Introduction
The main feature of malignant disease is metastasis,
the ability of cancer cells to spread from a primary site to form
tumors at distant sites in the body. However, even when certain
non-cancerous tumors appear to be benign, a number exhibit
low-grade clinically malignant behavior and present as rare
manifestations. For example, uterine leiomyoma, the most common
type of uterine tumor, is known as a benign tumor. However, an
unusual growth pattern is evident at presentation in a small
proportion of uterine leiomyomas, which are subsequently termed
benign metastasizing leiomyomas (BMLs). BML is a poorly-defined
clinicopathological condition that features histologically benign
metastatic smooth muscle tumors (1)
characterized by the proliferation of, usually multiple, smooth
muscle nodules. BML is a rare cause of pulmonary nodules that occur
upon the metastasis of uterine leiomyomas to the lung (2). As the majority of tumors are
asymptomatic, they are therefore identified incidentally on routine
chest X-rays, however, certain of these tumors induce coughing,
hemoptysis, dyspnea and decreased pulmonary function (3,4). Although
~100 cases have been reported in the literature (5), the incidence, pathogenesis and treatment
remain ambiguous. Due to the low morbidity and scarcity of reports
on this condition, there is no consensus on which methods should be
used to treat this disease. The current study reports a case of
pulmonary BML, with a history of hysterectomy due to uterine
leiomyoma, and presents a brief review with regard to the diagnosis
and treatment of this disease.
Case report
