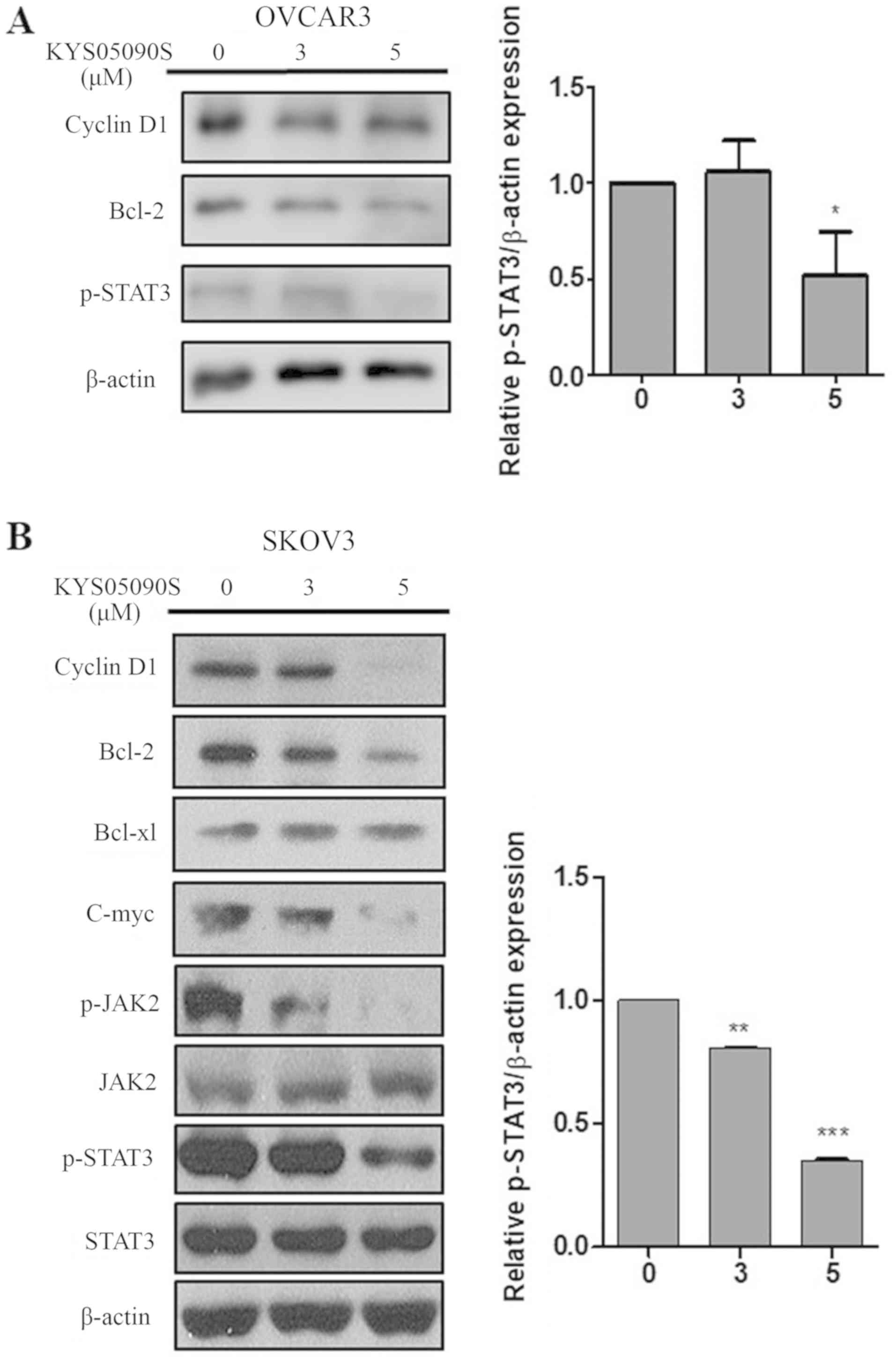 | Figure 4KYS05090S reduces the expression of
