Introduction
Lung cancer is the main cause of all
cancer-associated mortalities worldwide (1). Non-small cell lung cancer (NSCLC)
accounts for almost 80% of all lung cancers, and 50% of NSCLC cases
are classified as adenocarcinoma (ADC). There is a trend that the
incidence of lung ADC is increasing worldwide, particularly in
women (2,3). In addition, ADC is the most common
histological type of lung cancer that arises in never and former
smokers (4,5). Despite advances in treatment, the
five-year survival rate for patients with all stages of NSCLC is
only ~15% (6). Due to the
life-threatening nature of lung cancers, it is important to obtain
an improved understanding of the underlying mechanisms with respect
to the functional roles of the molecules involved in the
development and advancement of the cancer (7). Although numerous markers have been
reported using gene expression profiling analysis of lung cancers
(8), the current understanding of
lung cancer development and evolution remains unclear, and critical
genes and molecular pathways involved in lung cancer development
and progression require further exploration.
Sex determining region Y-box 9 (SOX9) is a
transcription factor and member of the SOX family that is emerging
as an important transcriptional regulator. SOX9 function was first
identified as a key regulator in the development of cartilage and
the testes, with mutations in SOX9 causing campomelic dysplasia and
autosomal sex reversal (9,10). Subsequently, it emerged that SOX9 is
upregulated in several tumor types, including lung ADC, breast
carcinoma, colorectal cancer and prostate cancer (11–14).
Although an association between the upregulation of SOX9 and lung
ADC has been reported (15), the role
of SOX9 in the proliferation, migration and invasion of cancer
cells remains unclear. In the present study, the expression of SOX9
was detected in 163 human ADC tissues, and it was found that SOX9
was upregulated in the majority of ADC tissues compared with the
corresponding adjacent non-cancerous lung tissues. The present
study also revealed that knockdown of SOX9 in lung ADC cell lines
resulted in a marked decrease in the growth, migration and invasion
of the cells. Accordingly, the ectopic expression of SOX9 may
dramatically promote cell growth, migration and invasion. The
present study offers evidence that SOX9 may act as a novel marker
for lung ADC and play a role in cell proliferation, migration and
invasion
Materials and methods
Patients and tissue specimens
In total, 163 paired ADC lung samples and adjacent
non-tumorous lung tissues were obtained from patients undergoing
surgical resection at The Second Hospital of Shandong University
(Jinan, Shandong, China) between 2009 and 2013. Prior to the
present study, patient consent and approval from the Institutional
Research Ethics Committee at the Second Hospital of Shandong
University (Jinan, China) were obtained to use these clinical
materials for research purposes. Sections of the tissue samples
were immediately frozen in liquid nitrogen until protein
extraction. The remaining tissue samples were fixed in 4% formalin
and embedded into wax blocks for immunohistochemistry.
Cell culture and DNA construction
The human lung ADC A549 cell line was obtained from
the American Type Culture Collection. The cells were routinely
cultured in Dulbecco's Modified Eagle Medium (DMEM; Invitrogen,
Carlsbad, CA, USA) supplemented with 10% heat-inactivated fetal
bovine serum (Invitrogen), 100 U/ml penicillin (Invitrogen) and 100
µg/ml streptomycin (Invitrogen) at 37°C in a humidified cell
incubator with a 5% CO2 atmosphere. The full-length
human SOX9 plasmid was constructed as described by a previous study
(11). The coding sequences of SOX9
in the EST clone were amplified by polymerase chain reaction, using
the following primer sequences: Forward, 5′-GGATCCCAT
GAATCTCCTGGACCCCT-3′, and reverse, 5′-GAATTCTCA
AGGTCGAGTGAGCTGTGTGT-3′. The sequences were then subcloned into a
pCMV-Tag2V expression vector (Stratagene, Inc., La Jolla, CA, USA).
Cell transfection was performed using Lipofectamine 2000
(Invitrogen).