A 47-year-old female presented with multiple
bilateral lung nodules discovered on pre-operative imaging. Upon
admission, the patient was in a good general condition. The patient
had undergone a hysterectomy 9 years previously for leiomyoma of
the uterus, but had not previously suffered from pulmonary disease,
such as pulmonary tuberculosis or pneumonia. No chest symptoms,
including coughing, dyspnea, chest pain or tightness, were present.
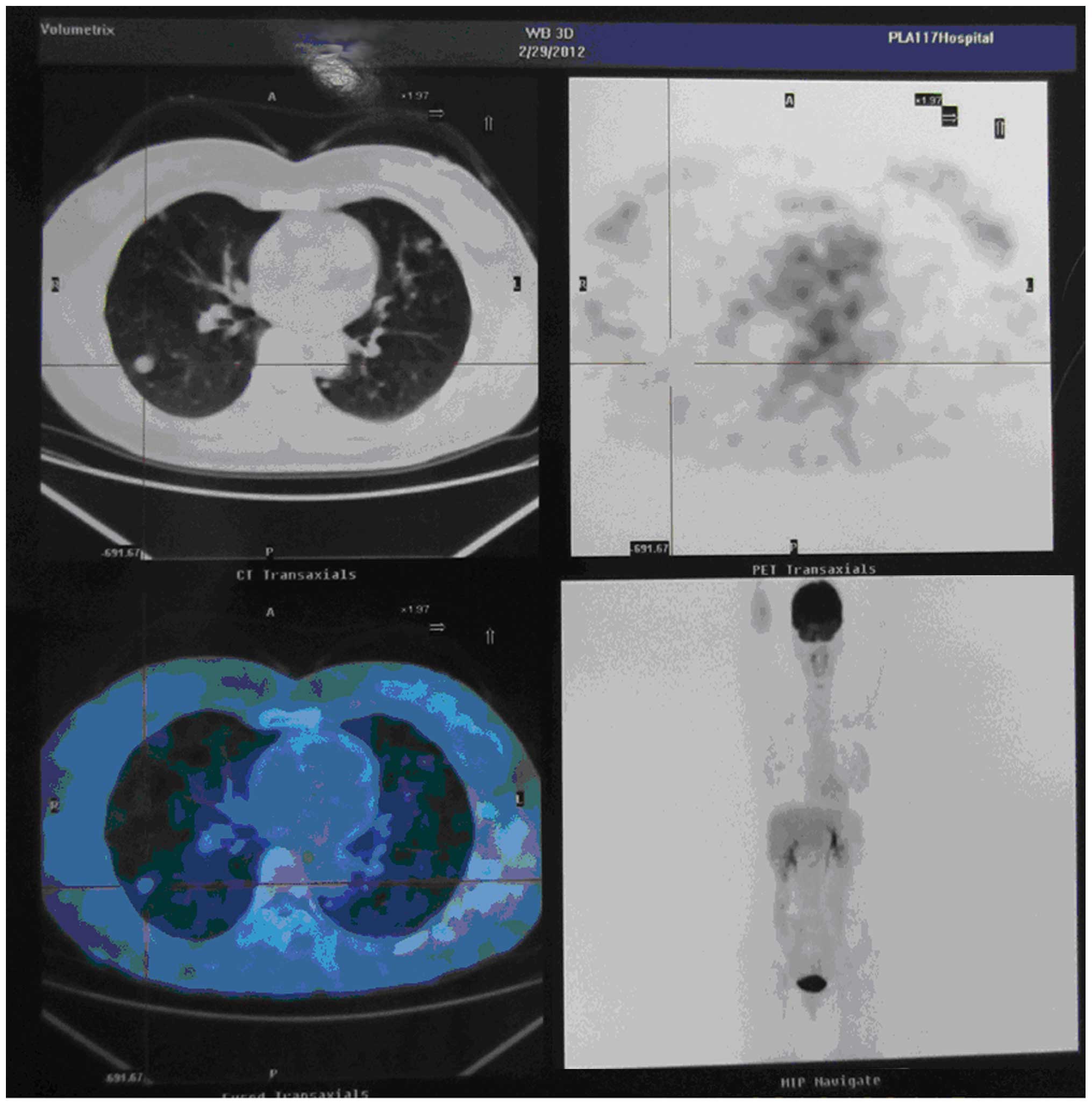
Chest tomography (CT) identified multiple well-defined nodular
shadows in the lungs (Fig. 1). These
lesions showed no avidity upon positron emission tomography
(Fig. 2). The patient was otherwise
in good health. The blood chemistry data were unremarkable, and the
carcinoembryonic antigen, neuron-specific enolase, squamous cell
carcinoma-related antigen and carbohydrate antigen 125 tumor marker
values were all within normal limits. BML, malignant metastatic
tumors or epithelioid hemangioendothelioma were suspected when
admitted to the Zhejiang Cancer Hospital (Hangzhou, Zhejiang,
China). The patient subsequently underwent video-assisted wedge
resection of the left upper lobe supported the suspected diagnosis.
The post-operative recovery was uneventful. The lesions excised
from the left upper lung lobe indicated low-grade spindle cell
proliferation consistent with BML (Fig.
3). The post-operative pathological report revealed epithelial
adenomatoid hyperplasia of the smooth muscle and the hyperplasia of
the alveolar epithelium, which was initially considered to be
leiomyomatous hamartoma and alveolar epithelial hyperplasia