anti-apoptotic proteins in ovarian cancer cells. (A) OVCAR3 cells
were exposed to KYS05090S (3 and 5 μM) for 24 h and
subjected to western blot analysis with antibodies against cyclin
D1, Bcl-2 and p-STAT3. (B) SKOV3 cells were exposed to KYS05090S (3
and 5 μM) for 24 h and subjected to western blotting with
antibodies against cyclin D1, Bcl-2, Bcl-xl, c-Myc, p-JAK2, JAK2,
STAT3, p-STAT3 and β-actin. *P<0.05, **P<0.01 and
***P<0.001 vs. untreated control by Student's t-test.
Bcl-2, B-cell lymphoma-2; Bcl-xl, B-cell lymphoma-extra large; p-,
phospho-; JAK2, Janus kinase 2; STAT3, signal transducer and
activator of transcription 3. |
KYS05090S suppresses the phosphorylation
of JAK2 and STAT3, and nuclear translocation of STAT3 in SKOV3 and
OVCAR3 cells
Subsequently, the role of JAK2/STAT3 signaling in
KYS05090S-induced apoptosis in SKOV3 cells was investigated, as
JAK2/STAT3 signaling is involved in cancer cell proliferation and
progression (16). It is well
established that IL-6 activates JAK2/STAT3 signaling (16-18).
In the present study, p-JAK2 and p-STAT3 levels were increased by
30 ng/ml IL-6 treatment and the phosphorylation effect was maximal
at 15 min after IL-6 treatment (Fig.
5A). Therefore, SKOV3 cells were exposed to IL-6 for 15 min to
activate the JAK2/STAT3 pathway in the subsequent experiments.
KYS05090S treatment significantly suppressed the IL-6-induced
phosphorylation of JAK2 and its downstream mediator, STAT3, in
SKOV3 cells (P<0.05; Fig.
5B).
Furthermore, nuclear translocation is essential for
the anti-apoptotic function of STAT3 within the JAK2/STAT3 pathway
(19). Therefore,
immunofluorescence staining for STAT3 and PI staining were
performed to observe nuclear translocation of STAT3 in SKOV3 cells.
IL-6 treatment successfully induced STAT3 nuclear translocalization
in SKOV3 cells (P<0.05; Fig.
6). However, KYS05090S treatment significantly reversed the
increased nuclear translocation of STAT3 induced by IL-6, compared
with untreated control cells.
Discussion
The treatment options for ovarian cancer have been
limited to surgery (for stage I-IVA epithelial ovarian cancer),
chemotherapy (primarily using paclitaxel, docetaxel and carboplatin
or bevacizumab), immunotherapy and hormonal therapy (20,21).
Despite improvements in early detection and development of
potential effective therapeutics for ovarian cancer, the prognosis
of patients following treatment is considered poor (22); therefore, more effective anticancer
agents are required for ovarian cancer therapy.
The T-type Ca2+ channel blocker KYS05090S
was previously reported to induce autophagy and apoptosis in A549
non-small lung cancer cells (10).
In the present study, the underlying anticancer mechanism of
KYS05090S was investigated in ovarian cancer cell lines.
KYS05090S exerted significant cytotoxicity in SKOV3
and OVCAR3 cells, which was increased compared with HEK293 cells.
To confirm whether the cytotoxicity of KYS05090S is attributed to
its apoptotic effect, DAPI staining, a TUNEL assay and cell cycle
analysis were conducted. As expected, KYS05090S treatment increased
the number of DAPI- and TUNEL-positive cells, and increased the
sub-G1 population in SKOV3 and OVCAR3 cells, indicating the
apoptotic effect of KYS05090S.
In general, two major apoptotic pathways have been
well established: The intrinsic (mitochondrial-mediated) pathway
and the extrinsic (death receptor-dependent) pathway, which result
in caspase activation (23,24).
