Introduction
Non-small cell lung cancer (NSCLC) accounts for ~85%
of all diagnosed lung cancers, and is the leading cause of
cancer-related mortality worldwide with an estimated 1.8 million
deaths in 2020 (1,2). Despite recent advances in surgery,
radiotherapy, chemotherapy and targeted therapy, the prognosis of
NSCLC remains dismal and the 5-year survival rate is lower than 20%
(3). Programmed death-ligand 1
(PD-L1) or programmed death-1 (PD-1) blockade immunotherapy has
resulted in striking clinical benefits in NSCLC, since nivolumab
and pembrolizumab have been approved as first- or second-line
treatments for advanced NSCLC, either as monotherapy or in
combination with chemotherapy (4-7).
However, due to the primary or acquired resistance and adverse
effects, only a small fraction of patients with NSCLC can benefit
from immune-related therapies (8-10).
Tumor PD-L1 expression detected by
immunohistochemistry (IHC) is the only approved biomarker for
predicting response to anti-PD-1/PD-L1 immunotherapy (11). Several clinical trials have
demonstrated superior overall survival (OS) for PD-1/PD-L1 blockade
in NSCLC patients with high PD-L1 expression, compared with those
with low PD-L1 expression (12,13). Alternative predictive biomarkers,
such as tumor mutational burden and the tumor microenvironment
(TME), have also been intensively investigated; however, conclusive
evidence is lacking (14,15). It is noteworthy that the
predictive value of PD-L1 expression is affected by multiple
variables, including the different testing platforms and cut-off
criteria for positivity, intra-tumoral and inter-tumoral
heterogeneity and the dynamic change of PD-L1 expression (16-18). In fact, clinical efficacy was also
observed in patients with cancer among the
PD-L1low/negative group (6,19),
suggesting that tumor PD-L1 expression alone is insufficient to
recognize patients sensitive to PD-1/PD-L1 blockade. Future studies
may help develop new predictors, particularly in identifying
potential responding candidates to anti-PD-1/PD-L1 among the
PD-L1low/negative patients.
Farnesoid X receptor (FXR) is a member of the
nuclear receptor superfamily that is predominantly expressed in the
liver and gastrointestinal tract (20). As a bile acid (BA)-activated
transcription factor, FXR regulates the expression of target genes
involved in BA homeostasis, lipid and glucose metabolism (21,22). Previous studies have demonstrated
the important role of FXR, either as an oncogene or as a
tumor-suppressive gene, in the tumorigenesis of liver, colorectal,
esophageal and breast cancer (23-26). It was previously reported that FXR
is upregulated in NSCLC, compared with pericarcinous lung tissues
and that FXR contributes to NSCLC cell proliferation via
transactivating CCND1 (27). In a recent study, an inhibitory
role of FXR in PD-L1 expression in NSCLC was identified (28). Critically,
FXRhighPD-L1low mouse Lewis lung carcinoma
(LLC) tumors were more vulnerable to anti-PD-1 therapy than mock
LLC tumors (28). However,
whether or not FXRhighPD-L1low phenotype
predicts clinical response to immune-related therapies in clinical
patients with NSCLC has yet to be investigated. The present study
aimed to determine the predictive value of FXR for anti-PD-1-based
chemo-immunotherapy in the setting of clinical NSCLC, mainly
focusing on the PD-L1low/negative group. In addition,
the potential correlation between FXR expression and
tumor-infiltrating CD8+ T cells was also revealed.
Materials and methods
Patients and data collection
From January 2019 to April 2021, 149 patients (119
men and 30 women; median age, 64; range, 38 to 75 years) with
pathologically confirmed NSCLC who were scheduled to receive
anti-PD-1-based chemo-immunotherapy at Shandong Provincial Hospital
(Jinan, China) were screened. Certain patients who relapsed after
complete resection were also included in the cohort, and their
tumor-node-metastasis (TNM) staging information was determined at
the beginning of anti-PD-1-based chemo-immunotherapy. Individuals
who were aged 18-75 years, had pathologically confirmed stage
III-IV NSCLC (according to the 8th edition of the AJCC/UICC
classification for NSCLC) and were ineligible for radical surgery
or radiotherapy, had at least one measurable lesion per the
Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST
1.1) (29), had an Eastern
Cooperative Oncology Group (ECOG) performance status (PS) (30) no more than 2, and had archival
tumor tissues obtained within 6 months before chemo-immunotherapy
or fresh tumor samples, were included. Those who had symptomatic
central nervous system metastasis, had used immunosuppressants
within 2 weeks before chemo-immunotherapy, had received neoadjuvant
chemotherapy, radiotherapy or immunotherapy, or had a history of
other malignant tumors were excluded. Patients were administered
intravenous anti-PD-1 agents, including camrelizumab, sintilimab,
tislelizumab, pembrolizumab (at a dose of 200 mg, every 3 weeks)
and toripalimab (at a dose of 240 mg, every 3 weeks), combined with
chemotherapies such as cisplatin, carboplatin, pemetrexed,
gemcitabine and paclitaxel according to the standards of relevant
guidelines. Clinical and pathological information, including age,
sex, smoking history, histologic type, TNM stage, ECOG PS and lines
of therapy, were retrospectively obtained from the medical
records.
The present study was approved (approval no.
NSFC-2019-05) by the Institutional Review Board of Shandong
Provincial Hospital affiliated to Shandong First Medical University
(Jinan, China) and complied with all relevant ethical regulations
of the Declaration of Helsinki.
Tumor response and survival analysis
For the evaluation of tumor response, CT scans were
reviewed by specialized radiologists. Tumor assessment was
performed at baseline and every 2 cycles according to the RECIST
1.1. On the basis of the best overall response, patients with
complete or partial response were considered responders, while
others with stable or progressive disease were considered
non-responders. The progression-free survival (PFS) and OS were
defined as the interval from treatment initiation to the date of
clinical progression or death, and to the date of death from any
cause, respectively (31).
Survival status was obtained through medical records or telephone
follow-up every 2 cycles of chemo-immunotherapy. Patients who had
not progressed or succumbed to the disease were censored for PFS
and OS at last follow-up (August 31, 2021).
IHC staining and assessment
All of the cases had available formalin-fixed
paraffin-embedded (FFPE) specimens of primary tumors which were
obtained from the most recent biopsy before treatment. Tissue
sections (4-µm thick) from each FFPE block were used for
FXR, PD-L1 and CD8 IHC staining as previously described (32). Slides were incubated overnight at
4°C with the following primary antibodies: Anti-bile acid receptor
NR1H4 antibody (1:100; product code ab187735), anti-PD-L1 antibody
(1:500; product code ab205921) and anti-CD8α antibody (1:100;
product code ab101500; all from Abcam). Isotype controls were
conducted simultaneously using concentration-matched non-specific
mouse or rabbit IgG (product codes ab37355 and ab172730,
respectively; both from Abcam). All stained slides were scanned
using a high-resolution digital slide scanner (TissueFAXS plus;
TissueGnostics Ltd.) up to a magnification of ×200.