(multicentric, with a 2-cm diameter for the larger nodule and a
0.2-cm diameter for the smaller nodule). The lesions were entirely
mesenchymal with entrapped epithelial elements. Histopathological
examination showed that the tumor consisted of well-differentiated
spindle-shaped cells, with low nuclear and cellular variance in
size and shape; there was no evident nuclear atypia and mitotic
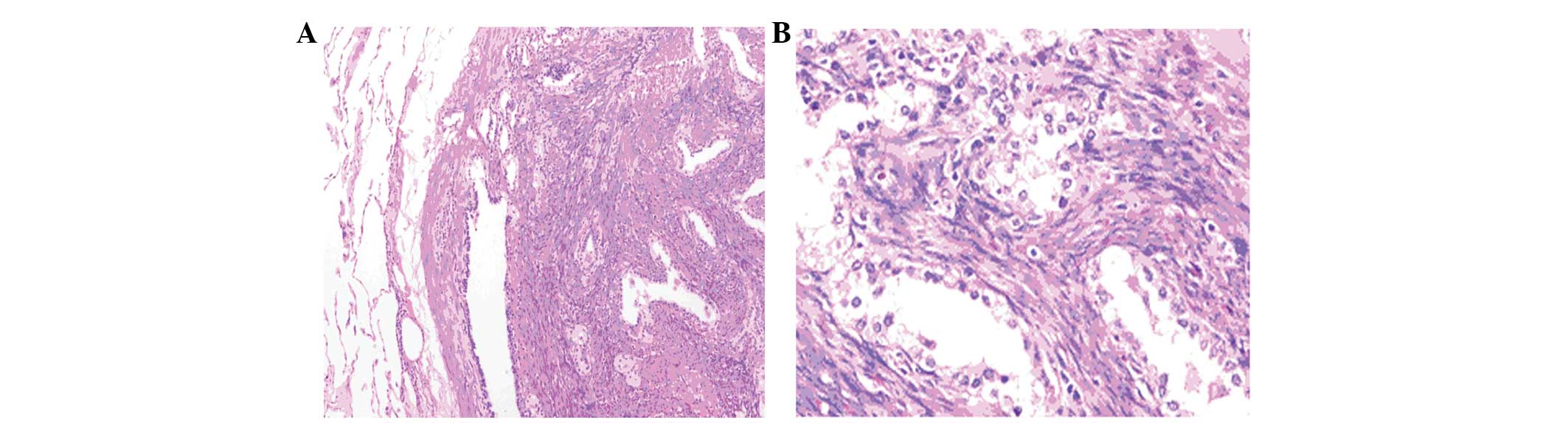
figures. Immunohistochemical studies revealed spindle cells
positive for actin (Fig. 4A), desmin
(Fig. 4B) and smooth muscle actin
(Fig. 4C). The entrapped epithelium
in the tissue was positive for cytokeratin 7 (Fig. 4D), surfactant protein A (Fig. 4E), and thyroid transcription factor 1
(Fig. 4F). The Ki-67 positivity rate
was low, at ~5% of the spindle cells. This profile supported the
light microscopic impression. In the pathological report, the
pathologist indicated that the disease of this patient may be BML,
and decided that a diagnosis could be formed by combining the
pathological report with the clinical data.
The patient followed an uneventful post-operative
course and was discharged on the third post-operative day. The
patient is currently being followed up on an outpatient basis
without any treatment. At present, more than two years after
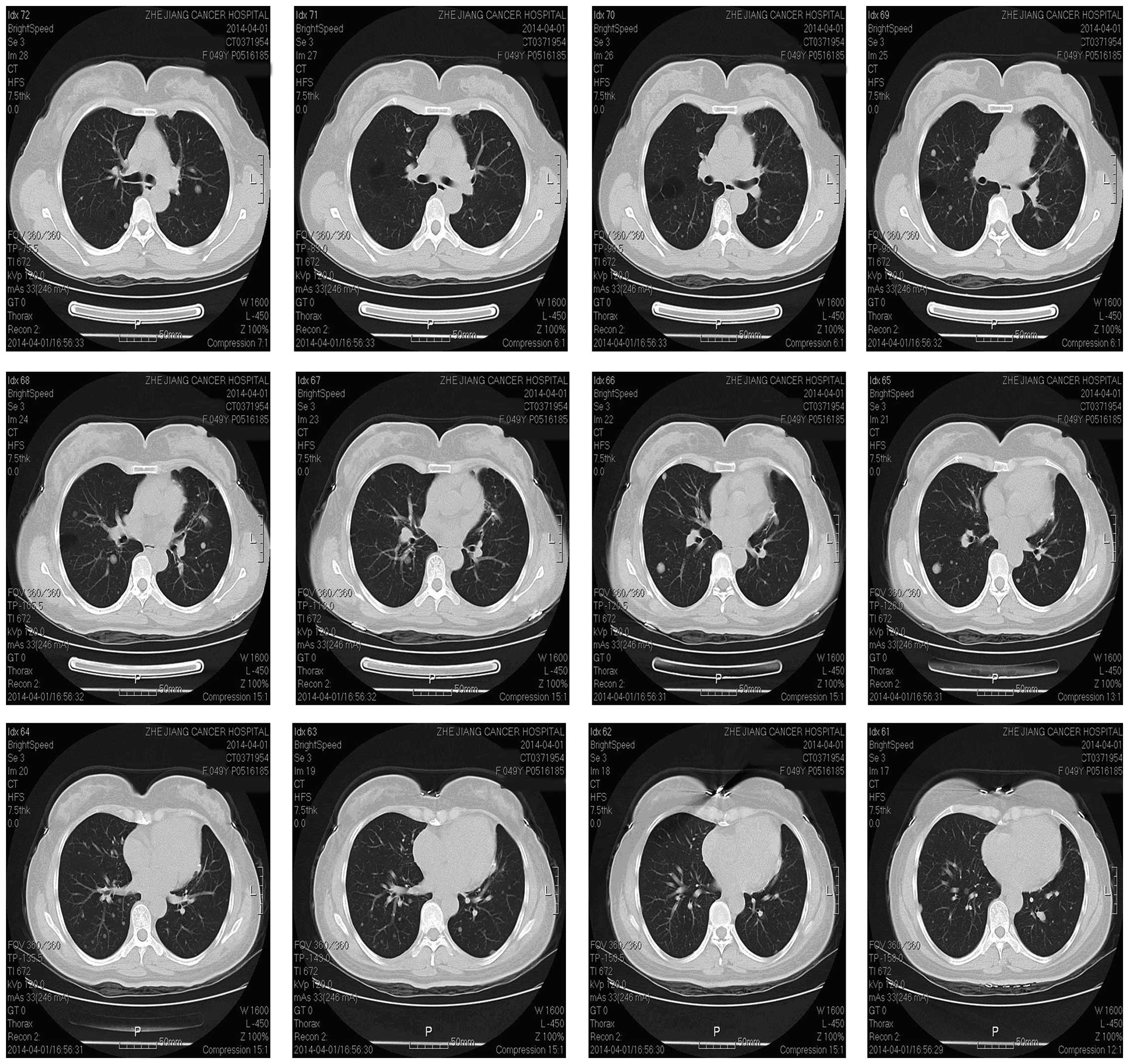
surgery, repeated CT (Fig. 5) scans
showed that the number and size of the lung nodules remain
unchanged.
Discussion
BML is a rare condition often occurring in
middle-aged women with a history of uterine leiomyomata. Although
the biological behavior of BML suggested malignancy, the tumor is
