Introduction
Acute lymphoblastic leukemia (ALL) is a malignant
disorder of hematological progenitor cells that arises from the
dysregulated clonal expansion of immature lymphoid progenitor cells
that have encountered a series of catastrophic alternations within
key regulatory genes (1). Similar to
the majority of other cancers, the pathogenesis of ALL is complex,
which probably originates from the complicated interactions between
endogenous exposures, hereditary susceptibility and chance
(2).
The complexity of ALL has also been reflected in the
various clinical characteristics of the two genders, which
demonstrates a regional and ethnic variance (3–7). The
majority of previous studies on Chinese patients conveyed limited
data regarding gender difference (4,8–11). In addition, the patients in these
studies were from Northern China, such as Beijing (12), Eastern China, such as Shanghai
(5,6,9–11), and central China, such as Wuhan
(10). To the best of our knowledge,
only one study has reported the characteristics of ALL patients
between 1985 and 1994 in Southern China, in Hong Kong (3). Updated studies are also limited in
number (13–16).
The present study aimed to compare the clinical
characteristics of acute lymphoblastic leukemia (ALL) in male and
female patients from one institution in Southern China. In
addition, the current study aims to provide clinical staff with
additional information on the characteristics of ALL in China.
Materials and methods
Study design
The present study was designed as a retrospective
analysis for the comparison between the clinical characteristics of
ALL present in male and female patients at one institution in
Southern China.
Data sources and search strategy
The patient data were collected from the Departments
of Hematology and Pediatrics of Nanfang Hospital, affiliated to the
Southern Medical University of Guangzhou (Guangzhou, Guangdong,
China). The data search was performed using the medical electronic
records in the aforementioned two departments. The index term
‘acute lymphoblastic leukemia’ was used and a time limit between
January 1, 2001 and December 31, 2012 was set for the search.
Subsequent to the initial retrieval of the patient records, the
records were reviewed for their assessment eligibility.
Inclusion and exclusion criteria
Eligible patients were those with a definite
diagnosis of ALL made at the aforementioned two departments, and
with records that contained at least one aspect of the following
information: Patient gender; age at the time of first diagnosis;
month of the initial onset of ALL symptoms; ABO blood group;
immunophenotype; and the results of a Philadelphia (Ph) chromosome
test, if available. Patients were excluded from the present study
if ALL had been suspected, but final cell morphology and
immunophenotype tests resulted in a different final diagnosis. The
present study was approved by the Ethical Board of Nanfang
Hospital.
Analysis of data
The overall clinical data of ALL was analyzed to
identify the gender ratio, average age at the first diagnosis,
month of the initial onset of ALL symptoms, distribution of ABO
blood groups, positive ratio of Ph chromosome and immunophenotype
distribution.
Statistical analysis
Statistical analysis was performed using SPSS 13.0
software (SPSS, Inc., Chicago, IL, USA). Continuous variables were
expressed as the mean ± standard deviation. Dichotomous variables
were expressed as percentages. An independent sample t-test
was performed to evaluate the differences in continuous variables
between the two genders. The χ2 test was applied to
assess the differences in dichotomous variables. P≤0.05 was
considered to indicate a statistically significant difference.