Immunohistochemistry
Paraffin-embedded specimens were cut into 4-µm
slices and baked at 65°C for 30 min. The sections were
deparaffinized with xylenes and rehydrated. The sections were
submerged into EDTA antigenic retrieval buffer and microwaved (800
W, 15 min) for antigenic retrieval. The sections were treated with
3% hydrogen peroxide in methanol to quench the endogenous
peroxidase activity, followed by incubation in 1% bovine serum
albumin to block non-specific binding. The slides were incubated
with the mouse anti-human primary monoclonal antibody against SOX9
(1:500; Abcam, Cambridge, MA, USA) overnight at 4°C, and incubated
with a horseradish peroxidase (HRP)-conjugated goat anti-mouse
secondary antibody (1:50, A0216, Shanghai Beyotime Biotechnology
Co., Ltd., Shanghai, China) at room temperature for 1 h. DAB was
applied for color development. The immunoreactivity pattern and
histological appearance of all tissues were examined and scored by
two independent pathologists. Nuclear SOX9 staining was evaluated
according to a previously described scoring system (16). Briefly, the staining intensity, which
was scored as 0, 1+, 2+ or 3+, and the proportion of positively
stained tumor cells were recorded for each tissue. A final staining
score was obtained from these two parameters, as follows: Negative,
intensity of 0; weak staining, intensity of 1+ in ≤70% of tumor
cells or intensity of 2+ in ≤30% of tumor cells; moderate staining,
intensity of 1+ in >70% of tumor cells, intensity of 2+ in
>30 and ≤70% of tumor cells or intensity of 3+ in ≤30% of tumor
cells; and strong staining, intensity of 2+ in >70% of tumor
cells or intensity of 3+ in >30% of tumor cells.
Western blotting
Cell and tissue lysates were prepared in RIPA buffer
and cleared by centrifugation. In total, 40 µg of protein lysate
per lane was separated on 12% SDS-PAGE gels and transferred onto
nitrocellulose membranes (Millipore, Billerica, MA, USA). For
immunodetection, the membranes were incubated with antibodies
against SOX9 (Abcam). Signals from HRP-coupled secondary antibodies
were generated by enhanced chemiluminescence solution (Amersham,
Piscataway, NJ, USA) and were detected by exposure of the membranes
to X-ray films (Kodak, Rochester, NY, USA). The relative signal
intensity was quantified by densitometry using UVIphoto and the
UVIsoft UVIband Application V97.04 software (Uvitec, Cambridge,
UK).
Small interfering RNA
For the protocol using small interfering (si)RNA,
pooled siRNA (SMARTpool; Dharmacon, Inc., Lafayette, CO, USA) was
used to target SOX9 gene expression according to the manufacturer's
instructions, using the following sequences:
5′-GGAACAACCCGUCUACACA-3′; 5′-GAACAAGCCGCACGUCAAG-3′;
5′-GACCUUCGAUGUCAACGAG-3′; and 5′-GGAAGUCGGUGAAGAACGG-3′
(Dharmacon, Inc.).
Cell proliferation assay
Cells treated with various conditions were seeded
onto a 96-well plate. Subsequent to overnight incubation, the
medium was removed and 20 µl MTT (5 mg/ml; Sigma-Aldrich, St.
Louis, MO, USA) was added to each well. The 96-well plates were
incubated at 37°C for 4 h. The plates were centrifuged, and the
formazan precipitates were dissolved in 150 µl of dimethyl
sulfoxide. The absorbance of the solution was measured at 490 nm
using a MRX II absorbance reader (DYNEX Technologies, Chantilly,
VA, USA).
Scratch assay
The lung ADC A549 cell line was transfected with the
SOX9 plasmid or treated with SOX9 siRNA. The cells were plated in a
12-well plate and scratched with a p200 pipet tip to generate a
cell-free zone (20 mm in length and 700 µm in width). The cells
were then incubated in 1% serum culture medium to avoid further
cell proliferation. The migration of cells into the cell-free zone
was monitored after 24 h using a Leica DFC 420c camera (Leica
Microsystems, Wetzlar, Germany) and the migration rates (length of
cell migration/migration time) were determined. The relative
migration activity was determined using the value of the negative
control as 1.