borderline, with benign histological features. A previous stuy
demonstrated that the lungs are the most common site of metastases
(6). Pulmonary BML may present as
asymptomatic pulmonary nodules diagnosed incidentally, be
synchronous with uterine leiomyomas or arise following
hysterectomy. Surgical palliation could be an option in highly
selected patients, but the results are debatable. Thoracic surgeons
should be aware of BML as a cause of multiple pulmonary
nodules.
Uterine leiomyoma is the most frequently occurring
form of uterine tumor and is typically considered to be benign,
with a favorable long-term prognosis. BML has been described as
incidental pulmonary nodules in women with a history of uterine
leiomyomas (7). The pathogenesis of
BML of the lung has not yet been completely identified. The disease
occurs predominantly in women of reproductive age (8), particularly during the premenopausal
period. There is lack of clinicopathological details and follow-up
data on these neoplasms, and the histological diagnosis is
challenging and usually problematic. The first case of pulmonary
BML was described in a study by Steiner (9) in 1939, which reported the case of a
36-year-old female who succumbed to cor pulmonale arising secondary
to multiple pulmonary metastases from benign uterine leiomyomas. In
1983, Martin (10) classified
leiomyomatous lesions of the lung into three large groups:
Leiomyomatosis in women, metastatic leiomyoma in men/children, and
multiple pulmonary fibroleiomyomatous hamartomas occurring in any
subjects. To date, the youngest reported patient with pulmonary BML
has been 23 years old (3).