For FXR and PD-L1, staining intensity was classified
as negative (0), weak (1),
moderate (2) and intense
(3) according to the degree of
dyeing. As for the percentage of stained cells, 0% (0), 1 to 25%
(1), 26 to 50% (2), 51 to 75% (3) and 76 to 100% (4) was defined. The IHC score was
generated by multiplying the staining intensity and percentage
(27). If the staining intensity
in a section was diverse, the highest was selected from the scores
of different intensities and ratios. Since the median IHC score of
FXR and PD-L1 was 6 and 4, respectively, an IHC score of 5 was used
as the cut-off value to discriminate low and high expression of FXR
and PD-L1, to ensure that the number of NSCLC patients with FXR or
PD-L1 low-expression was generally equal to the number of NSCLC
patients with FXR or PD-L1 high-expression. For CD8, 4-6
independent high-power fields (HPFs; ×200) which represented the
densest lymphocytic infiltrates were selected to reflect the extent
of CD8+ T-cell infiltration. The average CD8+
T-cell density (cells/HPF) was calculated as the mean value of the
4-6 areas (33). Two proficient
pathologists who were blinded to the clinical data independently
evaluated the IHC results and reached a final consensus.
Statistical analysis
Shapiro-Wilk method was used to assess the normality
of the quantitative data. Comparisons between skewed distribution
data were performed using Mann-Whitney U test. Categorical
variables were compared using chi-square tests or Fisher's exact
test. Spearman's rank correlation test was used to assess the
correlation between FXR and PD-L1 and between FXR and CD8 in NSCLC.
The comparison of infiltrating CD8+ T cells in four
groups, according to FXR staining intensity, was performed using
Kruskal-Wallis (K-W) rank sum test. Survival curves were estimated
by Kaplan-Meier analysis and the log-rank test was utilized to
examine the differences between groups. In addition, prognostic
factors were evaluated based on univariate and multivariate Cox
regression analyses. The variables with univariate regression
P<0.1 were included in multivariate regression analysis.
Statistical analyses were performed using the statistical software
SPSS Statistics 26.0 (IBM Corp.) and GraphPad Prism 8.0 (GraphPad
Software, Inc.). All tests were two-sided, and P<0.05 was
considered to indicate a statistically significant difference.
Results
Baseline characteristics of patients
A total of 149 patients treated with anti-PD-1-based
chemo-immunotherapy were eventually enrolled in the present study.
Their clinical and pathological characteristics are listed in
Table I. The cohort included 119
men and 30 women with a higher proportion of elderly, and most of
the patients were smokers. The majority of these NSCLC cases were
non-squamous cell carcinoma (93/149, 62.4%), of which 90 were
adenocarcinoma, 2 were sarcomatoid carcinoma and 1 was large cell
neuroendocrine carcinoma. More than half of the patients (103/149,
69.1%) were in stage IV at the beginning of anti-PD-1-based
chemo-immunotherapy. In the present cohort, all the patients
received PD-1 inhibitors combined with chemotherapy as the
first-line or higher lines with refractory progression after
chemotherapy, radiation or targeted therapy. Among them, the most
widely used PD-1 inhibitor was camrelizumab (111/149, 74.5%). There
were 40 patients who had oncogene mutations among 67 patients with
available gene analysis data. A total of 78 patients (52.3%) and 54
patients (36.2%) were classified as high FXR and PD-L1 expression,
respectively (Table I).
 | Table IBaseline clinical characteristics
according to FXR and PD-L1 protein expression of patients with
NSCLC in the present cohort. |
Table I
Baseline clinical characteristics
according to FXR and PD-L1 protein expression of patients with
NSCLC in the present cohort.
| Variables | All patients no.
(%) | Expression level of
FXR
| P-value | Expression level of
PD-L1
| P-value |
|---|
| Low (%) | High (%) | Low (%) | High (%) |
|---|
| N | 149 | 71 (47.7) | 78 (52.3) | | 95 (63.8) | 54 (36.2) | |
| Age, years | | | | 0.728a | | | 0.467a |
| <60 | 44 (29.5) | 20 (45.5) | 24 (54.5) | | 30 (68.2) | 14 (31.8) | |
| ≥60 | 105 (70.5) | 51 (48.6) | 54 (51.4) | | 65 (61.9) | 40 (38.1) | |
| Sex | | | | 0.348a | | | 0.711a |
| Male | 119 (79.9) | 59 (49.6) | 60 (50.4) | | 75 (63) | 44 (37) | |
| Female | 30 (20.1) | 12 (40.0) | 18 (60.0) | | 20 (66.7) | 10 (33.3) | |
| Smoking
history | | | | 0.844a | | | 0.275a |
| No | 41 (27.5) | 19 (46.3) | 22 (53.7) | | 29 (70.7) | 12 (29.3) | |
| Yes | 108 (72.5) | 52 (48.1) | 56 (51.9) | | 66 (61.1) | 42 (38.9) | |
| Histology | | | | 0.656a | | | 0.804a |
| Squamous | 56 (37.6) | 28 (50.0) | 28 (50.0) | | 35 (62.5) | 21 (37.5) | |
| Non-squamous | 93 (62.4) | 43 (46.2) | 50 (53.8) | | 60 (64.5) | 33 (35.5) | |
| TNM stage | | | | 0.300a | | | 0.110a |
| III | 46 (30.9) | 19 (41.3) | 27 (58.7) | | 25 (54.3) | 21 (45.7) | |
| IV | 103 (69.1) | 52 (50.5) | 51 (49.5) | | 70 (68.0) | 33 (32.0) | |
| ECOG PS | | | | 0.985a | | | 0.874a |
| 0 | 23 (15.4) | 11 (47.8) | 12 (52.2) | | 15 (65.2) | 8 (34.8) | |
| ≥1 | 126 (84.6) | 60 (47.6) | 66 (52.4) | | 80 (63.5) | 46 (36.5) | |
| Therapy line | | | | 0.453a | | | 0.014a |
| 1st | 94 (63.1) | 47 (50.0) | 47 (50.0) | | 53 (56.4) | 41 (43.6) | |
| ≥2nd | 55 (36.9) | 24 (43.6) | 31 (56.4) | | 42 (76.4) | 13 (23.6) | |
| PD-1
inhibitors | | | | 0.562a | | | 0.794a |
| Camrelizumab | 111 (74.5) | 53 (47.7) | 58 (52.3) | | 70 (63.1) | 41 (36.9) | |
| Tislelizumab | 14 (9.4) | 5 (35.7) | 9 (64.3) | | 9 (64.3) | 5 (35.7) | |
| Sintilimab | 22 (14.8) | 12 (54.5) | 10 (45.5) | | 15 (68.2) | 7 (31.8) | |
| Pembrolizumab | 1 (0.7) | 1 (100) | 0 (0) | | 0 (0) | 1 (100) | |
| Toripalimab | 1 (0.7) | 0 (0) | 1 (100) | | 1 (100) | 0 (0) | |
| Gene mutations | | | | 0.882b | | | 0.580b |
| EGFR mutation | 24 (16.1) | 11 (45.8) | 13 (54.2) | | 15 (62.5) | 9 (37.5) | |
| KRAS mutation | 10 (6.7) | 7 (70.0) | 3 (30.0) | | 5 (50.0) | 5 (50.0) | |
| BRAF mutation | 2 (1.3) | 1 (50.0) | 1 (50.0) | | 1 (50.0) | 1 (50.0) | |
| HER-2
mutation | 2 (1.3) | 1 (50.0) | 1 (50.0) | | 2 (100) | 0 (0) | |
| ALK fusion | 1 (0.7) | 0 (0) | 1 (100) | | 0 (0) | 1 (100) | |
| PIK3CA
mutation | 1 (0.7) | 0 (0) | 1 (100) | | 1 (100) | 0 (0) | |
| Wild type | 27 (18.1) | 13 (48.1) | 14 (51.9) | | 18 (66.7) | 9 (33.3) | |
| Unknown | 82 (55.0) | 38 (46.3) | 44 (53.7) | | 53 (64.6) | 29 (35.4) | |
Associations between FXR, PD-L1
expression and response to anti-PD-1-based chemo-immunotherapy in
all patients
As visualized using IHC, the expression of FXR was
mainly localized in the nucleus and cytoplasm, while PD-L1 was
expressed on the membrane of tumor cells (Fig. 1A). A total of 46 patients were
classified as responders according to the RECIST 1.1, and the
objective response rate (ORR) to chemo-immunotherapy in the present
study was 30.9%. It was revealed that responsive tumors expressed
higher levels of both FXR and PD-L1 compared with those
irresponsive to chemo-immunotherapy (P=0.01 and 0.003,
respectively; Fig. 1B and C).