Results
Patients
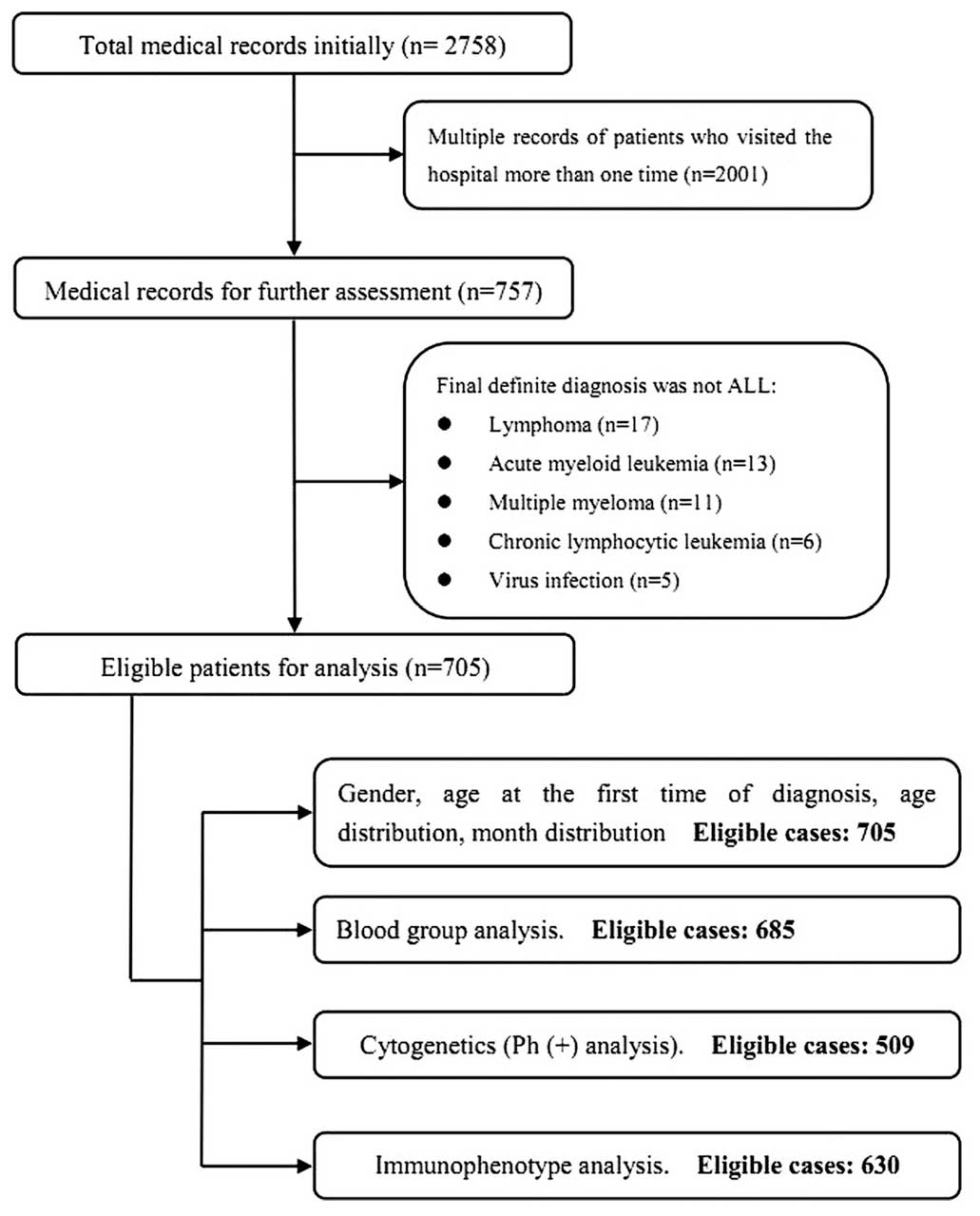
A total of 2,758 patients were initially identified.
Subsequent to reviewing the records, 705 eligible patients were
finally included for the present study. The identification and
inclusion process was illustrated in Fig.
1.
Gender ratio
The 705 eligable patients consisted of 457 males and
248 females, resulting in a male to female gender ratio of
1.84:1.
Average age at first diagnosis
As shown in Fig. 2,
363 patients (51.49%) were <15 years old when diagnosed with
ALL, with an incidence peak between 2 and 4 years of age. The
overall average age at first diagnosis was 17.52 years. The average
age at diagnosis was found to be significantly higher for female
patients compared with male patients (19.54 vs. 16.43 years;
P=0.007; Table I).
 | Table I.Clinical data of 705 patients with
acute lymphoblastic leukemia. |
Table I.