Invasion assay
The lung ADC A549 cell line was transfected with
human SOX9 plasmids or treated with SOX9 siRNA. The invasion assays
were then performed using the QCM ECMatrix Cell Invasion Assay kit
(Millipore, Billerica, MA). Briefly, ~2×104 of the cells
were grown in the upper chamber in serum-free medium, with 20%
serum medium in the bottom chamber. The invasive migration of cells
through the extracellular matrix layer was then determined by
crystal violet staining in the bottom of the upper chamber. The
relative invasion activity was determined by the measurement of the
optical density at 560 nm and using the value of the negative
control as 1.
Statistical analysis
Student's t-test was used to assess the
significance of the differences between groups. All statistical
analyses were performed using the SPSS statistical software,
version 17.0 (SPSS, Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Increased SOX9 protein expression in
lung ADC tissues
To investigate the role of SOX9 in human lung ADC,
the expression of SOX9 was compared between 163 lung ADC tissue
samples and adjacent non-tumorous lung tissues by
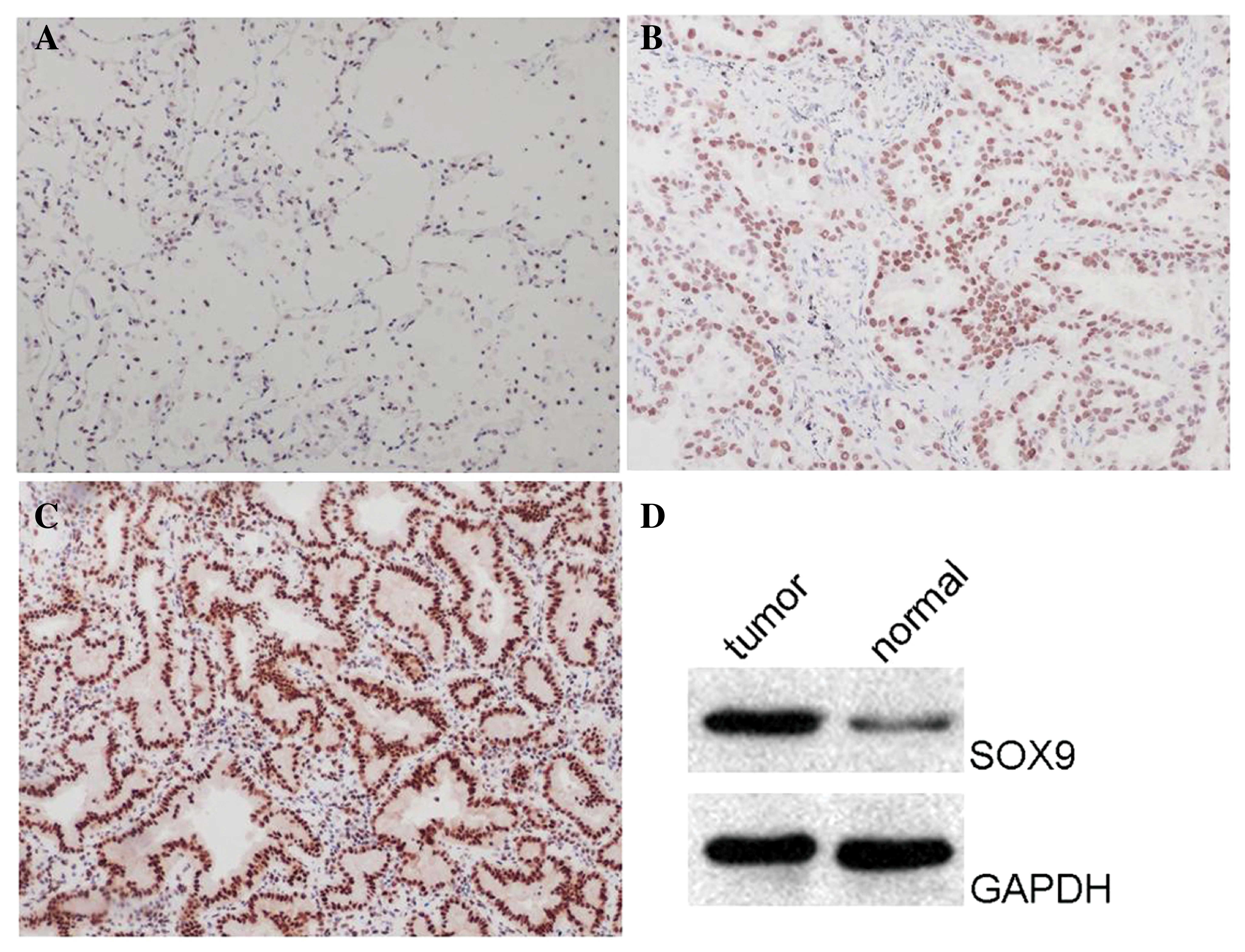
immunohistochemistry. Examples of SOX9 immunostaining are shown in
Fig. 1. Weak SOX9 expression was
observed in all adjacent non-tumorous lung tissues (Fig. 1A). By contrast, the majority of lung
ADC tissues (59.7%) demonstrated moderate (Fig. 1B) or strong (Fig. 1C) SOX9 expression (P<0.01). This
result indicates that the SOX9 protein is over-expressed in the
majority of lung ADC tissues. To further validate the hypothesis,
western blot analysis was performed on the tissues, and revealed
that the protein level of SOX9 was upregulated in cancerous tissues
compared with the corresponding non-cancerous controls (Fig. 1D). This result was consistent with the
data obtained from immunohistochemistry. The aforementioned results
indicate that SOX9 protein expression is upregulated in the
majority of lung ADC tissues.
Overexpression of SOX9 promotes cell
proliferation and knockdown of SOX9 inhibits cell growth in the
lung ADC cell line
Having established the association between SOX9