Meanwhile, the chi-square test revealed that high FXR and PD-L1
expression levels were associated with a higher ORR in all patients
(P=0.036 and 0.002, respectively; Fig. 1D and E). These findings underlined
the utility of high expression of FXR as a predictive biomarker for
immunotherapy in addition to PD-L1.
 | Figure 1FXR and PD-L1 expression are
associated with tumor response to anti-PD-1-based
chemo-immunotherapy in patients with NSCLC. (A) Representative IHC
images of FXR (upper panel) and PD-L1 (lower panel) expression in
NSCLC specimens (Scale bar, 50 µm). Isotype control: the
primary antibody was replaced by nonspecific mouse or rabbit IgG.
(B and C) Representative IHC images (upper graphs) and IHC scores
(lower graphs) of FXR (B) and PD-L1 (C) in responders vs.
non-responders (P=0.01 and P=0.003, respectively; Mann-Whitney U
test). Scale bars indicate 50 µm. Error bars indicate the
median and interquartile range. (D) Objective response in patients
with low FXR vs. high FXR (ORR 22.5 vs. 38.5%, P=0.036; chi-square
test). (E) Objective response in patients with low PD-L1 vs. high
PD-L1 (ORR 22.1 vs. 46.3%, P=0.002; chi-square test). The objective
response rate (n/N), is shown above each bar. FXR, farnesoid X
receptor; PD-L1, programmed death-ligand 1; PD-1, programmed
death-1; NSCLC, non-small cell lung cancer; IHC,
immunohistochemistry. |
Prognostic significance of FXR, PD-L1 and
clinicopathological parameters in all patients
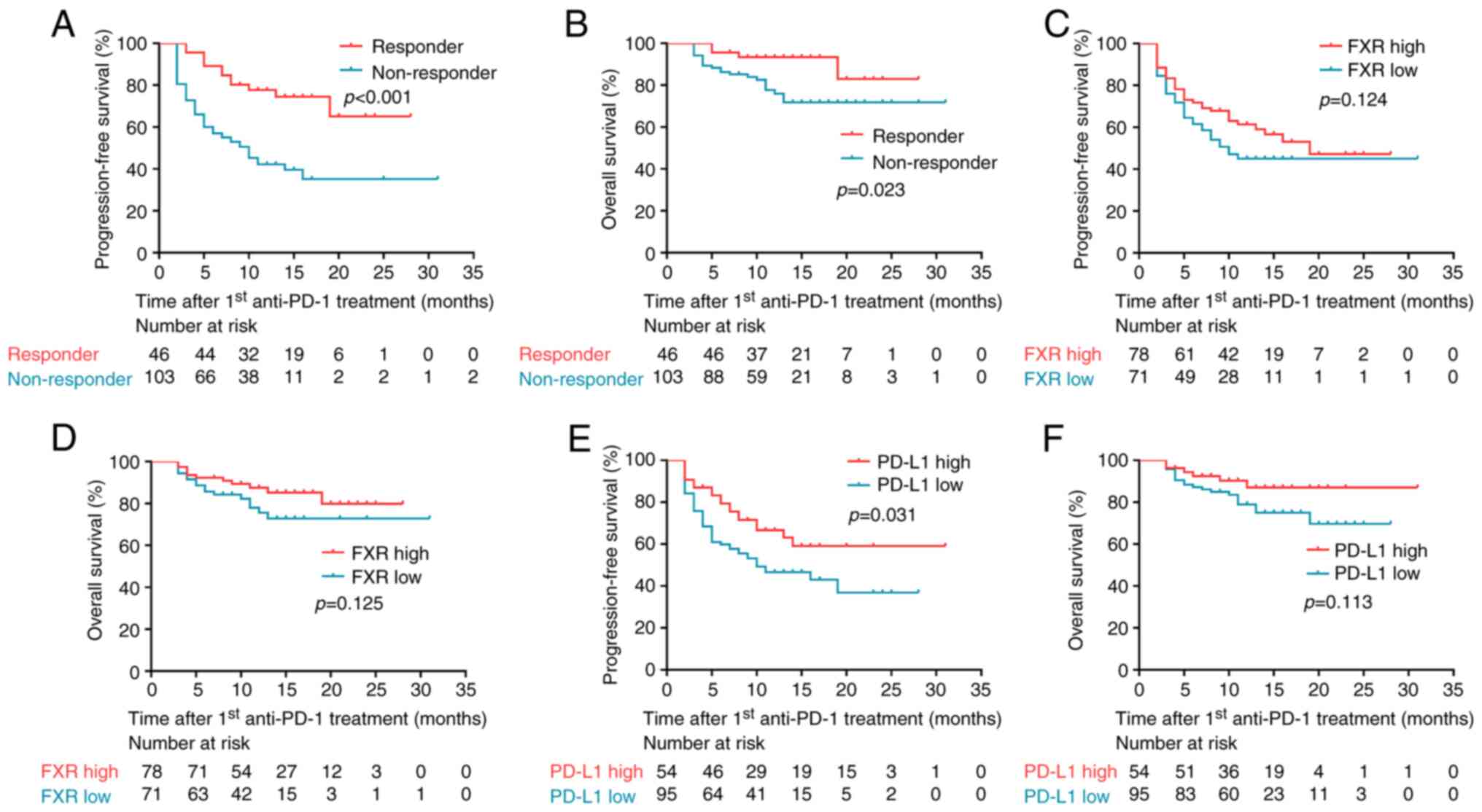
As illustrated in Fig.
2A and B, the Kaplan-Meier and log-rank tests demonstrated that
responders to anti-PD-1-based chemo-immunotherapy had both
significantly longer PFS and OS than non-responders (P<0.001 and
P=0.023, respectively). There was a non-significant trend towards
improved PFS and OS in patients with high FXR expression as
compared with their FXR low counterparts (Fig. 2C and D). In addition, PD-L1
high-expression patients were found to have a significantly longer
PFS (P=0.031; Fig. 2E), as
compared with PD-L1 low-expression patients; however, there was no
statistical association between PD-L1 expression and OS (Fig. 2F).