Clinical data of 705 patients with
acute lymphoblastic leukemia.
| Characteristics | Males, n (%) | Females, n (%) | Total, n (%) | P-value |
|---|
| Total | 457 (100.00) | 248 (100.00) | 705 (100.00) | – |
| Average age at
diagnosis, years | 16.43±13.84 | 19.54±15.12 | 17.52±14.37 | 0.007 |
| Seasonal
presentation |
|
|
|
|
|
Total | 457 (64.82) | 248 (35.18) | 705 (100.00) | – |
|
December-February | 120 (26.26) | 59 (23.79) | 179 (25.39) | 0.472 |
|
March-May | 114 (24.94) | 65 (26.21) | 179 (25.39) | 0.713 |
|
June-August | 118 (25.82) | 71 (28.63) | 189 (26.81) | 0.421 |
|
September-November | 105 (22.98) | 53 (21.37) | 158 (22.41) | 0.626 |
| Blood group
distribution |
|
|
|
|
|
Total | 445 (64.96) | 240 (35.04) | 685 (100.00) | – |
| A | 124 (27.86) | 65 (27.08) | 189 (27.59) | 0.827 |
| B | 108 (24.27) | 61 (25.42) | 169 (24.67) | 0.740 |
| O | 184 (41.35) | 97 (40.42) | 281 (41.02) | 0.813 |
| AB | 29 (6.52) | 17 (7.08) | 46 (6.72) | 0.777 |
| Cytogenetic
test |
|
|
|
|
|
Total | 326 (64.05) | 183 (35.95) | 509 (100.00) | – |
| Ph
chromosome(+) | 58 (17.79) | 32 (17.49) | 90 (17.68) | 0.931 |
| Ph
chromosome(–) | 268 (82.21) | 151 (82.51) | 419 (82.32) | – |
| Immunophenotype
distribution |
|
|
|
|
|
Total | 405 (64.29) | 225 (35.71) | 630 (100.00) | – |
| B
cell | 308 (76.05) | 182 (80.89) | 490 (77.78) | 0.162 |
| T
cell | 64 (15.80) | 21 (9.33) | 85 (13.49) | 0.023 |
|
Other | 33 (8.15) | 22 (9.78) | 55 (8.73) | 0.487 |
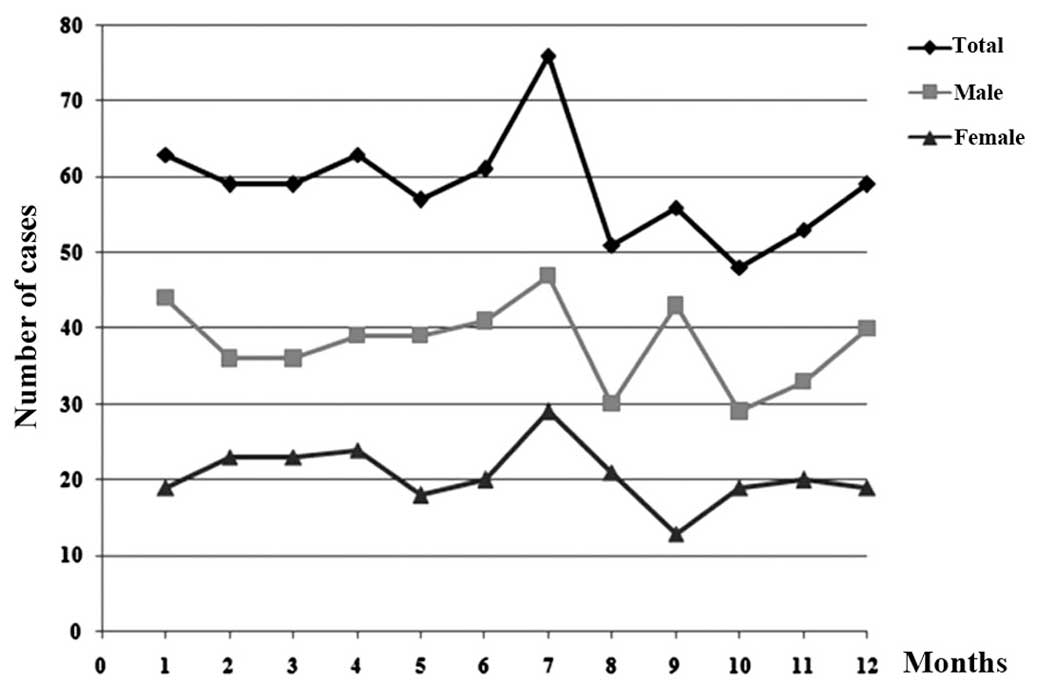
Distribution of the month of
presentation
The peak time for presentation with the initial
onset of ALL symptoms was July, for male and female patients
(Fig. 3). No significant difference
in seasonal distribution was observed between the two genders
(Table I).
