Introduction
Berberine (BBR) is an isoquinoline alkaloid found in
the rhizome of numerous valuable medicinal plants (1). BBR has been reported to exhibit
anti-inflammatory activity via the inhibition of transforming
growth factor-β-activated kinase 1 (TAK1) (2). TAK1 is an important mediator of nuclear
factor-κB activation, which in turn has important roles in cell
survival and inflammation (3).
Tumor necrosis factor (TNF)-related
apoptosis-inducing ligand (TRAIL) has potential antitumor activity,
due to its high selectivity for various types of cancer cells over
normal cells (4). However, the
clinical efficacy of TRAIL has been limited due to primary or
acquired resistance (5,6). TRAIL-based combination therapy
approaches have therefore been introduced as a novel strategy
against resistance (1,7,8). Of note,
several studies have reported the contribution of the activated
epidermal growth factor (EGF) receptor (EGFR) pathway in
TRAIL-resistance (9,10). TRAIL activates TAK1 in tumor cells
(11), which may result in the
phosphorylation of downstream mitogen-activated protein kinases
(MAPKs), most importantly p38 and extracellular signal-regulated
kinase (ERK), and the subsequent regulation of the EGFR pathway
(12,13).
EGFR (ErbB-1) is a member of the ErbB family of
receptors, which is activated through the binding of its specific
ligands (14). In canonical EGFR
activation, EGF binds to EGFR to induce the preferential
phosphorylation of tyrosine residues, which subsequently results in
the internalization of EGFR into the cytoplasm to enhance the
survival and proliferation of cells (14). By contrast, the TNF-α ligand, a member
of the TNF family, binds to its relevant receptor to induce the
TAK1-mediated activation of MAPKs and the subsequent
phosphorylation of serine/threonine residues of EGFR. This
non-canonical pathway of MAPKs/EGFR may eventually result in the
p38/serine-dependent internalization of EGFR (14). However, the functional role of the
p38/serine-dependent internalization remains to be fully elucidated
(12,15).
A previous study demonstrated the efficiency of a
BBR/TRAIL combination therapy against triple negative breast cancer
(TNBC) cells as well as its effect on the sensitization of
TRAIL-resistant TNBC cells to TRAIL (11). The aim of the present study was to
investigate a novel pathway for potentiating the apoptotic effect
of BBR/TRAIL combination therapy through p38 MAPKs.
Materials and methods
Antibodies and reagents
Phospho-specific antibodies against p38 (Thr-180,
Tyr-182; rabbit anti-human; cat. no., 9211), ERK (Thr-202, Tyr-204;
rabbit anti-human; cat. no. 9101), and EGFR (Tyr-1068, Thr-669 and
Ser-1046/1047; mouse anti-human; cat. nos. 2236, 3056 and 2238,
respectively) as well as antibodies against poly-(adenosine
diphosphate-ribose) polymerase 1 (PARP-1; rabbit anti-human; cat.
no. 9542) and caspase-3 (rabbit anti-human; cat. no. 9662) were
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Antibodies against EGFR (1005; rabbit anti-human; cat. no. sc-03)
and β-actin (C-11; goat anti-human; cat. no. sc-1615) were obtained
from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). All
antibodies were polyclonal, with the exception of EGFR (Tyr-1068)
that was monoclonal. Primary antibodies were used at 1:1,000
dilution. Recombinant human TRAIL Apo II ligand was obtained from
PeproTech, Inc. (Rocky Hill, NJ, USA). SB203580, U0126 and
PD153035, which are inhibitors of p38, ERK and EGFR tyrosine
kinase, respectively, were obtained from Merck Biosciences
(Danvers, MA, USA), while 5Z-7-oxozeaenol, a selective TAK1
inhibitor, was obtained from Chugai Pharmaceutical Co., Ltd.
(Tokyo, Japan). BBR chloride was purchased from Wako Pure Chemical
Industries, Ltd. (Osaka, Japan). All chemical inhibitors, in
addition to, BBR chloride were dissolved in dimethyl sulfoxide
(Wako Pure Chemical Industries, Ltd.).