To study the prognostic role of FXR and PD-L1 in all
patients, a Cox regression model was applied including several
clinical characteristics (age, sex, smoking history, histologic
type, TNM stage, ECOG PS, lines of therapy and gene mutation
state). Given that the number of NSCLC patients with an ECOG PS
score of 2 was quite small (4/149, 2.7%), the ECOG PS data were
analyzed as a binary variable (0 vs. ≥1). The univariate analysis
revealed that TNM stage, line of therapy and PD-L1 expression were
associated to PFS (P-values <0.1; Table SI). In multivariate analysis, TNM
stage [P=0.011; hazard ratio (HR), 2.146; 95% confidence interval
(CI), 1.188-3.874)] remained an independent prognostic indicator
for PFS. Conversely, only age was defined as an independent
prognostic factor for OS (P=0.021; HR, 4.117; 95% CI, 1.235-13.727;
Table SII). Collectively,
neither FXR nor PD-L1 expression could stratify PFS and OS in the
present cohort.
Subgroup analysis of tumor responses and
prognosis based on the correlation between FXR and PD-L1
It was previously reported that
FXRhighPD-L1low mouse LLC tumors exhibited an
increased susceptibility to PD-1 blockade compared with mock LLC
tumors (28). To further
investigate whether FXR could be an effective predictor of clinical
response to anti-PD-1-based chemo-immunotherapy among
PD-L1low patients with NSCLC, a subgroup analysis of
tumor responses and prognosis based on the correlation between FXR
and PD-L1 was conducted. Firstly, a significant increase of PD-L1
expression in 'FXR low' tumors was found (P=0.016; Fig. 3A), which was consistent with a
previous study (28). The
chi-square test revealed that FXR was inversely associated with
PD-L1 expression in specimens with NSCLC (P=0.005; Fig. 3B). In addition, the result of
Spearman's correlation analysis demonstrated that there was a
significant inverse correlation between FXR and PD-L1 expression in
the entire cohort (r=−0.236, P=0.004; Fig. 3C). Furthermore, it was
investigated whether PD-L1 expression was different between
squamous cell carcinoma and adenocarcinoma in the present study.
There was no statistically significant difference in PD-L1
expression between squamous cell carcinoma and adenocarcinoma in
149 specimens with NSCLC enrolled in the present study (data not
shown).
Then, four subgroups based on the IHC levels of FXR
and PD-L1 were defined (Fig. 4A).
Notably, patients with high expression of both PD-L1 and FXR
exhibited the highest ORR (60%), followed by the
FXRlowPD-L1high group (38.2%). In these PD-L1
high-expression patients, although responsive tumors expressed
higher levels of FXR than that of non-responsive tumors (Fig. S1), no significant association was
identified between FXR expression and tumor response (Fig. 4A). Of note, patients with high
expression of FXR demonstrated a higher ORR as compared with FXR
low-expression patients in the presence of PD-L1 low-expression
(31.0 vs. 8.1%, P=0.009). Additionally,
PD-L1low-responsive tumors expressed significantly
increased FXR compared with the PD-L1low-non-responsive
tumors (P<0.001; Fig. 4B).
Collectively, these results suggested FXR as a promising predictive
biomarker for clinical efficacy to anti-PD-1-based
chemo-immunotherapy when PD-L1 is low or negative.
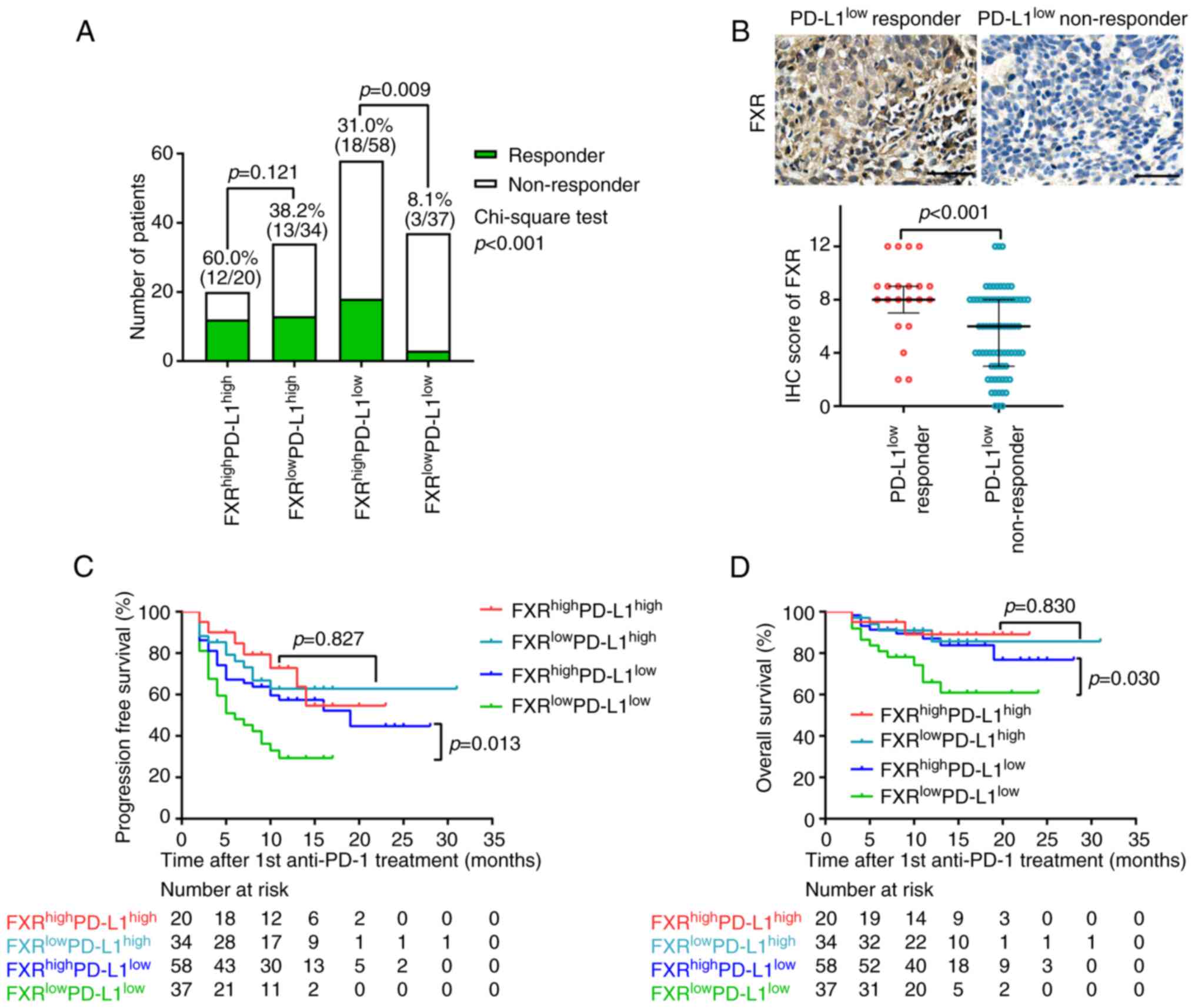 | Figure 4Subgroup analysis of tumor responses
and prognosis based on the IHC levels of FXR and PD-L1 in patients
with NSCLC receiving anti-PD-1-based chemo-immunotherapy. (A)
Objective response in patients with
FXRhighPD-L1high,
FXRlowPD-L1high,
FXRhighPD-L1low, or
FXRlowPD-L1low (ORR in
FXRhighPD-L1low vs.