Blood group distribution
A total of 685 patient records were suitable for
analysis of the blood group distribution. As listed in Table I, blood group O was the most frequent
blood type in ALL patients, not only overall (41.02%), but also in
male (41.35%) and female (40.42%) patients. However, no significant
difference was identified in the distribution of blood group
between the two genders (Table
I).
Immunophenotype distribution
Out of the 705 ALL patients, 630 patients had
medical records that were suitable for analysis of the
immunophenotype in the two genders. A total of 490 ALL patients
possessed B-cell ALL (77.78%), accounting for the largest
proportion of cases. No significant difference was identified in
the immunophenotype distribution, with the exception of a higher
percentage of T-cell ALL in male patients (15.80%) compared with
female patients (9.33%) (P=0.023).
Philadelphia (Ph) chromosome
A total of 509 medical records contained the outcome
of testing for the Ph chromosome. The present study found that a
total of 90 ALL patients possessed the Ph chromosome, giving an
overall Ph chromosome-positive rate of 17.68%. The Ph
chromosome-positive rates in males and females were 17.79 and
17.49%, respectively (P=0.931; Table
I).
Discussion
The findings of the present study based on 705 ALL
patients in Southern China indicated a male predominance of ALL and
a predilection of ALL to occur in children. Females appeared to be
older at the time of the first diagnosis compared with males. The
peak seasonal onset of ALL symptoms was in July. The O blood group
and B-cell immunophenotype were the most frequent in ALL. No
significant difference was identified in the distributions of
seasonality and blood type, incidence of the Ph chromosome or
immunophenotype distribution between the genders, but a
significantly higher proportion of T-cell ALL was identified in
male patients.
The present finding of the gender ratio indicated a
male predominance, which is consistent with previous studies of
Chinese ALL patients (3,4,8–11,17–21). As
shown in Table II, the gender ratio
of ALL patients from various areas of China ranged between 1.37
(20) and 1.92 (3), indicating that regional differences
exerted a minor influence on the gender predominance. With respect
to other Asian countries, the majority of studies obtained similar
outcomes of male predominance in ALL, including male to female
ratios of 1.88 in India (22), 1.49
in Indonesia (23), 1.70 in Jordan
(24) and 1.85 in Iran (25). However, Matsumura et al
(6) reported that the incidence of
ALL in Japan demonstrated a female predominance. The present study
considers that this difference may be associated with two factors.
The first one lies in the sample size of the study by Matsumura
et al (n=256), which may have affected the outcome. The
second factor is that the predominance may result from
environmental pollution, including nuclear radiation.
 | Table II.Previous studies of the
epidemiological characteristics of acute lymphoblastic leukemia in
Chinese patients. |
Table II.
Previous studies of the
epidemiological characteristics of acute lymphoblastic leukemia in
Chinese patients.
|
|
|
|
|
|
|
|
| Ph chromosome |
Immunophenotype |
|---|
|
|
|
|
|
|
|
|
|
|
|
|---|
| First author
(Ref.) | Study period | City | Patients, n | Males | Females | Ratio | Median age, years
(range) | Yes, n | No, n | Incidence, % | B-cell | T-cell |
|---|
| Chen et al
(8) | Nov 1989 - Dec
2010 | Shanghai | 1,346 | 812 | 534 | 1.52 | 10.0 (10
days-85.0) | 218 | 1,346 | 16.20 | 1,022 | 164 |
| Gao et al
(4) | Jan 2003 - Feb
2010 | Beijing | 1,004 | 606 | 398 | 1.52 | 5.0
(0.33–16.0) | 55 | 1,004 | 5.48 | 902 | 102 |
| Gu et al
(9) | Jan 1998 - Mar
2003 | Shanghai | 158 | 92 | 66 | 1.39 | NP | 7 | 133 | 5.26 | 120 | 28 |
| Li et al
(10) | Mar 2003 - Dec
2010 | Wuhan | 1,175 | 699 | 476 | 1.47 | 18.0
(0.2–85.0) | 211 | 1,175 | 17.96 | 828 | 170 |
| Mi et al
(11) | Nov 1989 - Dec
2010 | Shanghai | 1,091 | 657 | 434 | 1.51 | 10.0 (10
days-75.0) | 174 | 1,091 | 15.95 | 869 | 140 |
| Ma et al
(3) | Jan 1985 - Dec