Cell cultures
MDA-MB-468 cells (ATCC, Rockville, MD, USA) were
maintained in Dulbecco's modified Eagle's medium (DMEM; Invitrogen
Life Technologies, Carlsbad, CA, USA) supplemented with 10% fetal
calf serum (Invitrogen Life Technologies), 2 mM glutamine (Life
Technologies, Gaithersburg, MD, USA), 100 U/ml penicillin and 100
µg/ml streptomycin (Life Technologies) at 37°C in a humidified
atmosphere of 5% CO2. PC-9 cells were provided by Dr
Kiura (Okayama University, Okayama, Japan) and were maintained in
RPMI-1640 medium (Invitrogen Life Technologies), with identical
supplements and under identical conditions.
Cell viability assay
A WST-1 Cell Counting kit (Dojindo, Kumamoto, Japan)
was used to detect cell viability. In brief, cell suspensions in
DMEM supplemented with 10% fetal calf serum were seeded into a
96-well plate (5–8×103/70 µl/well) and incubated at 37°C
in a humidified atmosphere of 5% CO2. Following 24 h of
incubation, 10 µl FBS-supplemented DMEM containing the inhibitors
at specified concentrations were added to the wells. After 30 min,
10 µl medium containing BBR (Wako Pure Chemical Industries, Ltd.)
was added to the respective wells and incubated at 37°C for 90 min,
followed by 10 µl medium containing TRAIL (PeproTech, Inc., Rocky
Hill, NJ, USA). Plates were then incubated for 24 h at 37°C. WST-1
solution (10 µl) was added to each well 2 h prior to the end of the
experiment. Absorbance was measured at 450 nm using a microplate
reader (Multiskan Plus Model 355 Microplate Reader; Thermo
Labsystems, Helsinki, Finland). Cell viability was determined from
the absorbance of soluble formazan dye generated by living
cells.
Transfection of small interfering RNAs
(siRNAs)
Duplex siRNAs were synthesized at Hokkaido System
Science Co., Ltd. (Sapporo, Japan). The target sequences were as
follows: p38α, 5′-GCAUUACAACCAGACAGUUGAUAUU-3′; and firefly
luciferase (GL2), 5′-CGUACGCGGAAUACUUCGA-3′. MDA-MB-468 cells were
transfected with siRNAs in a final concentration of 50 nM using
lipofectamine (Life Technologies). At 72 h post-transfection, the
cells were treated.
Immunoblotting
Following stimulation, whole-cell lysates were
prepared as described previously (16). Cell lysates were resolved by 7.5, 10
or 12.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis
and transferred to an Immobilon-P nylon membranes (EMD Millipore,
Billerica, MA, USA). The membranes were treated with BlockAce
(Dainippon Pharmaceutical Co., Ltd., Suita, Japan) and probed with
primary antibodies (dilution, 1:1,000). The antibodies were then
detected using polyclonal goat anti-rabbit (cat. no. P0448),
polyclonal rabbit anti-mouse (cat. no. P0260) or polyclonal rabbit
anti-goat (cat. no. P0449) horseradish peroxidase-conjugated
immunoglobulin G antibody (dilution, 1:2,000; Dako, Carpinteria,
CA, USA) and visualized using an enhanced chemiluminescence system
(Amersham Biosciences, Piscataway, NJ, USA). Certain antibody
reactions were performed using in Can Get Signal® immunostain
solution (Toyobo Co., Ltd, Osaka, Japan).
Statistical analysis
Data are presented as the mean ± standard deviation
of 3 experiments. The statistical analysis was performed using JMP
software (version 10; SAS Institute Inc., Cary, NC, USA). The
statistical significance was determined by applying the Bonferroni
method (compared to BBR/TRAIL). P<0.05 was considered to
indicate a statistically significant difference.
Results
TRAIL enhances BBR-mediated p38
activation and counteracts BBR-mediated pERK downregulation in a
TAK1-dependent manner
In light of the previous findings of BBR and TRAIL
studies (1,17), the present study aimed to investigate
the effect of the BBR/TRAIL combination therapy on MAPK activation
in EGFR-overexpressing breast cancer cells. The results
demonstrated that TRAIL alone markedly upregulated ERK
phosphorylation and slightly upregulated p38 phosphorylation in
MDA-MB-468 cells (Fig. 1). This
effect was not associated with any changes in apoptosis, which may
be due to the resistance of MDA-MB-468 cells to TRAIL (Fig. 1, lane 2). By contrast, BBR decreased
ERK phosphorylation and markedly increased p38 phosphorylation
(Fig. 1). In addition, the BBR/TRAIL
combination further enhanced p38 phosphorylation and decreased ERK
phosphorylation. TAK1 inhibition using the specific inhibitor
5Z-7-oxozeaenol demonstrated an inhibition of the combination
therapy-mediated upregulation of p38; comparably, decreased ERK
phosphorylation was observed following 5Z-7-oxozeaenol treatment.