overexpression and lung ADC, the functional association between
SOX9 expression and cancer phenotypes was assessed. The full-length
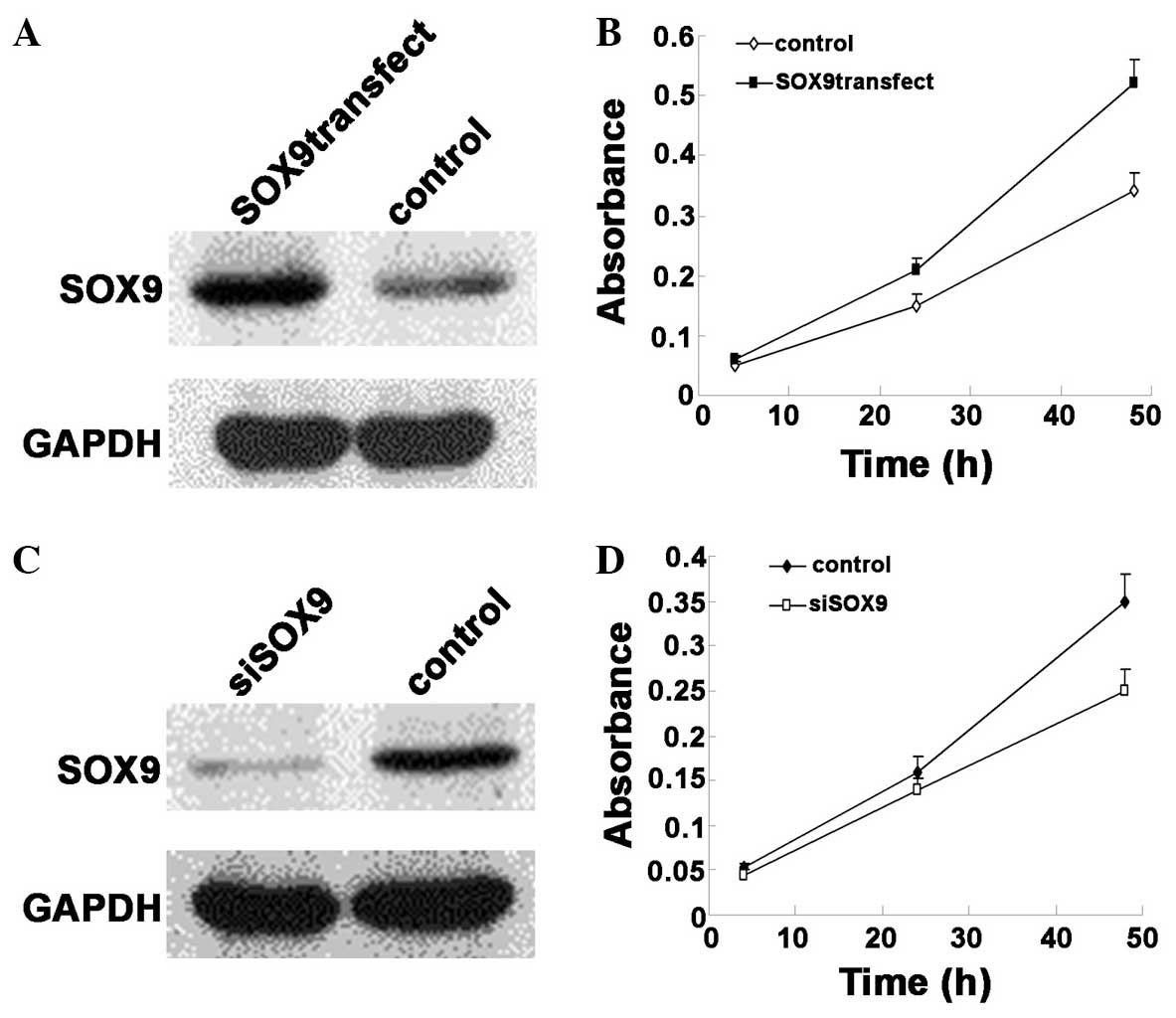
human SOX9 plasmid or control was transfected into the lung ADC
A549 cell line. After 2 days, the cells were reseeded and subjected
to an MTT cell proliferation assay. Western blot analysis was also
performed to confirm the overexpression of SOX9 (Fig. 2A). As revealed by the cell
proliferation assay, the monolayer cell growth of A549 was
significantly increased when SOX9 expression was upregulated
(Fig. 2B). To further study the
biological significance of SOX9 in cell growth, SOX9 expression was
knocked down by RNA interference The A549 cell line was treated
with SOX9 siRNA or non-targeting control siRNA and incubated for 48
h prior to performing the MTT assay. The silencing of SOX9 was
confirmed by western blotting (Fig.
2C). The MTT assay revealed that knock-down of SOX9 resulted in
a significant decrease of growth capability (Fig. 2D). The aforementioned results indicate
that SOX9 is important for the proliferation of lung ADC cells.