The intrinsic apoptotic pathway causes the activation of caspase-9
and the extrinsic pathway is mediated by the activation of
caspase-8 (25). The western blot
analysis in the present study demonstrated that KYS05090S induced
PARP cleavage and activation of caspase-9/3, but not caspase-8
activation (data not shown), in SKOV3 and OVCAR3 cells, indicating
that apoptosis induced by KYS05090S treatment is mediated primarily
via the intrinsic pathway.
During the intrinsic pathway, activation of
pro-apoptotic Bcl-2 family proteins subsequently inactivate
anti-apoptotic Bcl-2 proteins (26). In the present study, KYS05090S
attenuated the expression of cyclin D1, c-Myc, Bcl-xl and Bcl-2 as
anti-apoptotic proteins. It is well documented that JAK2/STAT3
signaling is critically involved in cancer progression,
proliferation, differentiation, apoptosis, oncogenesis and immunity
(16,27-29).
Furthermore, STAT3 dimerization and its transcriptional activity
are known to inhibit cell growth and induce apoptosis (29). Additionally, accumulating evidence
has revealed that IL-6 directly activates the JAK2/STAT3 signaling
pathway (16,30), which suppresses major
histocompatibility complex class II expression on DC and
CD4+ Th cells during immune responses through activation
of lysosomal protease (31). Serum
IL-6 levels are also associated with the tumor stage and size,
metastasis and survival of patients with colon cancer (32). Notably, in the present study,
KYS05090S suppressed the phosphorylation of JAK2 and its downstream
mediator STAT3 in SKOV3 and OVCAR3 cells, and also blocked nuclear
translocation of STAT3 even in IL-6-treated SKOV3 cells, indicating
that the JAK2/STAT3 signaling pathway has a crucial role in the
effects of KYS05090S (33).
Nonetheless, considering that the half maximal inhibitory
concentration of the T-type Ca2+ channel
blocker-KYS05090S was reported to be 0.51±0.02 μM in A549
cells (10), KYS05090S may be a
multi-target compound, including JAK2/STAT3 pathway components,
Ca2+ channels and other targets. Taking previous
evidence from 3,4-dihydroquinazoline derivatives into consideration
(34), further study is required
for determining nontoxic optimal doses in animals and to identify
the mechanism of KYS05090S along with pharmacokinetics and DMEM
studies for future clinical trials.
Overall, the T-type Ca2+ channel blocker
KYS05090S significantly increased cytotoxicity, the number of
apoptotic bodies and sub-G1 cellular population, activated PARP
cleavage and caspase-9/3, and reduced the protein levels of cyclin
D1, Bcl-2 and p-JAK2/p-STAT3 in SKOV3 and OVCAR3 cells.
Furthermore, KYS05090S blocked the nuclear translocation of STAT3
and suppressed phosphorylation of JAK2/STAT3 in IL-6-treated SKOV3
cells. Collectively, the data support that KYS05090S induces
apoptosis via activation of caspase-9/3 and inhibition of
JAK2/STAT3 signaling in ovarian cancer cells, and may be used as a
potent antitumor agent (Fig.
7).
Funding
This work was supported by the National Research
Foundation of Korea grant funded by the Korea Government (grant no.
2017R1A2A1A17069297).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BIK and JHK conducted analysis and interpretation of
data and contributed to drafting the manuscript. BIK, JHK, DYS, MN,
JHJ, BS and JL designed and performed the experiments for
acquisition of data. SHK made acquisition of funding, conception,
design and supervision for this study. JL synthesized and supplied
KYS05090S. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Abbreviations:
|
IL-6
|
interleukin-6
|
|
JAK2
|
Janus kinase 2
|
|
STAT3
|
signal transducer and activator of
transcription 3
|
|
PARP
|
poly(ADP-ribose) polymerase
|
|
BSA
|
bovine serum albumin
|
Acknowledgments
Not applicable.