FXRlowPD-L1low patients, 31 vs. 8.1%,
P=0.009; chi-square test). The objective response rate (n/N), is
shown above each bar. (B) Representative IHC images (upper panels)
and IHC score (lower panel) of FXR in PD-L1low
responders vs. PD-L1low non-responders (P<0.001;
Mann-Whitney U test). Scale bars indicate 50 µm. Error bars
indicate the median and interquartile range. (C and D) Kaplan-Meier
survival curves for PFS and OS of four subgroups (PFS and OS in
FXRhighPD-L1low vs.
FXRlowPD-L1low patients, P=0.013 and P=0.03,
respectively; log-rank test). IHC, immunohistochemistry; FXR,
farnesoid X receptor; PD-L1, programmed death-ligand 1; NSCLC,
non-small cell lung cancer; PD-1, programmed death-1; PFS,
progression-free survival; OS, overall survival. |
The Kaplan-Meier survival curves in Fig. 4C and D revealed that high FXR
expression was associated with both longer PFS (P=0.013) and OS
(P=0.03) among the PD-L1low patients with NSCLC.
However, consistent with the association with therapeutic
responses, the extent of FXR expression in PD-L1high
patients was not associated with either PFS or OS (Fig. 4C and D).
Prognostic significance of FXR in
PD-L1low patients
Cox regression models were then applied, including
clinical variables to verify the prognostic value of FXR in
PD-L1low patients. As presented in Table II, TNM stage and FXR expression
were found to be significantly correlated with PFS in univariate
Cox regression analysis. These two variables were then analyzed in
a multivariate Cox regression model. Intriguingly, FXR expression
was still identified as an independent predictor for PFS in
PD-L1low patients with NSCLC (P=0.038; HR, 0.552; 95%
CI, 0.315-0.967).
 | Table IIUnivariate and multivariate cox
regression analysis for progression-free survival in
PD-L1low patients. |
Table II
Univariate and multivariate cox
regression analysis for progression-free survival in
PD-L1low patients.
| Variables | Univariate analysis
| Multivariate
analysis
|
|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age, years | | | | | | |
| <60 | 1 | | | | | |
| ≥60 | 0.775 | 0.433-1.389 | 0.392 | | | |
| Sex | | | | | | |
| Male | 1 | | | | | |
| Female | 0.847 | 0.424-1.693 | 0.639 | | | |
| Smoking
history | | | | | | |
| No | 1 | | | | | |
| Yes | 0.846 | 0.472-1.515 | 0.573 | | | |
| Histology | | | | | | |
| Squamous | 1 | | | | | |
| Non-squamous | 1.076 | 0.603-1.918 | 0.805 | | | |
| TNM stage | | | | | | |
| III | 1 | | | 1 | | |
| IV | 2.174 | 1.052-4.495 | 0.036 | 2.017 | 0.971-4.189 | 0.060 |
| ECOG PS | | | | | | |
| 0 | 1 | | | | | |
| ≥1 | 1.127 | 0.507-2.503 | 0.770 | | | |
| Therapy line | | | | | | |
| 1st | 1 | | | | | |
| ≥2nd | 1.25 | 0.720-2.171 | 0.428 | | | |
| Gene mutations | | | | | | |
| Wild | 1 | | | | | |
| Mutant | 1.725 | 0.728-4.086 | 0.215 | | | |
| Expression level of
FXR | | | | | | |
| Low | 1 | | | 1 | | |
| High | 0.515 | 0.295-0.901 | 0.020 | 0.552 | 0.315-0.967 | 0.038 |
As for the univariate Cox regression analysis for OS
in PD-L1low patients, age and FXR expression were found
to stratify OS significantly at the level of P<0.1 (Table III). Additionally, multivariate
analysis showed that FXR expression remained an independent
prognostic indicator for OS in PD-L1low patients with
NSCLC (P=0.029; HR, 0.377; 95% CI, 0.157-0.905).
 | Table IIIUnivariate and multivariate cox
regression analysis for overall survival in PD-L1low
patients. |
Table III
Univariate and multivariate cox
regression analysis for overall survival in PD-L1low
patients.
| Variables | Univariate analysis
| Multivariate
analysis
|
|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age, years | | | | | | |
| <60 | 1 | | | 1 | | |
| ≥60 | 2.954 | 0.870-10.035 | 0.083 | 3.149 | 0.925-10.723 | 0.067 |
| Sex | | | | | | |
| Male | 1 | | | | | |
| Female | 0.546 | 0.161-1.858 | 0.333 | | | |
| Smoking
history | | | | | | |
| No | 1 | | | | | |
| Yes | 1.555 | 0.568-4.257 | 0.39 | | | |
| Histology | | | | | | |
| Squamous | 1 | | | | | |
| Non-squamous | 0.567 | 0.239-1.343 | 0.197 | | | |
| TNM stage | | | | | | |
| III | 1 | | | | | |
| IV | 1.817 | 0.609-5.422 | 0.284 | | | |
| ECOG PS | | | | | | |
| 0 | 1 | | | | | |
| ≥1 | 1.898 | 0.442-8.157 | 0.389 | | | |
| Therapy line | | | | | | |
| 1st | 1 | | | | | |
| ≥2nd | 0.844 | 0.355-2.006 | 0.701 | | | |
| Gene mutations | | | | | | |
| Wild | 1 | | | | | |
| Mutant | 0.265 | 0.047-1.486 | 0.131 | | | |
| Expression level of
FXR | | | | | | |
| Low | 1 | | | 1 | | |
| High | 0.398 | 0.167-0.953 | 0.039 | 0.377 | 0.157-0.905 | 0.029 |
Correlation between FXR and infiltrating
CD8+ T cells in NSCLC
The predictive value of FXR on anti-PD-1-based
chemo-immunotherapy in the perspective of TME was then sought to be
explained. Since CD8+ T cells represent the most crucial
tumoricidal effector cells and the main target of the PD-L1/PD-1
checkpoint pathway in the TME (34,35), the infiltration of CD8+
T cells in specimens with NSCLC was examined. Representative
microphotographs of the infiltration levels of different
CD8+ T cells are shown in Fig. 5A. The cells positively stained for
CD8 were semi-quantified and low or high groups were defined
according to the median value. IHC evaluation revealed a
statistically significant decrease of infiltrating CD8+
T cells in FXRhigh NSCLC specimens (P=0.014; Fig. 5B). Chi-square analysis
demonstrated that FXR expression was inversely associated with the
infiltration of CD8+ T cells in specimens with NSCLC
(P=0.004; Fig. 5C). Importantly,
the Spearman's correlation analysis revealed that there was a
significant inverse correlation between FXR expression and
infiltrating CD8+ T cells in the enrolled 149 specimens
with NSCLC (r=−0.217, P=0.008; Fig.