Cell migration and invasion is
promoted by overexpression of SOX9 and knockdown of SOX9 inhibits
migration and invasion in the lung ADC cell line
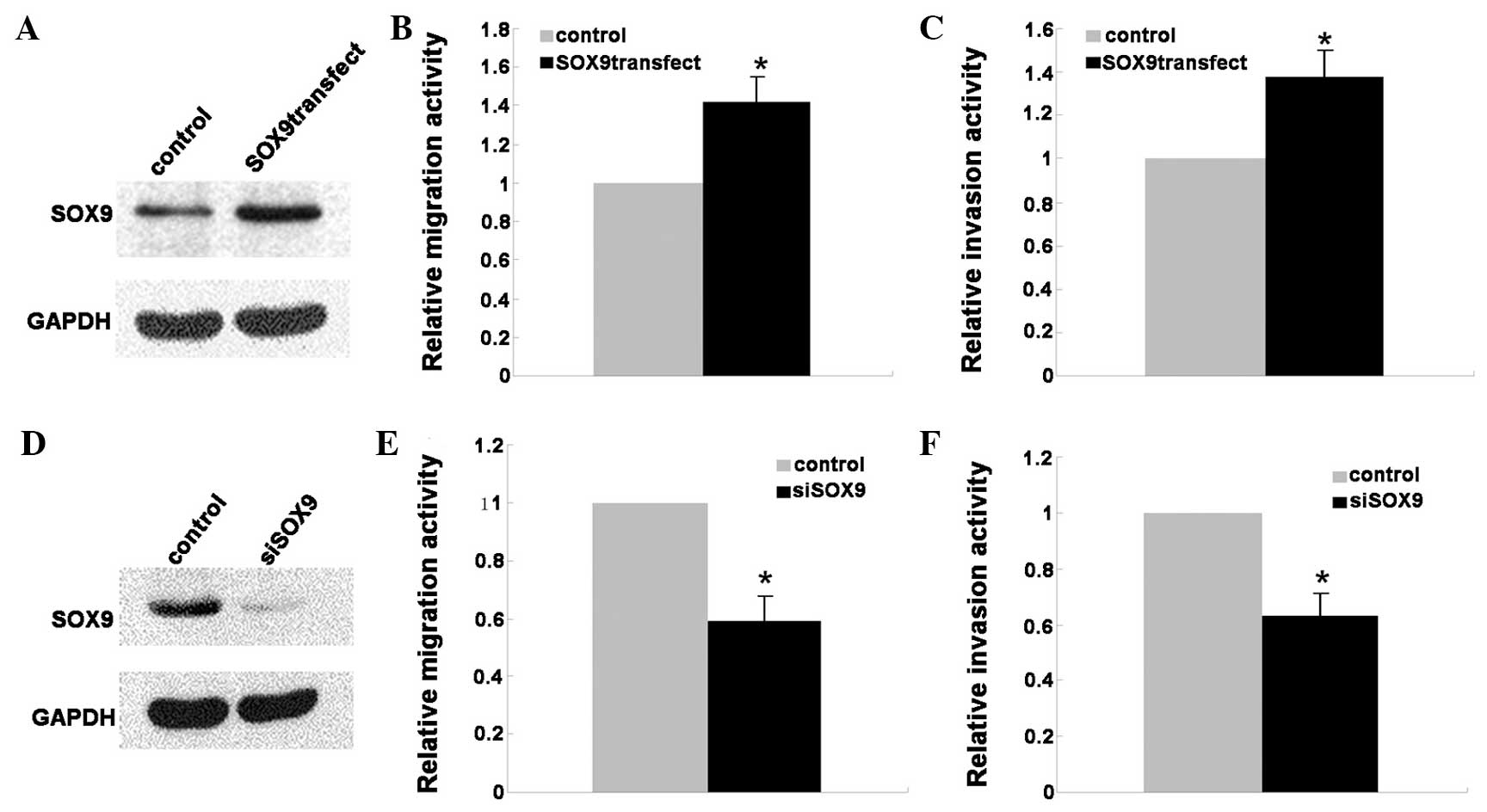
To investigate the effect of SOX9 on the migration
and invasion of lung ADC cells, the A549 cells were transfected
with the full-length human SOX9 plasmid or negative control (NC)
and incubated for 2 days. To verify the over-expression of SOX9,
western blotting was performed and the results revealed
significantly increased expression of SOX9 in the A549 cells that
were transfected with the SOX9 plasmid compared with the cells that
were transfected wit the NC (Fig.
3A). The scratch assay was used to study the effect of SOX9 on
A549 cell migration, and the result demonstrated that the ectopic
expression of SOX9 may significantly promote cell migration
compared with the control group (Fig.