This indicated that TAK1 was involved in the regulation of the
phosphorylation of p38 and ERK in MDA-MB-468 cells. However, TAK1
inhibition did not induce any changes in combination
therapy-mediated apoptosis (Fig. 1,
lane 5 vs. 4). In order to further demonstrate the effect of p38
and ERK inhibition on cell apoptosis, cells were pretreated with
specific inhibitors, SB203580 and U0126, respectively. The results
revealed that p38 inhibition enhanced apoptosis in cells treated
with BBR/TRAIL combination therapy, whereas ERK inhibition did not
exhibit notable effects on apoptosis (Fig. 1).
 | Figure 1.p38 counteracts the apoptotic effect
mediated by BBR/TRAIL combination therapy in human breast cancer
cells. TRAIL-resistant MDA-MB-468 cells were pretreated with
dimethyl sulfoxide, 0.3 µM 5-oxo (transforming growth
factor-β-activated kinase 1), 10 µM SB (p38 inhibitor) or 5 µM U
(ERK inhibitor) for 30 min, followed by incubation with 55 µM BBR
for 90 min. Cells were then further incubated in the presence or
absence of recombinant human TRAIL (50 ng/ml) for 3 h. Whole cell
lysates were analyzed by immunoblotting for the indicated proteins.
TRAIL, tumor necrosis factor-related apoptosis-inducing ligand;
BBR, berberine; 5-oxo, 5Z-7-oxozeaenol; SB, SB203580; U, U0126;
ERK, extracellular signal-regulated kinase; PARP, poly-(adenosine
diphosphate-ribose) polymerase 1. |
p38 MAPK rescues cancer cells from
BBR/TRAIL combination therapy
In order to confirm the efficient inhibition of p38
in EGFR-expressing cells, the previous experiment was repeated with
EGFR tyrosine kinase inhibition instead of TAK1 inhibition. EGFR
tyrosine kinase is well-known to be crucial for cell survival and
proliferation (18). Although
blocking the EGFR tyrosine kinase may indirectly block ERK and EGFR
threonine phosphorylation, the present results demonstrated that
p38 inhibition downregulated EGFR serine phosphorylation as well as
enhanced apoptosis compared with that of EGFR tyrosine kinase
inhibition. In addition, ERK inhibition did not promote apoptosis
in MDA-MB-468 cells (Fig. 2A). This
confirmed that p38, but not ERK, may contribute to cell apoptosis
in EGFR-expressing MDA-MB-468 cells. In order to confirm these
results, the effects of the EGFR tyrosine kinase inhibitor and p38
inhibitor were examined in EGFR-mutant non-small-cell lung
carcinoma (NSCLC) PC-9 cells, the results of which demonstrated a
comparable pattern to that of the EGFR-expressing breast cancer
cells (Fig. 2B). The consequences of
enhancing cellular apoptosis following combination therapy were
demonstrated using cell viability assays in these
EGFR-overexpressing cell lines; MDA-MB-468 and PC-9. In this
experiment, cells were exposed to a lower concentration of BBR, in
addition to TRAIL, and the specific inhibitors for extended time
periods in order to monitor their significance on cell viability.
As shown in Fig. 3A and B, only p38
inhibition in addition to BBR/TRAIL therapy induced a significant
decrease in cell viability, compared to ERK inhibition or EGFR
inhibition. In addition, the specificity of p38 action was
confirmed using specific knockdown of p38 with siRNA. The results
demonstrated that p38 knockdown induced EGFR serine downregulation,
which resulted in the cleavage of caspase-substrate, PARP-1
(Fig. 3C); this therefore confirmed
the value of using p38 inhibition in conjunction with BBR/TRAIL
combination therapy in EGFR-expressing cancer cells.