5D). The CD8 expression data were analyzed in four groups,
according to FXR staining intensity (negative, weak, moderate and
intense). However, the K-W analysis showed that there was no
difference in the degree of infiltration of CD8+ T cells
among the four FXR staining groups in the present study (data not
shown). Thus, it was considered that the immunosuppressive effects
of FXR previously reported (28)
in in vitro co-culture and mouse models could also be
recapitulated in clinical patients with NSCLC.
Discussion
In the past decade, the emergence of
anti-PD-1/PD-L1-directed immunotherapy has significantly changed
the clinical management and outcome of patients with advanced NSCLC
(4-7). High expression of tumor PD-L1
predicts clinical efficacy of PD-1/PD-L1 blockade (12,13), meanwhile a few
PD-L1low/negative patients still benefit from these
drugs (6,19). Thus, there is an urgent need to
further stratify patients who can derive benefit from immunotherapy
from those who cannot within the PD-L1low/negative
group. In the present study, 149 clinical NSCLC specimens were
screened for FXR and PD-L1 expression to determine their predictive
value for anti-PD-1-based chemo-immunotherapy. The present results
showed that high FXR and PD-L1 expression levels were associated
with a higher ORR in the entire cohort. The inverse correlation
between the expression of FXR and PD-L1 in NSCLC specimens was also
verified, consistent with a previous study (28). Notably, subgroup analysis revealed
that high FXR expression was associated with a higher ORR, as well
as longer PFS and OS among PD-L1low patients with NSCLC.
Mechanistically, a statistically significant decrease of
infiltrating CD8+ T cells in FXRhigh NSCLC
specimens was observed. The present study provided a brand-new
stratification, recommending FXRhighPD-L1low
as a potential predictive biomarker for
PD-L1low/negative NSCLC patients who can benefit from
anti-PD-1-based chemo-immunotherapy.
Previously, emerging evidence supported differential
roles for FXR in carcinogenesis. It was previously reported that
FXR overexpression contributed to lymphatic metastasis of human
pancreatic cancer (36). Another
study demonstrated a vital role of FXR in endothelial cell motility
and vascular tube formation, essential for tumor angiogenesis
(37). It was previously found
that FXR promotes NSCLC cell proliferation through increasing
CCND1 transcription (27),
and that enforced FXR expression constructs an immunosuppressive
microenvironment in mouse LLC tumors (28). The present study provided
compelling clinical evidence to extend FXR function as an indicator
of sensitivity to immune-related therapies. The data showed that
high FXR expression was associated with a higher ORR in patients
with NSCLC undergoing anti-PD-1-based chemo-immunotherapy.
Additionally, there was a non-significant trend toward improved PFS
and OS in FXR high-expression group as compared with FXR
low-expression group with the same treatment. Consistent with the
present findings, previous studies have reported that FXR
activation enhanced the chemo-sensitivity of biliary tract and
colorectal cancer cells to oxaliplatin and cisplatin, respectively
(38,39).
PD-L1 is currently approved as a predictive
biomarker for anti-PD-1/PD-L1 response in cancer treatment,
including NSCLC (11,40). However, the predictive value of
tumoral PD-L1 is discordant since in clinical trials it was
identified that a proportion of PD-L1low/negative
patients can also derive clinical benefit from PD-1/PD-L1 blockade
(6,19). In the present study, 30.9%
(46/149) of the patients were classified as responders to
chemo-immunotherapy, which is consistent with the ORR reported by
Carbone et al (41) in
stage IV or recurrent patients with NSCLC treated with the
combination of nivolumab and platinum doublet chemotherapy.
Interestingly, it was observed that 22.1% (21/95) of the patients
with low PD-L1 expression responded to anti-PD-1-based
chemo-immunotherapy, consistent with a previous study which
revealed that the anti-PD-L1 antibody MPDL3280A resulted in an ORR
of 20% in PD-L1 low-expression patients with NSCLC (42). Possible explanations for this
discordance may include the fact that PD-L1 expression is dynamic
and heterogeneous, both within the same tumor and between primary
and metastatic lesions in the same patient (16). An alternative explanation could
rely on the different testing platforms and different cut-off
values for PD-L1 positivity (17,18). There are currently no approved
predictors that can guide treatment decision for the
PD-L1low/negative patients. In the present study, the
inverse correlation between FXR and PD-L1 expression in NSCLC
specimens was verified. Consistently, a previous study demonstrated
that FXR can suppress PD-L1 transcription by binding to the
putative FXR element in PD-L1 promoter. In addition, SHP, a
downstream target gene of FXR, and EGFR signals are also involved
in FXR-induced PD-L1 downregulation in NSCLC cells (28). Notably, stratifying by FXR and
PD-L1 expression showed that FXRhighPD-L1low
patients with NSCLC displayed a significantly higher ORR, as well
as longer PFS and OS, compared with
FXRlowPD-L1low patients among the
PD-L1low group. High FXR expression was established to
be an independent predictor for PFS and OS in PD-L1low
patients with NSCLC receiving anti-PD-1-based chemo-immunotherapy.
In line with the present findings, baseline serum IL-6 level was
demonstrated to be a potential biomarker for predicting the
efficacy and survival outcome of PD-1/PD-L1 inhibitors, even in
PD-L1low/negative patients with NSCLC (43). Similarly, SWI/SNF chromatin
remodeling gene alterations were positively associated with
objective responses in immune checkpoint inhibitor-treated advanced
pancreatic cancer in the presence of low PD-L1 expression (44). Based on the encouraging results,
combining FXR with PD-L1 IHC testing could be considered to
identify NSCLC subsets with high likelihood of deriving benefit
from immune-related therapies.
Finally, the underlying mechanisms of
chemo-immunotherapy responsiveness in FXR high-expression patients
with NSCLC in the perspective of the TME was investigated. It is
well-known that the tumor-infiltrating CD8+ T cells
represent the most crucial tumoricidal effector cells, as well as
the main target of the PD-L1/PD-1 checkpoint pathway (34,35). In accordance with a previous study
(28), a statistically
significant decrease of infiltrating CD8+ T cells in the
more responding FXRhigh NSCLC was observed. There was a
significantly inverse correlation between FXR and CD8 expression in
NSCLC specimens, suggesting that the restrained tumor-infiltrating
CD8+ T cells, rather than the fully activated ones, are
more readily to be rescued by anti-PD-1 in FXE high-expression
tumors. This theory is supported by a previous study, which
revealed that objective response to PD-1/PD-L1 blockade mainly
occurs in tumors with adaptive immune-resistant infiltrating T
cells (45). The present study
cannot exclude the possibility that other immune cell populations
also contribute to the increased responsiveness to anti-PD-1-based
chemo-immunotherapy in FXR high-expression NSCLC. Future studies
are required to elucidate the interplay between tumoral FXR
expression and other tumor-infiltrating immune cells in the
TME.
There are certain limitations in the present study.