Occasionally, the pulmonary lesions can be found at the same time
as the uterine leiomyoma (11). The
mean duration between hysterectomy and the appearance of lung
lesions is ~15 years (12). The
majority of reported cases occur in women of reproductive age
following hysterectomy or surgery for uterine leiomyomas. The
natural history of pulmonary BML remains uncertain (13). Although the disease has benign
histological features and often presents with indolent features,
its metastatic behavior suggests its malignant potential. It has
been suggested that pulmonary BML represents a low-grade,
slow-growing leiomyosarcoma (14,15). Giove
et al (16) reported a case of
BML in a 55-year-old female who remained alive with lung, lymph
node, skin, bone and possible brain metastases 14 years after the
first uterine myomectomy. Jautzke et al (17) reviewed 74 cases of BML and found the
lungs to be the most common site of involvement, similar to the
study by Rivera et al, which found pulmonary lesions in 20
out of 33 cases (8). Although the
lungs are the most common sites for metastasis, extrapulmonary
lesions have been documented in the lymph nodes, deep soft tissues,
omentum and mesentery, bone, spine, skull base and heart (5). The majority of tumors are asymptomatic
and are found incidentally on routine chest roentgenograms,
however, coughing, dyspnea and chest pain have also been reported
as symptoms (18). Recently, Miyazaki
et al (19) presented a case
of massive hemoptysis from pulmonary BML, and transarterial
embolization was attempted in the treatment as the thoracic surgeon
expected that resection of the tumor may be difficult due to the
tumor location. The majority of reports of BML describe a chronic,
benign, indolent course, but Bachman and Wolff reported a case of
mortality due to of acute respiratory distress syndrome from
multiple lesions with massive pulmonary and hilar lymphatic
metastasis (20).
Pathological features of pulmonary BML are often of
a benign nature, as observed in the present case. Absence of high
cellularity coagulative tumor cell necrosis, cytological atypia and
increased mitosis (﹥5 per 10 high-powered fields) with a low Ki-67
index support the low proliferative state and benign nature of
these tumors (5). Interlacing
fascicles of smooth muscle cells lacking anaplasia or vascular
invasion, with entrapped respiratory epithelium are revealed upon
histological examination. A range of immunohistochemical markers,
including desmin and muscle-specific actin, are present to confirm
the mesenchymal derivation of these tumors with smooth muscle
differentiation. In addition, the presence of estrogen and
progesterone receptors supports the derivation of BML from the
uterus (21,22), which reinforces the use of treatment
with hormonal agents. Radiographically, BML presents as solitary or
multiple lesions scattered within the normal interstitium; these
well-circumscribed nodules range from a few millimeters to a few
centimeters in size. Intravenous contrast medium does not enhance
the nodules. Endobronchial and pleural sparing is also
characteristic of BML. Rare cases have been reported with a miliary
pattern (23), cavitary lung nodules,
interstitial lung disease and multiloculated fluid-containing cystic
lesions (24). The present patient
was finally diagnosed with pulmonary BML by combining the
pathological report with the clinical data.
No standard management guidelines have been
formulated with regard to the treatment of BML. Careful
observation, surgical resection, hysterectomy and bilateral
oophorectomy, administration of progestins and aromatase
inhibitors, and medical castration using luteinizing
hormone-releasing hormone analogs have all been reported as
potential treatment modalities (25).
Lesions that increase in size may require surgical resection to
prevent potentially fatal complications such as massive hemoptysis.
However, the effect of reducing the tumor burden through surgical
palliation should be carefully evaluated. Smaller subcentimeter
lesions can be followed with surveillance scans. The presence of
estrogen and progesterone receptors makes these tumors susceptible
to hormonal manipulation by surgical or medical castration.