 | Figure 2.Crosstalk between EGFR and the
apoptotic effect mediated by BBR/TRAIL combination therapy in
EGFR-expressing breast cancer cells. (A) EGFR-overexpressing
MDA-MB-468 and (B) mutant EGFR-overexpressing PC-9 cells were
pretreated with dimethyl sulfoxide, 1 µM PD (EGFR tyrosine kinase
inhibitor), 10 µM SB (p38 inhibitor) or 5 µM U (ERK inhibitor) for
30 min, followed by incubation with 55 µM BBR for 90 min. Cells
were then further incubated in the presence or absence of 50 ng/ml
recombinant human TRAIL for 3 h. Whole cell lysates were analyzed
by immunoblotting for the indicated proteins. TRAIL, tumor necrosis
factor-related apoptosis-inducing ligand; BBR, berberine; EGFR,
epidermal growth factor receptor; PD, PD153035; SB, SB203580; U,
U0126; ERK, extracellular signal-regulated kinase; PARP,
poly-(adenosine diphosphate-ribose) polymerase 1. |
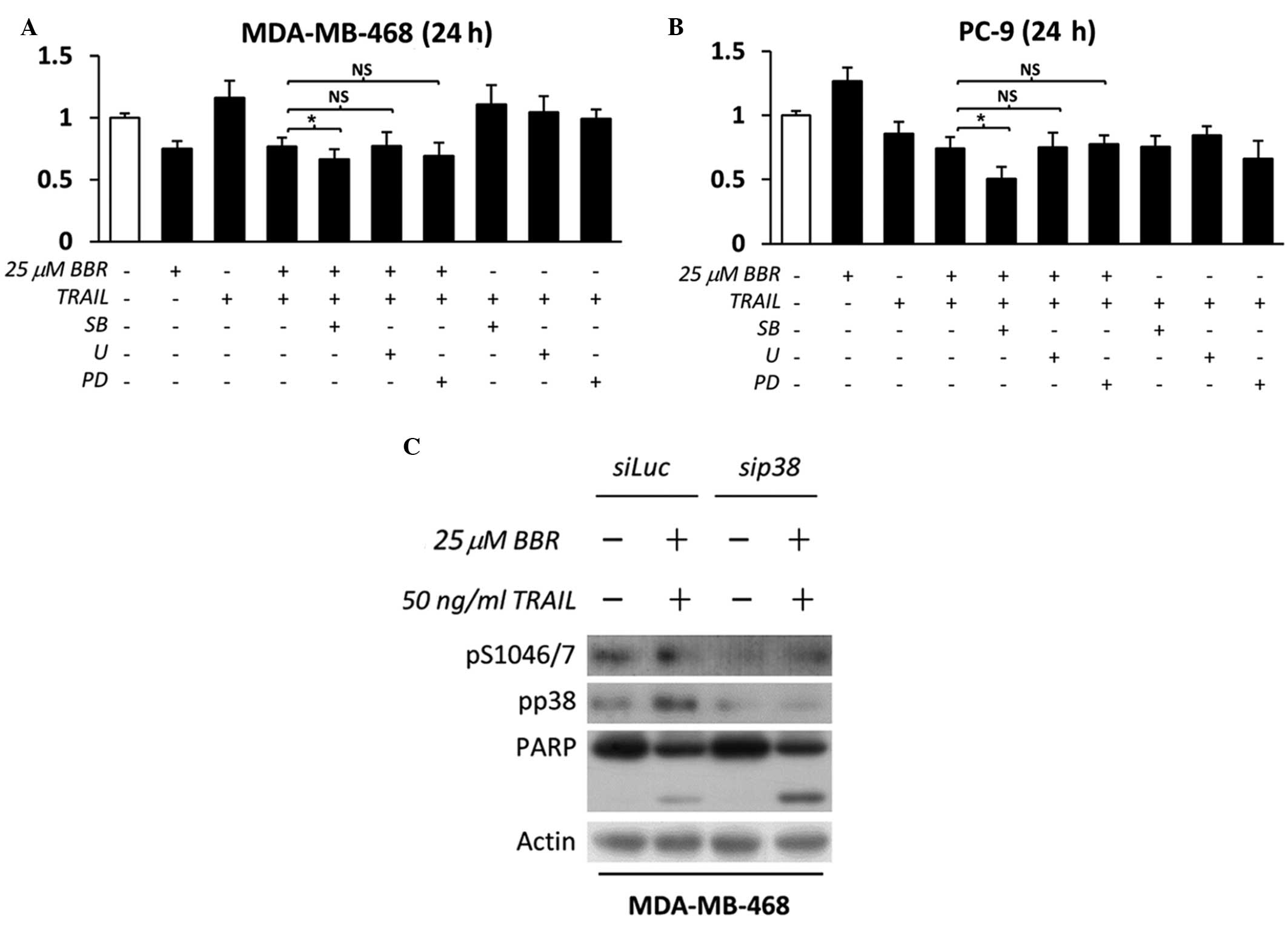 | Figure 3.p38 inhibition enhances the apoptotic
effect mediated by BBR/TRAIL combination therapy in EGFR-expressing
cancer cells. (A) EGFR-overexpressing MDA-MB-468 and (B) mutant
EGFR-overexpressing PC-9 cells were pretreated with dimethyl
sulfoxide, 1 µM PD (EGFR tyrosine kinase inhibitor), 10 µM SB (p38
inhibitor) or 5 µM U (extracellular signal-regulated kinase
inhibitor) for 30 min, followed by incubation with 25 µM BBR for 90
min. Cells were then further incubated in the presence or absence
of 50 ng/ml recombinant human TRAIL for 24 h in the presence or
absence of specific inhibitors.. Absorbance was measured following
incubation with WST-1 reagent and cell viability was calculated as
a percentage relative to the untreated control cells. Data are
expressed as the mean ± standard deviation of triplicate
experiments. *P<0.05 between groups; NS, not significant. (C)
MDA-MB-468 cells were transfected with Luc (control) or p38α siRNA
in the presence or absence of BBR/TRAIL. Whole cell lysates were
analyzed by immunoblotting for the indicated proteins. TRAIL, tumor
necrosis factor-related apoptosis-inducing ligand; BBR, berberine;
EGFR, epidermal growth factor receptor; siRNA, small interfering
RNAs; PD, PD153035; SB, SB203580; U, U0126; PARP, poly-(adenosine
diphosphate-ribose) polymerase 1; Luc, luciferase. |
Discussion
A previous study demonstrated the role of BBR in
synergizing TRAIL action in TRAIL-sensitive MDA-MB-231 TNBC cells,