1994 | Hong Kong | 73 | 48 | 25 | 1.92 | 4.3 (0.4–14.2) | NP | NP | NP | 52 | 10 |
| Guo et al
(17) | Oct 2003 - Jun
2006 | Tianjin | 400 | 255 | 145 | 1.76 | 6.0
(6.87±4.12)a | 7 | 154 | 4.54 | 218 | 34 |
| Li et al
(18) | Jul 1996 - Dec
2007 | Tianjin | 389 | 73 | 37 | 1.97 | 34.0
(15.0–59.0) | 110 | 389 | 28.28 | 90 | 15 |
| Tong et al
(19) | Jan 2007 - Jul
2010 | Shenyang | 207 | 126 | 81 | 1.56 | 4.0 (1.0–14.0) | 7 | 146 | 4.79 | 183 | 24 |
| Yang (20) | Oct 1994 - Dec
2008 | Fuzhou | 401 | 232 | 169 | 1.37 | 26.0
(14.0–78.0) | NP | NP | NP | 161 | 25 |
| Zeng et al
(21) | Apr 2004 - Apr
2010 | Beijing | 784 | 504 | 280 | 1.80 | NP | 47 | 631 | 7.45 | 562 | 57 |
| Ni et al
(5) | Jan 2002 - Dec
2006 | Shanghai | 544 | NP | NP | NP | 32.0
(1.2–89.0) | 30 | 253 | 11.86 | 242 | 59 |
| Present study | Jan 2001 - Dec
2012 | Guangzhou | 705 | 457 | 248 | 1.84 | 14.0
(0.5–73.0) | 90 | 509 | 17.68 | 490 | 85 |
The present study found more than one-half of the
patients in the present study were under 15 years of age,
particularly between 2 and 4 years of age, indicating that children
and specifically infants are a high-risk group for the development
of ALL. The present finding was in accordance with previous
conclusions that ~60% of patients in ALL were younger than 20 years
of age (26–28). In addition, it was also observed that
female patients were, on average, three years older than male
patients at the time of the first diagnosis.
The month distribution for the initial onset of ALL
symptoms found a peak presentation in July, overall and in each of
the genders (Fig. 3), indicating that
seasonality may affect the morbidity of ALL. However, controversy
remains over the effect of seasonal factors in the incidence,
diagnosis and treatment of ALL (7,24,29). Gao et al (12) performed an international survey in
Singapore, the USA and Sweden to provide evidence for the seasonal
diagnosis of ALL. It was concluded that little evidence exsisted
for a seasonal influence on ALL diagnosis. Kulkarni et al
(30) indicated in a retrospective
study that seasonality was not an independent prognostic factor and
did not demonstrate a significant association with survival in
univariate analysis. Therefore, future studies may focus on the
influence of seasonality on ALL. Although the peak onset of ALL
symptoms was in July, no significant differences in seasonal
presentation were identified between the two genders.
Previous studies have reported that the ABO blood
groups are closely associated with numerous tumors, including
ovarian, endometrial and cervical cancer (31), nasopharyngeal carcinoma (32) and pancreatic cancer (33). Therefore, the present study
investigated the association between the ABO blood groups and the
incidence of ALL in Chinese patients. The present results indicated
that the O blood group accounted for the highest proportion of
cases, not only overall (41.02%), but also individually in male
(41.35%) and female (40.42%) patients. The AB blood group accounted
for the lowest proportion of cases. The finding implies that O
blood group may lead to a increased risk of ALL. Considering that a
control group was not used in the present study, future studies
should be based on a case-control design. Although the proportions
of the ABO blood groups varied between the genders, no significant
difference was identified in the distribution of the blood types.
Disputes continue on the difference between the ABO blood group
distributions of the two genders, which may be in assoication with
various regions as well as different ethnicities. Jackson et
al (34) found that the
proportion of Malay male patients with the O blood group was
significantly higher compared with the proportion of female
patients, which was considered to explain the increased incidence
of acute leukemia in males. Similar to the present outcomes, Alavi
et al (35) reported that the
most frequent blood type of paediatric patients with ALL in Iran
was the O blood group, overall (56.5%) and in male (56.7%) and
female (54.4%) patients. There was also no statistical difference
in blood type distribution between the genders.