Hormonal relative treatment is a commonly chosen therapy for BML
when estrogen and progesterone receptors are identified on tumor
histology (7,18). Certain patients have been shown to be
sensitive to treatment with progestin, goserelin, ovarian ablation
and oophorectomy. This suggests that estrogen and progesterone may
play a significant role in the pathogenesis of BML. Bilateral
oophorectomy or medical reversible castration with luteinizing
hormone-releasing hormone analogs control gonadal hormone secretion
(1,26), which may control the growth of already
established lesions. However, hormone therapy does not generate a
response in all patients, and the side-effects of flushes, fatigue
and nausea can be aggravating to the patient (3). In a previous study, even in the presence
of positive estrogen and progesterone receptors on smooth muscle
cells, no significant change was noted in the size of the BML
lesions following 6–12 months of treatment with tamoxifen,
progesterone and an aromatase inhibitor (7). A combination of medical and surgical
treatment would exert a synergistic effect and should be considered
in the management of progressive and symptomatic lesions. One
previous study proposed that BML may naturally decrease following
the menopause (27). The majority of
BML lesions remain at one size, however, a small percentage display
an aggressive course (28). It is
currently unclear why the tumor progress in BML. As the majority of
lesions stain positive for estrogen and progesterone receptors, and
as the present patient refused hormone therapy, the estrogen and
progesterone receptors were not detected in the histological
chemistry examination. Therefore, a wait-and-see strategy was
decided upon for the patient.
In the current study, the case of a female with
pulmonary BML arising after hysterectomy, who was followed up after
surgery, was reviewed. Although a rare disease, BML should be
considered by physicians for asymptomatic women of reproductive age
with a history of uterine leiomyoma, who present with solitary or
multiple pulmonary nodules. A standard strategy remains to be
established for the treatment of this disease, but as the clinical
course of BML varies among cases, an individual treatment approach
should be considered. Thoracic surgeons should be aware of this
unusual cause of multiple pulmonary nodules.
References
|
1
|
Egberts JH, Schafmayer C, Bauerschlag DO,
Jänig U and Tepel J: Benign abdominal and pulmonary metastasizing
leiomyoma of the uterus. Arch Gynecol Obstet. 274:319–322. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Banner AS, Carrington CB, Emory WB, et al:
Efficacy of oophorectomy in lymphangioleiomyomatosis and benign
metastasizing leiomyoma. N Engl J Med. 305:204–209. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Goyle KK, Moore DF Jr, Garrett C and Goyle
V: Benign metastasizing leiomyomatosis: Case report and review. Am
J Clin Oncol. 26:473–476. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Säynäjäkängas O, Maiche AG and Liakka KA:
Multiple progressive pulmonary leiomyomatous metastases treated
with tamoxifen - a case report with a review of the literature.
Acta Oncol. 43:113–114. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rege AS, Snyder JA and Scott WJ: Benign
metastasizing leiomyoma: A rare cause of multiple pulmonary
nodules. Ann Thorac Surg. 93:e149–e151. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen S, Zhang Y, Zhang J, et al: Pulmonary
benign metastasizing leiomyoma from uterine leiomyoma. World J Surg
Oncol. 11:1632013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Abramson S, Gilkeson RC, Goldstein JD,
Woodard PK, Eisenberg R and Abramson N: Benign metastasizing
leiomyoma: Clinical, imaging and pathologic correlation. AJR Am J
Roentgenol. 176:1409–1413. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rivera JA, Christopoulos S, Small D and
Trifiro M: Hormonal manipulation of benign metastasizing
leiomyomas: Report of two cases and review of the literature. J
Clin Endocrinol Metab. 89:3183–3188. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Steiner PE: Metastasizing fibroleiomyoma
of the uterus: Report of a case and review of the literature. Am J
Pathol. 15:89–110. 1939.PubMed/NCBI
|
|
10
|
Martin E: Leiomyomatous lung lesions: A
proposed classification. AJR Am J Roentgenol. 141:269–272. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moon H, Park SJ, Lee HB, et al: Pulmonary
benign metastasizing leiomyoma in a postmenopausal woman. Am J Med
Sci. 338:72–74. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kayser K, Zink S, Schneider T, et al:
Benign metastasizing leiomyoma of the uterus: Documentation of
clinical, immunohistochemical and lectin-histochemical data of ten
cases. Virchows Arch. 437:284–292. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ng JS, Han A, Chew SH and Low J: A
clinicopathologic study of uterine smooth muscle tumours of
uncertain malignant potential (STUMP). Ann Acad Med Singapore.