as well as sensitizing the TRAIL-resistant MDA-MB-468 TNBC cells to
TRAIL (1). In addition to the
difference in TRAIL sensitivity pattern, MDA-MB-468, and not
MDA-MB-231 cells, were reported to preferentially overexpress EGFR
(1). Furthermore, through comparing
data from the publicly available microarray (GSE41313; http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE41313),
it was revealed that MDA-MB-468 exhibited the highest expression of
EGFR among other breast cancer cells (data not shown). In the
present study, the role of MAPK in cellular apoptosis was
investigated in EGFR-expressing cells. MDA-MB-468 cells were
primarily used in the present study, with a secondary focus on PC-9
EGFR-mutant NSCLC cells. The selected cell lines facilitated the
exploration of the present findings regardless of the EGFR status.
In concurrence with previous studies (12,14,19), the
present study suggested that the role of p38 in EGFR serine
phosphorylation is of increasing importance for cell survival. In
order to explore the role of p38 in BBR/TRAIL-mediated apoptosis,
independent of the EGFR-expression status, the present study used
MDA-MB-468 and PC-9 cells; the results of which confirmed the role
of p38 in counteracting BBR/TRAIL-mediated apoptosis. The role of
p38 in EGFR serine was not thoroughly investigated in the present
study; however, these results provided certain evidence for this
reproducible association. It was therefore suggested that p38 may
counteract the BBR/TRAIL-mediated apoptosis as well as suppress the
apoptotic effect of other therapies.
The role of p38 in cell death/survival has been a
controversial issue for decades. We believe that the type, strength
and duration of specific stimuli towards p38 may significantly
affect the eventual outcome experienced by the cell. However, in
cancer cells expressing EGFR, growing evidence indicates the
prominence of the p38-EGFR serine axis on cell survival. Our recent
studies demonstrated the role of various stimuli on the activation
of this survival axis (12,18). Notably, in a recent study, we
demonstrated a similar apoptotic response subsequent to inhibiting
the p38-EGFR serine axis (14).
Therefore, studies involving the use of specific p38 inhibitors may
be of potential future use in anticancer combination therapies.