References
|
1
|
Coward JI, Middleton K and Murphy F: New
perspectives on targeted therapy in ovarian cancer. Int J Womens
Health. 7:189–203. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yan H, Guo BY and Zhang S:
Cancer-associated fibroblasts attenuate Cisplatin-induced apoptosis
in ovarian cancer cells by promoting STAT3 signaling. Biochem
Biophys Res Commun. 470:947–954. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lim HJ and Ledger W: Targeted therapy in
ovarian cancer. Wom Health Lond. 12:363–378. 2016. View Article : Google Scholar
|
|
4
|
Dai L, Xia P and Di W: Sphingosine
1-phosphate: A potential molecular target for ovarian cancer
therapy? Cancer Invest. 32:71–80. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mabuchi S, Kuroda H, Takahashi R and
Sasano T: The PI3K/AKT/mTOR pathway as a therapeutic target in
ovarian cancer. Gynecol Oncol. 137:173–179. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cheaib B, Auguste A and Leary A: The
PI3K/Akt/mTOR pathway in ovarian cancer: Therapeutic opportunities
and challenges. Chin J Cancer. 34:4–16. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang S, Cui B, Lai H, Liu G, Ghia EM,
Widhopf GF II, Zhang Z, Wu CC, Chen L, Wu R, et al: Ovarian cancer
stem cells express ROR1, which can be targeted for
anti-cancer-stem-cell therapy. Proc Natl Acad Sci USA.
111:17266–17271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Horvath CM: STAT proteins and
transcriptional responses to extracellular signals. Trends Biochem
Sci. 25:496–502. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Siddiquee K, Zhang S, Guida WC, Blaskovich
MA, Greedy B, Lawrence HR, Yip ML, Jove R, McLaughlin MM, Lawrence
NJ, et al: Selective chemical probe inhibitor of Stat3, identified
through structure-based virtual screening, induces antitumor
activity. Proc Natl Acad Sci USA. 104:7391–7396. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rim HK, Cho S, Shin DH, Chung KS, Cho YW,
Choi JH, Lee JY and Lee KT: T-type Ca2+ channel blocker,
KYS05090 induces autophagy and apoptosis in A549 cells through
inhibiting glucose uptake. Molecules. 19:9864–9875. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dziegielewska B, Gray LS and Dziegielewski
J: T-type calcium channels blockers as new tools in cancer
therapies. Pflugers Arch. 466:801–810. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jang SJ, Choi HW, Choi DL, Cho S, Rim HK,
Choi HE, Kim KS, Huang M, Rhim H, Lee KT, et al: In vitro
cytotoxicity on human ovarian cancer cells by T-type calcium
channel blockers. Bioorg Med Chem Lett. 23:6656–6662. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jung SY, Lee SH, Kang HB, Park HA, Chang
SK, Kim J, Choo DJ, Oh CR, Kim YD, Seo JH, et al: Antitumor
activity of 3,4-dihydroquinazoline dihydrochloride in A549
xenograft nude mice. Bioorg Med Chem Lett. 20:6633–6636. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Byun JS, Sohn JM, Leem DG, Park B, Nam JH,
Shin DH, Shin JS, Kim HJ, Lee KT and Lee JY: In vitro synergistic
anticancer activity of the combination of T-type calcium channel
blocker and chemotherapeutic agent in A549 cells. Bioorg Med Chem
Lett. 26:1073–1079. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Telford WG, King LE and Fraker PJ:
Comparative evaluation of several DNA binding dyes in the detection
of apoptosis-associated chromatin degradation by flow cytometry.