First, this is a retrospective, single-center study, and the sample
size is relatively small to perform an elaborate statistical
analysis. Large-scale prospective multi-center studies could be
helpful to validate the present findings. Secondly, the OS data
were slightly immature since the majority of patients did not reach
the primary endpoint of death, which may limit the prognostic value
of OS. Third, the molecular basis by which FXR suppresses
tumor-infiltrating CD8+ T cells in NSCLC remains to be
further explored. In FXR high-expression patients with NSCLC, the
majority (58/78, 74.4%) were classified as
FXRhighPD-L1low, while the minority (20/78,
25.6%) were classified as FXRhighPD-L1high,
which can be explained by the fact that FXR suppresses PD-L1
expression in NSCLC (28).
However, in FXR low-expression patients with NSCLC,
FXRlowPD-L1low accounted for 52.1% (37/71)
and FXRlowPD-L1high accounted for 47.9%
(34/71), which indicated that the expression of PD-L1 may be
regulated by other factors in FXR low-expression NSCLC, beyond the
scope of this manuscript. Despite these limitations, this
represents the first study investigating the predictive value of
FXR expression in cancer immunotherapy.
In conclusion, it was reported that high FXR and
PD-L1 expression levels were associated with higher ORR in patients
with NSCLC. It is noteworthy that FXR was inversely correlated with
PD-L1 expression in specimens with NSCLC, and that
FXRhighPD-L1low phenotype was associated with
a higher ORR, as well as longer PFS and OS among the
PD-L1low group. Mechanistic insights revealing that the
infiltrating CD8+ T cells were significantly decreased
in FXRhigh NSCLC tumors were also provided. The present
study recommended the FXRhighPD-L1low
signature as a promising predictor of response to anti-PD-1-based
chemo-immunotherapy in PD-L1low/negative NSCLC,
providing clinical evidence for the development of complementary
biomarkers for immune-related therapies.
Supplementary Data
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
WY and SJ designed and conceived the study. LW and
XX collated and analyzed the clinicopathological data and wrote the
manuscript. BS and JS performed the IHC experiments. BL and XW
performed the statistical analysis and data interpretation. WY and
SJ reviewed and revised the manuscript and confirmed the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The study protocol was approved (approval no.
NSFC-2019-05) by the Ethics Committee of Shandong Provincial
Hospital affiliated to Shandong First Medical University (Jinan,
China) and complied with the Helsinki declaration and the approved
guidelines of our institution. Written informed consent was
obtained from all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgments
The authors would like to thank Dr Jiawen Xu and Dr
Zhenhui Su at Shandong Provincial Hospital (Jinan, China) for
evaluating the IHC staining.
Funding
The present study was supported in part by the National Natural
Science Foundation of China (grant no. 81902325), the Shandong
Provincial Natural Science Foundation (grant nos. ZR2020MH005 and
ZR2021ZD35) and the Jinan Science and Technology Plan Project
(grant no. 202019201).
Abbreviations:
|
NSCLC
|
non-small cell lung cancer
|
|
PD-L1
|
programmed death-ligand 1
|
|
PD-1
|
programmed death-1
|
|
IHC
|
immunohistochemistry
|
|
TMB
|
tumor mutational burden
|
|
TME
|
tumor microenvironment
|
|
FXR
|
farnesoid X receptor
|
|
BA
|
bile acid
|
|
LLC
|
Lewis lung carcinoma
|
|
RECIST 1.1
|
response evaluation criteria in solid
tumors version 1.1
|
|
PFS
|
progression-free survival
|
|
OS
|
overall survival
|
|
FFPE
|
formalin-fixed paraffin-embedded
|
|
S-W
|
Shapiro-Wilk
|
|
K-W
|
Kruskal-Wallis
|
|
ORR
|
objective response rate
|
|
HR
|
hazard ratio
|
|
CI
|
confidence interval
|
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Duma N, Santana-Davila R and Molina JR:
Non-small cell lung cancer: Epidemiology, screening, diagnosis, and
treatment. Mayo Clin Proc. 94:1623–1640. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Garon EB, Rizvi NA, Hui R, Leighl N,
Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L,
et al: Pembrolizumab for the treatment of non-small-cell lung
cancer. New Engl J Med. 372:2018–2028. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gandhi L, Rodríguez-Abreu D, Gadgeel S,
Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ,
Powell SF, et al: Pembrolizumab plus chemotherapy in metastatic
non-small-cell lung cancer. New Engl J Med. 378:2078–2092. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brahmer J, Reckamp KL, Baas P, Crinò L,
Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE,
Holgado E, et al: Nivolumab versus docetaxel in advanced
squamous-cell non-small-cell lung cancer. New Engl J Med.
373:123–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus docetaxel in advanced nonsquamous non-small-cell
lung cancer. New Engl J Med. 373:1627–1639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qin S, Xu L, Yi M, Yu S, Wu K and Luo S:
Novel immune checkpoint targets: Moving beyond PD-1 and CTLA-4. Mol
Cancer. 18:1552019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Postow MA, Sidlow R and Hellmann MD:
Immune-related adverse events associated with immune checkpoint
blockade. New Engl J Med. 378:158–168. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Postow MA, Callahan MK and Wolchok JD:
Immune checkpoint blockade in cancer therapy. J Clin Oncol.
33:1974–1982. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shukuya T and Carbone DP: Predictive
markers for the efficacy of anti-PD-1/PD-L1 antibodies in lung
cancer. J Thorac Oncol. 11:976–988. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Reck M, Rodríguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: Pembrolizumab versus chemotherapy for PD-L1-positive
non-small-cell lung cancer. New Engl J Med. 375:1823–1833. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Herbst RS, Giaccone G, de Marinis F,
Reinmuth N, Vergnenegre A, Barrios CH, Morise M, Felip E, Andric Z,
Geater S, et al: Atezolizumab for first-line treatment of
PD-L1-selected patients with NSCLC. New Engl J Med. 383:1328–1339.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rizvi H, Sanchez-Vega F, La K, Chatila W,
Jonsson P, Halpenny D, Plodkowski A, Long N, Sauter JL, Rekhtman N,
et al: Molecular determinants of response to anti-programmed cell
death (PD)-1 and anti-programmed death-ligand 1 (PD-L1) blockade in
patients with non-small-cell lung cancer profiled with targeted
next-generation sequencing. J Clin Oncol. 36:633–641. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shirasawa M, Yoshida T, Shimoda Y,
Takayanagi D, Shiraishi K, Kubo T, Mitani S, Matsumoto Y, Masuda K,
Shinno Y, et al: Differential immune-related microenvironment
determines programmed cell death protein-1/programmed death-ligand
1 blockade efficacy in patients with advanced NSCLC. J Thorac
Oncol. 16:2078–2090. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mansfield AS, Murphy SJ, Peikert T, Yi ES,
Vasmatzis G, Wigle DA and Aubry MC: Heterogeneity of programmed
cell death ligand 1 expression in multifocal lung cancer. Clin
Cancer Res. 22:2177–2182. 2016. View Article : Google Scholar
|
|
17
|
Sacher AG and Gandhi L: Biomarkers for the
clinical use of PD-1/PD-L1 inhibitors in non-small-cell lung
cancer: A review. JAMA Oncol. 2:1217–1222. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Havel JJ, Chowell D and Chan TA: The
evolving landscape of biomarkers for checkpoint inhibitor
immunotherapy. Nat Rev Cancer. 19:133–150. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Daud AI, Wolchok JD, Robert C, Hwu WJ,
Weber JS, Ribas A, Hodi FS, Joshua AM, Kefford R, Hersey P, et al:
Programmed death-ligand 1 expression and response to the
anti-programmed death 1 antibody pembrolizumab in melanoma. J Clin
Oncol. 34:4102–4109. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Forman BM, Goode E, Chen J, Oro AE,
Bradley DJ, Perlmann T, Noonan DJ, Burka LT, McMorris T, Lamph WW,
et al: Identification of a nuclear receptor that is activated by
farnesol metabolites. Cell. 81:687–693. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu TT, Makishima M, Repa JJ, Schoonjans K,
Kerr TA, Auwerx J and Mangelsdorf DJ: Molecular basis for feedback
regulation of bile acid synthesis by nuclear receptors. Mol Cell.