3B). Similarly, the invasive capability of A549 cells
transfected with the NC or SOX9 plasmid was evaluated by the
ECMatrix cell invasion assay. As expected, the transfected SOX9
plasmid in A549 cells notably enhanced the invasive ability of the
A549 cells compared with NC-transfected cells (Fig. 3C). Furthermore, the migratory and
invasive capability of A549 cells transfected with siRNA of SOX9 or
non-targeting control siRNA were evaluated by scratch and ECMatrix
cell invasion assays. The results demonstrated that the silencing
of SOX9 caused significant suppression of the migratory and
invasive capability of A549 cells (Fig.
3D–F).
Discussion
SOX9 was originally considered a chondrogenic
transcription factor involved in bone formation and testis
development, whereas the mutation of the SOX9 gene has been
associated with campomelic dysplasia and autosomal sex reversal
(17). Subsequent studies revealed
SOX9 to be a multifaceted transcription factor that is vital for
the development of numerous other organs and tissues, including the
pancreas (18,19), the prostate (20,21), the
intestine (22) and pigment cells
(23). It has been well reported that
genes and pathways critical for development may also perform
important roles in cancer development and progression, and it is
therefore not notable that SOX9 is associated with cancer. At
present, SOX9 has been found to be upregulated in several tumor
types, including lung ADC, breast carcinoma, colorectal cancer and
prostate cancer (11–14). Despite the previously reported
association between upregulation of SOX9 and lung ADC, the role of
SOX9 in cancer cell proliferation, migration and invasion remains
unclear (11).
The present study described the SOX9 expression in
163 lung ADC tissue samples and adjacent non-tumorous lung tissues.
The data from immunohistochemistry revealed that weak SOX9
expression was observed in all adjacent non-tumorous lung tissues.
By contrast, the majority of lung ADC (59.7%) tissues demonstrated
moderate or strong SOX9 expression (P<0.01). The western
blotting analysis also revealed that SOX9 was upregulated in
cancerous tissues compared with the corresponding non-cancerous
controls. The differential expression of SOX9 between the lung ADC
tissues and controls suggested that SOX9 may have some functional
roles in cancer phenotypes. To assess this hypothesis, MTT, scratch
and invasion assays were performed in the lung ADC A549 cell line.
The results revealed that the overexpression of SOX9 promotes cell
proliferation, migration and invasion. Accordingly, knockdown of
SOX9 resulted in the inhibition of cell growth, migration and
invasion. Based on the findings from the present study, it was
hypothesized that SOX9, which is involved in the regulation of cell
proliferation, migration and invasion, may act as a novel marker
for lung ADC. Despite the present and previous studies, the precise
pathway that SOX9 uses to promote proliferation, migration and
invasion in lung ADC cells remains unclear. Additional
investigation is required to delineate the pathway and mechanism
underlying this involvement, and this may aid the understanding of
lung cancer formation and identification of potential novel targets
for cancer therapy.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (81000731) and the Promotive
Research Fund for Excellent Young and Middle-Aged Scientists of
Shandong Province (BS2010YY045).
References
|
1
|
Greenlee RT, Hill-Harmon MB, Murray T and
Thun M: Cancer statistics, 2001. CA Cancer J Clin. 51:15–36. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen KY, Chang CH, Yu CJ, Kuo SH and Yang
PC: Distribution according to histologic type and outcome by gender
and age group in Taiwanese patients with lung carcinoma. Cancer.