Cytometry. 13:137–143. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang SW and Sun YM: The IL-6/JAK/STAT3
pathway: Potential therapeutic strategies in treating colorectal
cancer (Review). Int J Oncol. 44:1032–1040. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shinagawa K, Yanamoto S, Naruse T,
Kawakita A, Morishita K, Sakamoto Y, Rokutanda S and Umeda M:
Clinical roles of interleukin-6 and STAT3 in oral squamous cell
carcinoma. Pathol Oncol Res. 23:425–431. 2017. View Article : Google Scholar
|
|
18
|
Ni CW, Hsieh HJ, Chao YJ and Wang DL:
Interleukin-6-induced JAK2/STAT3 signaling pathway in endothelial
cells is suppressed by hemodynamic flow. Am J Physiol Cell Physiol.
287:C771–C780. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang HF and Lai R: STAT3 in cancer-friend
or foe? Cancers (Basel). 6:1408–1440. 2014. View Article : Google Scholar
|
|
20
|
Aravantinos G and Pectasides D:
Bevacizumab in combination with chemotherapy for the treatment of
advanced ovarian cancer: A systematic review. J Ovarian Res.
7:572014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sopik V, Rosen B, Giannakeas V and Narod
SA: Why have ovarian cancer mortality rates declined? Part III
Prospects for the future. Gynecol Oncol. 138:757–761. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu J and Matulonis UA: New strategies in
ovarian cancer: Translating the molecular complexity of ovarian
cancer into treatment advances. Clin Cancer Res. 20:5150–5156.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kiraz Y, Adan A, Kartal Yandim M and Baran
Y: Major apoptotic mechanisms and genes involved in apoptosis.
Tumour Biol. 37:8471–8486. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lesinski GB, Raig ET, Guenterberg K, Brown
L, Go MR, Shah NN, Lewis A, Quimper M, Hade E, Young G, et al:
IFN-alpha and bortezomib overcome Bcl-2 and Mcl-1 overexpression in
melanoma cells by stimulating the extrinsic pathway of apoptosis.
Cancer Res. 68:8351–8360. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fadeel B and Orrenius S: Apoptosis: A
basic biological phenomenon with wide-ranging implications in human
disease. J Intern Med. 258:479–517. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hata AN, Engelman JA and Faber AC: The
BCL2 Family: Key mediators of the apoptotic response to targeted
anticancer therapeutics. Cancer Discov. 5:475–487. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Imada K and Leonard WJ: The Jak-STAT
pathway. Mol Immunol. 37:1–11. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Matsui F and Meldrum KK: The role of the
Janus kinase family/signal transducer and activator of
transcription signaling pathway in fibrotic renal disease. J Surg
Res. 178:339–345. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang X, Crowe PJ, Goldstein D and Yang JL:
STAT3 inhibition, a novel approach to enhancing targeted therapy in
human cancers (Review). Int J Oncol. 41:1181–1191. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jung IH, Choi JH, Chung YY, Lim GL, Park
YN and Park SW: Predominant activation of JAK/STAT3 pathway by
interleukin-6 is implicated in hepatocarcinogenesis. Neoplasia.
17:586–597. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kitamura H, Kamon H, Sawa S, Park SJ,
Katunuma N, Ishihara K, Murakami M and Hirano T: IL-6-STAT3
controls intracellular MHC class II alphabeta dimer level through
cathepsin S activity in dendritic cells. Immunity. 23:491–502.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Knüpfer H and Preiss R: Serum
interleukin-6 levels in colorectal cancer patients--a summary of
published results. Int J Colorectal Dis. 25:135–140. 2010.
View Article : Google Scholar
|
|
33
|
Chung CD, Liao J, Liu B, Rao X, Jay P,
Berta P and Shuai K: Specific inhibition of Stat3 signal
transduction by PIAS3. Science. 278:1803–1805. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim JH, Jeong HR, Jung DW, Yoon HB, Kim
SY, Kim HJ, Lee KT, Gadotti VM, Huang J, Zhang FX, et al: Synthesis
and biological evaluation of fluoro-substituted
3,4-dihydroquin-azoline derivatives for cytotoxic and analgesic
effects. Bioorg Med Chem. 25:4656–4664. 2017. View Article : Google Scholar : PubMed/NCBI
|