An increasing number of genetic aberrations, which
are considered to be predictive markers of prognoses, have been
detected in the treatment of ALL. One of the most frequently used
genetic aberrations is the Ph chromosome, also termed the BCR-ABL
fusion gene, which may indicate a poor prognosis (36,37). The
present investigation of the Ph chromosome based on 509 Chinese
patients found that the incidence of the Ph chromosome was 17.68%,
with no significant difference between the genders (P=0.931). The
incidence of the Ph chromosome in China varied between 4.54%
(17) and 28.28% (18) (Table
II). The present study hypothesizes that this wide range in the
incidence of the Ph chromosome may be due to the various study
objectives in the aforementioned studies. Studies that focused on
paediatric patients with ALL usually obtained a positive ratio
<10%, with the ratio mostly being ~5% (4,9,17,19,21).
However, studies involving adult or mixed-age patients tended to
find a positive ratio >10%, mostly >15% (5,8,10,11,18).
Therefore, adult ALL patients often demonstrated an increased
incidence of the Ph chromosome compared with the pediatric patients
with ALL. In the present study, the incidence of the Ph chromosome
in adult patients was 20.76% (71 out of 342 patients), which was
significantly higher than the incidence in paediatric patients
(5.23%) (P=0.000). This may be an explanation for the worse
prognosis of ALL in adult patients compared with paediatric
patients.
In addition, the present study found an increased
incidence of B-cell ALL (77.78%), which was consistent with
previously published studies performed in China. As can be observed
in Table II, all published studies
based on a Chinese population demonstrated an increased incidence
in the B-cell immunophenotype, accounting for an average of 86.27%
of cases (5739 of 6652 patients; range, 81.08–90.79%). The
immunophenotype of ALL may be another predictive factor of
prognosis, as T-cell ALL usually demonstrates a worse prognosis
compared with B-cell ALL (38). In
the present study, a total of 85 patients were identified as
possessing T-cell ALL, with a significantly higher percentage in
males (P=0.023). Considering the limited sample size, additional
studies should be performed.
There are two main limitations of the present study.
No control group was used in the current study, which may reduce
the reliability of the outcomes, particularly those with
significant gender differences. In addition, patients were not
sorted as children and adults, which may exert certain influences
on the conclusions. However, the present study continues to be
valuable, as it enriches the knowledge of the clinical
characteristics of ALL in China.
In summary, the present study provides medical
professionals with the clinical characteristics of ALL based on
patients in Southern China. The present study indicates a male
predominance and similar clinical characteristics of ALL between
the two genders in Southern China. Whether significant gender
differences reflected in the present study needs more clinical data
to certify.
References
|
1
|
Pui CH: Childhood leukemias. N Engl J Med.
332:1618–1630. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Inaba H, Greaves M and Mullighan CG: Acute
lymphoblastic leukaemia. Lancet. 381:1943–1955. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ma SK, Chan GC, Ha SY, Chiu DC, Lau YL and
Chan LC: Clinical presentation, hematologic features and treatment
outcome of childhood acute lymphoblastic leukemia: a review of 73
cases in Hong Kong. Hematol Oncol. 15:141–149. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gao F, Nordin P, Krantz I, Chia KS and
Machin D: Variation in the seasonal diagnosis of acute
lymphoblastic leukemia: evidence from Singapore, the United states
and Sweden. Am J Epidemiol. 162:753–763. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ni X, Shen ZX, Chen FY, Liang H, Lu FJ,
Chen J, Wang C, Shao JB, Hou J, Zou SH and Wang JM: Trend in the
incidence and geographic variations of acute lymphoblastic leukemia
in Shanghai, China from 2002 to 2006. Chin Med J (Engl).
124:2406–2410. 2011.PubMed/NCBI
|
|
6
|
Matsumura T, Kami M, Yamaguchi T, Yuji K,
Kusumi E, Taniguchi S, Takahashi S, Okada M, Sakamaki H, Azuma H,
et al: Allogeneic cord blood transplantation for adult acute
lymphoblastic leukemia: retrospective survey involving 256 patients
in Japan. Leukemia. 26:1482–1486. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Goujon-Bellec S, Mollie A, Rudant J,
Guyot-Goubin A and Clavel J: Time trends and seasonal variations in
the diagnosis of childhood acute lymphoblastic leukaemia in France.