103:2566–2574. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yoshimi I, Ohshima A, Ajiki W, Tsukuma H
and Sobue T: A comparison of trends in the incidence rate of lung
cancer by histological type in the Osaka cancer registry, Japan and
in the Surveillance, Epidemiology and End Results Program, USA. Jpn
J Clin Oncol. 33:98–104. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Toh CK and Lim WT: Lung cancer in
never-smokers. J Clin Pathol. 60:337–340. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu NS, Spitz MR, Kemp BL, Cooksley C,
Fossella FV, et al: Adenocarcinoma of the lung in young patients:
The M. D. Anderson experience. Cancer. 88:1837–1841. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chan BA and Hughes BG: Targeted therapy
for non-small cell lung cancer: Current standards and the promise
of the future. Transl Lung Cancer Res. 4:36–54. 2015.PubMed/NCBI
|
|
7
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Roh MS: Molecular pathology of lung
cancer: Current status and future directions. Tuberc Respir Dis
(Seoul). 77:49–54. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wagner T, Wirth J, Meyer J, Zabel B, Held
M, et al: Autosomal sex reversal and campomelic dysplasia are
caused by mutations in and around the SRY-related gene SOX9. Cell.
79:1111–1120. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Foster JW, Dominguez-Steglich MA, Guioli
S, Kwok C, Weller PA, et al: Campomelic dysplasia and autosomal sex
reversal caused by mutations in an SRY-related gene. Nature.
372:525–530. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jiang SS, Fang WT, Hou YH, Huang SF, Yen
BL, et al: Upregulation of SOX9 in lung adenocarcinoma and its
involvement in the regulation of cell growth and tumorigenicity.
Clin Cancer Res. 16:4363–4373. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Müller P, Crofts JD, Newman BS,
Bridgewater LC, Lin CY, et al: SOX9 mediates the retinoic
acid-induced HES-1 gene expression in human breast cancer cells.
Breast Cancer Res Treat. 120:317–326. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lü B, Fang Y, Xu J, Wang L, Xu F, et al:
Analysis of SOX9 expression in colorectal cancer. Am J Clin Pathol.
130:897–904. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang H, Leav I, Ibaragi S, Wegner M, Hu
GF, et al: SOX9 is expressed in human fetal prostate epithelium and
enhances prostate cancer invasion. Cancer Res. 68:1625–1630. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou CH, Ye LP, Ye SX, et al: Clinical
significance of SOX9 in human non-small cell lung cancer
progression and overall patient survival. J Exp Clin Cancer Res.
31:182012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sirma H, Broemel M, Stumm L, Tsourlakis T,
Steurer S, et al: Loss of CDKN1B/p27Kip1 expression is associated
with ERG fusion-negative prostate cancer, but is unrelated to
patient prognosis. Oncol Lett. 6:1245–1252. 2013.PubMed/NCBI
|
|
17
|
Südbeck P, Schmitz ML, Baeuerle PA and
Scherer G: Sex reversal by loss of the C-terminal transactivation
domain of human SOX9. Nat Genet. 13:230–232. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lynn FC, Smith SB, Wilson ME, Yang KY,
Nekrep N and German MS: Sox9 coordinates a transcriptional network
in pancreatic progenitor cells. Proc Natl Acad Sci USA.
104:10500–10505. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Piper K, Ball SG, Keeling JW, Mansoor S,
Wilson DI and Hanley NA: Novel SOX9 expression during human
pancreas development correlates to abnormalities in Campomelic
dysplasia. Mech Dev. 116:223–226. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Thomsen MK, Butler CM, Shen MM and Swain
A: Sox9 is required for prostate development. Dev Biol.
316:302–311. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang H, McKnight NC, Zhang T, Lu ML, Balk
SP and Yuan X: SOX9 is expressed in normal prostate basal cells and
regulates androgen receptor expression in prostate cancer cells.
Cancer Res. 67:528–536. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Blache P, van de Wetering M, Duluc I,
Domon C, Berta P, et al: SOX9 is an intestine crypt transcription
factor, is regulated by the Wnt pathway, and represses the CDX2 and
MUC2 genes. J Cell Biol. 166:37–47. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Passeron T, Valencia JC, Bertolotto C,
Hoashi T, Le Pape E, et al: SOX9 is a key player in ultraviolet
B-induced melanocyte differentiation and pigmentation. Proc Natl
Acad Sci USA. 104:13984–13989. 2007. View Article : Google Scholar : PubMed/NCBI
|