39:625–628. 2010.PubMed/NCBI
|
|
14
|
Burkhardt A, Otto HF and Kaukel E:
Multiple pulmonary (hamartomatous?) leiomyomas. Light and electron
microscopic study. Virchows Arch A Pathol Anat Histol. 394:133–141.
1981. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cohen DT, Oliva E, Hahn PF, Fuller AF Jr
and Lee SI: Uterine smooth-muscle tumors with unusual growth
patterns: Imaging with pathologic correlation. AJR Am J Roentgenol.
188:246–255. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Giove S, Scappaticci E, Baldi S, Ricci C
and Minetto E: Benign metastasizing leiomyoma of the uterus. Case
report. Minerva Med. 75:1819–1821. 1984.(In Italian). PubMed/NCBI
|
|
17
|
Jautzke G, Muller-Ruchholtz E and Thalmann
U: Immunohistological detection of estrogen and progesterone
receptors in multiple and well differentiated leiomyomatous lung
tumors in women with uterine leiomyomas (so-called benign
metastasizing leiomyomas). A report on 5 cases. Pathol Res Pract.
192:215–223. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ogawa M, Hara M, Ozawa Y, et al: Benign
metastasizing leiomyoma of the lung with malignant transformation
mimicking mediastinal tumor. Clin Imaging. 35:401–404. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Miyazaki M, Nakayama A, Noda D, Maehara Y
and Tsushima Y: Difficulty in complete transarterial embolization
for pulmonary benign metastasizing leiomyoma with massive
hemoptysis. Jpn J Radiol. 32:53–57. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bachman D and Wolff M: Pulmonary
metastases from benign-appearing smooth muscle tumors of the
uterus. AJR Am J Roentgenol. 127:441–446. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rao UN, Finkelstein SD and Jones MW:
Comparative immunohistochemical and molecular analysis of uterine
and extrauterine leiomyosarcomas. Mod Pathol. 12:1001–1009.
1999.PubMed/NCBI
|
|
22
|
McGinley KM, Bryant S, Kattine AA,
Fitzgibbon JF and Googe PB: Cutaneous leiomyomas lack estrogen and
progesterone receptor immunoreactivity. J Cutan Pathol. 24:241–245.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lipton JH, Fong TC and Burgess KR: Miliary
pattern as presentation of leiomyomatosis of the lung. Chest.
91:781–782. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Osadchy A, Zehavi T and Zissin R:
Pulmonary benign metastasising leiomyomas presenting as
fluid-containing masses on CT in a patient with two unrelated
malignancies. Br J Radiol. 78:639–641. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yoon G, Kim TJ, Sung CO, et al: Benign
metastasizing leiomyoma with multiple lymph node metastasis: A case
report. Cancer Res Treat. 43:131–133. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Arif S, Ganesan R and Spooner D:
Intravascular leiomyomatosis and benign metastasizing leiomyoma: An
unusual case. Int J Gynecol Cancer. 16:1448–1450. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Arai T, Yasuda Y, Takaya T and Shibayama
M: Natural decrease of benign metastasizing leiomyoma. Chest.
117:921–922. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sapmaz F, Ergin M, Katrancioglu O,
Gonlugur T, Gonlugur U and Elagoz S: Benign metastasizing
leiomyoma. Lung. 186:271–273. 2008. View Article : Google Scholar : PubMed/NCBI
|