6:507–515. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang YD, Chen WD, Moore DD and Huang W:
FXR: A metabolic regulator and cell protector. Cell Res.
18:1087–1095. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang F, Huang X, Yi T, Yen Y, Moore DD and
Huang W: Spontaneous development of liver tumors in the absence of
the bile acid receptor farnesoid X receptor. Cancer Res.
67:863–867. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
De Gottardi A, Touri F, Maurer CA, Perez
A, Maurhofer O, Ventre G, Bentzen CL, Niesor EJ and Dufour JF: The
bile acid nuclear receptor FXR and the bile acid binding protein
IBABP are differently expressed in colon cancer. Dig Dis Sci.
49:982–989. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guan B, Li H, Yang Z, Hoque A and Xu X:
Inhibition of farnesoid X receptor controls esophageal cancer cell
growth in vitro and in nude mouse xenografts. Cancer.
119:1321–1329. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Journe F, Durbecq V, Chaboteaux C, Rouas
G, Laurent G, Nonclercq D, Sotiriou C, Body JJ and Larsimont D:
Association between farnesoid X receptor expression and cell
proliferation in estrogen receptor-positive luminal-like breast
cancer from postmenopausal patients. Breast Cancer Res Treat.
115:523–535. 2009. View Article : Google Scholar
|
|
27
|
You W, Chen B, Liu X, Xue S, Qin H and
Jiang H: Farnesoid X receptor, a novel proto-oncogene in non-small
cell lung cancer, promotes tumor growth via directly
transactivating CCND1. Sci Rep. 7:5912017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
You W, Li L, Sun D, Liu X, Xia Z, Xue S,
Chen B, Qin H, Ai J and Jiang H: Farnesoid X receptor constructs an
immunosuppressive microenvironment and sensitizes
FXRhighPD-L1low NSCLC to anti-PD-1
immunotherapy. Cancer Immunol Res. 7:990–1000. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar
|
|
30
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern cooperative oncology group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cortellini A, Ricciuti B, Facchinetti F,
Alessi JVM, Venkatraman D, Dall'Olio FG, Cravero P, Vaz VR,
Ottaviani D, Majem M, et al: Antibiotic-exposed patients with
non-small-cell lung cancer preserve efficacy outcomes following
first-line chemo-immunotherapy. Ann Oncol. 32:1391–1399. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lantuejoul S, Sound-Tsao M, Cooper WA,
Girard N, Hirsch FR, Roden AC, Lopez-Rios F, Jain D, Chou TY, Motoi
N, et al: PD-L1 testing for lung cancer in 2019: Perspective from
the IASLC pathology committee. J Thorac Oncol. 15:499–519. 2020.
View Article : Google Scholar
|
|
33
|
Nakayama Y, Mimura K, Tamaki T, Shiraishi
K, Kua LF, Koh V, Ohmori M, Kimura A, Inoue S, Okayama H, et al:
Phospho-STAT1 expression as a potential biomarker for
anti-PD-1/anti-PD-L1 immunotherapy for breast cancer. Int J Oncol.
54:2030–2038. 2019.PubMed/NCBI
|
|
34
|
Weigelin B, Krause M and Friedl P:
Cytotoxic T lymphocyte migration and effector function in the tumor
microenvironment. Immunol Lett. 138:19–21. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Blank C and Mackensen A: Contribution of
the PD-L1/PD-1 pathway to T-cell exhaustion: An update on
implications for chronic infections and tumor evasion. Cancer
Immunol Immunother. 56:739–745. 2007. View Article : Google Scholar
|
|
36
|
Lee JY, Lee KT, Lee JK, Lee KH, Jang KT,
Heo JS, Choi SH, Kim Y and Rhee JC: Farnesoid X receptor,
overexpressed in pancreatic cancer with lymph node metastasis
promotes cell migration and invasion. Br J Cancer. 104:1027–1037.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Das A, Yaqoob U, Mehta D and Shah VH: FXR
promotes endothelial cell motility through coordinated regulation
of FAK and MMP-9. Arterioscler Thromb Vasc Biol. 29:562–570. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang W, Zhan M, Li Q, Chen W, Chu H, Huang
Q, Hou Z, Man M and Wang J: FXR agonists enhance the sensitivity of
biliary tract cancer cells to cisplatin via SHP dependent
inhibition of Bcl-xL expression. Oncotarget. 7:34617–34629. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Guo J, Zheng J, Mu M, Chen Z, Xu Z, Zhao
C, Yang K, Qin X, Sun X and Yu J: GW4064 enhances the
chemosensitivity of colorectal cancer to oxaliplatin by inducing
pyroptosis. Biochem Biophys Res Commun. 548:60–66. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fusi A, Festino L, Botti G, Masucci G,
Melero I, Lorigan P and Ascierto PA: PD-L1 expression as a
potential predictive biomarker. Lancet Oncol. 16:1285–1287. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Carbone DP, Reck M, Paz-Ares L, Creelan B,
Horn L, Steins M, Felip E, van den Heuvel MM, Ciuleanu TE, Badin F,
et al: First-line nivolumab in stage IV or recurrent non-small-cell
lung cancer. New Engl J Med. 376:2415–2426. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Herbst RS, Soria JC, Kowanetz M, Fine GD,
Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger
SN, et al: Predictive correlates of response to the anti-PD-L1
antibody MPDL3280A in cancer patients. Nature. 515:563–567. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kang DH, Park CK, Chung C, Oh IJ, Kim YC,
Park D, Kim J, Kwon GC, Kwon I, Sun P, et al: Baseline serum
interleukin-6 levels predict the response of patients with advanced
non-small cell lung cancer to PD-1/PD-L1 inhibitors. Immune Netw.
20:e272020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Botta GP, Kato S, Patel H, Fanta P, Lee S,
Okamura R and Kurzrock R: SWI/SNF complex alterations as a
biomarker of immunotherapy efficacy in pancreatic cancer. JCI
Insight. 6:e1504532021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ayers M, Lunceford J, Nebozhyn M, Murphy
E, Loboda A, Kaufman DR, Albright A, Cheng JD, Kang SP, Shankaran
V, et al: IFN-γ-related mRNA profile predicts clinical response to
PD-1 blockade. J Clin Invest. 127:2930–2940. 2017. View Article : Google Scholar : PubMed/NCBI
|