Cancer Epidemiol. 37:255–261. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen B, Wang YY, Shen Y, Zhang WN, He HY,
Zhu YM, Chen HM, Gu CH, Fan X, Chen JM, et al: Newly diagnosed
acute lymphoblastic leukemia in China (I): abnormal genetic
patterns in 1346 childhood and adult cases and their comparison
with the reports from Western countries. Leukemia. 26:1608–1616.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gu LJ, Li J, Xue HL, Tang JY, Chen J, Zhao
HJ, Ye H, Chen J and Pan C: Clinical outcome of children with newly
diagnosed acute lymphoblastic leukemia treated in a single center
in Shanghai, China. Leuk Lymphoma. 49:488–494. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li X, Li J, Hu Y, Xie W, Du W, Liu W, Li
X, Chen X, Li H, Wang J, et al: A comprehensive cytogenetic
classification of 1466 Chinese patients with de novo acute
lymphoblastic leukemia. Leuk Res. 36:720–726. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mi JQ, Wang X, Yao Y, Lu HJ, Jiang XX,
Zhou JF, Wang JH, Jiao B, Shen SH, Tang JY, et al: Newly diagnosed
acute lymphoblastic leukemia in China (II): prognosis related to
genetic abnormalities in a series of 1091 cases. Leukemia.
26:1507–1516. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gao C, Zhao XX, Li WJ, Cui L, Zhao W SG,
Yue ZX, Jiao Y, Wu MY and Li ZG: Clinical features, early treatment
responses and outcomes of pediatric acute lymphoblastic leukemia in
China with or without specific fusion transcripts: a single
institutional study of 1,004 patients. Am J Hematol. 87:1022–1027.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu Z, Liu XL, Du QF, et al: Clinical
characteristics and outcomes of 59 patients with acute
lymphoblastic leukemia positive for BCR/ABL. Nan Fang Yi Ke Da Xue
Xue Bao. 29:512–515. 2009.(In Chinese). PubMed/NCBI
|
|
14
|
Gu L, Ma Z, Dong S, Kuang S, Tong Y and
Xue H: Study on clinical and molecular biological characteristics
of infant acute leukemia. Zhonghua Xue Ye Xue Za Zhi. 21:349–351.
2000.(In Chinese). PubMed/NCBI
|
|
15
|
Gao SQ, Deng ZH, Jin SZ, Zhang SY, Zhang X
and Wu GG: Study on the correlation between acute lymphoblastic
leukemia and HLA genes in southern China Han population. Zhongguo
Shi Yan Xue Ye Xue Za Zhi. 13:210–214. 2005.(In Chinese).
PubMed/NCBI
|
|
16
|
Tien HF, Wang CH, Lee FY, et al:
Cytogenetic study of acute lymphoblastic leukemia and its
correlation with immunophenotype and genotype. Cancer Genet
Cytogenet. 59:191–198. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guo Y, Chen YM, Zou Y, Chen XJ, Zhang L,
Wang SC and Zhu XF: Biologic features of 688 cases of childhood
acute leukemia-a single centre retrospective study. Zhongguo Dang
Dai Er Ke Za Zhi. 11:793–796. 2009.(In Chinese). PubMed/NCBI
|
|
18
|
Li Y, Zou D, Zhao Y, Mi Y, Wang J and Qiu
L: Clinical characteristics and outcomes of adults with
Philadelphia chromosome positive and/or bcr-abl positive acute
lymphoblastic leukemia: a single center study from China. Leuk
Lymphoma. 51:488–496. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tong HX, Wang QS, Lu CW, Wang H and Liu
ZG: Immunophenotypes in 207 pediatric patients with ALL and theirs
correlation with cytogenetics and clinical features. Zhongguo Shi
Yan Xue Ye Xue Za Zhi. 19:696–701. 2011.(In Chinese). PubMed/NCBI
|
|
20
|
Yang S: Retrospective analysis of Clinical
data of 1039 patients with acute leukemia in the Hematology
Department of Fujian Medical University Union Hopsital. Fujian
Medical University; 2010
|
|
21
|
Zeng HM, Guo Y, Yi XL, Zhou JF, An WB and
Zhu XF: Large sample clinical analysis of patients with children
acute leukemia in single center. Zhongguo Shi Yan Xue Ye Xue Za
Zhi. 19:692–695. 2011.(In Chinese). PubMed/NCBI
|
|
22
|
Rajalekshmy KR, Abitha AR, Anuratha N and
Sagar TG: Time trend in frequency of occurrence of major
immunophenotypes in paediatric acute lymphoblastic leukemia cases
as experienced by Cancer Institute, Chennai, south India during the
period 1989–2009. Indian J Cancer. 48:310–315. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Supriyadi E, Widjajanto PH, Purwanto I,
Cloos J, Veerman AJ and Sutaryo S: Incidence of childhood leukemia
in Yogyakarta, Indonesia, 1998–2009. Pediatr Blood Cancer.
57:588–593. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Abbasi S, Maleha F and Shobaki M: Acute
lymphoblastic leukemia experience: epidemiology and outcome of two
different regimens. Mediterr J Hematol Infect Dis. 5:e20130242013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Karimi M and Yarmohammadi H: Seasonal
variations in the onset of childhood leukemia/lymphoma: April 1996
to March 2000, Shiraz, Iran. Hematol Oncol. 21:51–55. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hunger SP, Lu X, Devidas M, Camitta BM,
Gaynon PS, Winick NJ, Reaman GH and Carroll WL: Improved survival
for children and adolescents with acute lymphoblastic leukemia
between 1990 and 2005: a report from the children's oncology group.
J Clin Oncol. 30:1663–1669. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pui CH, Robison LL and Look AT: Acute
lymphoblastic leukaemia. Lancet. 371:1030–1043. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Stanulla M and Schrappe M: Treatment of
childhood acute lymphoblastic leukemia. Semin Hematol. 46:52–63.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nyari TA, Kajtar P, Bartyik K, Thurzo L,
McNally R and Parker L: Seasonal variation of childhood acute
lymphoblastic leukaemia is different between girls and boys. Pathol
Oncol Res. 14:423–428. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kulkarni KP and Marwaha RK: Seasonality in
diagnosis of childhood acute lymphoblastic leukemia: impact on
disease presentation, survival outcome and resources. J Pediatr
Hematol Oncol. 35:81–82. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yuzhalin AE and Kutikhin AG: ABO and Rh
blood groups in relation to ovarian, endometrial and cervical
cancer risk among the population of South-East Siberia. Asian Pac J
Cancer Prev. 13:5091–5096. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sheng L, Sun X, Zhang L and Su D: ABO
blood group and nasopharyngeal carcinoma risk in a population of
Southeast China. Int J Cancer. 133:893–897. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pelzer U, Klein F, Bahra M, Sinn M, Dorken
B, Neuhaus P, Meyer O and Riess H: Blood group determinates
incidence for pancreatic cancer in Germany. Front Physiol.
4:1182013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jackson N, Menon BS, Zarina W, Zawawi N
and Naing NN: Why is acute leukemia more common in males? A
possible sex-determined risk linked to the ABO blood group genes.
Ann Hematol. 78:233–236. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Alavi S, Ashraf H, Rashidi A, Hosseini N,
Abouzari M and Naderifar M: Distribution of ABO blood groups in
childhood acute leukemia. Pediatr Hematol Oncol. 23:611–617. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Arico M, Valsecchi MG, Camitta B, Schrappe
M, Chessells J, Baruchel A, Gaynon P, Silverman L, Janka-Schaub G,
Kamps W, et al: Outcome of treatment in children with Philadelphia
chromosome-positive acute lymphoblastic leukemia. N Engl J Med.
342:998–1006. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pieters R: Infant acute lymphoblastic
leukemia: Lessons learned and future directions. Curr Hematol Malig
Rep. 4:167–174. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Van Vlierberghe P, Pieters R, Beverloo HB
and Meijerink JP: Molecular-genetic insights in paediatric T-cell
acute lymphoblastic leukaemia. Br J Haematol. 143:153–168. 2008.
View Article : Google Scholar : PubMed/NCBI
|