Acknowledgements
The present study was supported, in part, by a
short-term fellowship grant from the Science and Technology
Development Fund, Egypt (no. 6093).
References
|
1
|
Refaat A, Abdelhamed S, Yagita H, Inoue H,
Yokoyama S, Hayakawa Y and Saiki I: Berberine enhances tumor
necrosis factor-related apoptosis-inducing ligand-mediated
apoptosis in breast cancer. Oncol Lett. 6:840–844. 2013.PubMed/NCBI
|
|
2
|
Zhang Y, Li X, Zhang Q, Li J, Ju J, Du N,
Liu X, Chen X, Cheng F, Yang L, et al: Berberine hydrochloride
prevents postsurgery intestinal adhesion and inflammation in rats.
J Pharmacol Exp Ther. 349:417–426. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sakurai H: Targeting of TAK1 in
inflammatory disorders and cancer. Trends Pharmacol Sci.
33:522–530. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Keane MM, Ettenberg SA, Nau MM, Russell EK
and Lipkowitz S: Chemotherapy augments TRAIL-induced apoptosis in
breast cell lines. Cancer Res. 59:734–741. 1999.PubMed/NCBI
|
|
5
|
Tolcher AW, Mita M, Meropol NJ, von Mehren
M, Patnaik A, Padavic K, Hill M, Mays T, McCoy T, Fox NL, et al:
Phase I pharmacokinetic and biologic correlative study of
mapatumumab, a fully human monoclonal antibody with agonist
activity to tumor necrosis factor-related apoptosis-inducing ligand
receptor-1. J Clin Oncol. 25:1390–1395. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dyer MJ, MacFarlane M and Cohen GM:
Barriers to effective TRAIL-targeted therapy of malignancy. J Clin
Oncol. 25:4505–4506. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Abdelhamed S, Yokoyama S, Refaat A, Ogura
K, Yagita H, Awale S and Saiki I: Piperine enhances the efficacy of
TRAIL-based therapy for triple-negative breast cancer cells.
Anticancer Res. 34:1893–1899. 2014.PubMed/NCBI
|
|
8
|
Refaat A, Abd-Rabou A and Reda A: TRAIL
combinations: The new ‘trail’ for cancer therapy (Review). Oncol
Lett. 7:1327–1332. 2014.PubMed/NCBI
|
|
9
|
Xu L, Zhang Y, Liu J, Qu J, Hu X, Zhang F,
Zheng H, Qu X and Liu Y: TRAIL-activated EGFR by Cbl-b-regulated
EGFR redistribution in lipid rafts antagonises TRAIL-induced
apoptosis in gastric cancer cells. Eur J Cancer. 48:3288–3299.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu L, Qu X, Li H, Li C, Liu J, Zheng H and
Liu Y: Src/caveolin-1-regulated EGFR activation antagonizes
TRAIL-induced apoptosis in gastric cancer cells. Oncol Rep.
32:318–324. 2014.PubMed/NCBI
|
|
11
|
Choo MK, Kawasaki N, Singhirunnusorn P,
Koizumi K, Sato S, Akira S, Saiki I and Sakurai H: Blockade of
transforming growth factor-beta-activated kinase 1 activity
enhances TRAIL-induced apoptosis through activation of a caspase
cascade. Mol Cancer Ther. 5:2970–2976. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nishimura M, Shin MS, Singhirunnusorn P,
Suzuki S, Kawanishi M, Koizumi K, Saiki I and Sakurai H:
TAK1-mediated serine/threonine phosphorylation of epidermal growth
factor receptor via p38/extracellular signal-regulated kinase:
NF-{kappa}B-independent survival pathways in tumor necrosis factor
alpha signaling. Mol Cell Biol. 29:5529–5539. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang C, Chen T, Zhang N, Yang M, Li B, Lü
X, Cao X and Ling C: Melittin, a major component of bee venom,
sensitizes human hepatocellular carcinoma cells to tumor necrosis
factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis
by activating CaMKII-TAK1-JNK/p38 and inhibiting IkappaBalpha
kinase-NFkappaB. J Biol Chem. 284:3804–3813. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Refaat A Aminullah, Zhou Y, Kawanishi M,
Tomaru R, Abdelhamed S, Shin MS, Koizumi K, Yokoyama S, Saiki I and
Sakurai H: Role of tyrosine kinase-independent phosphorylation of
EGFR with activating mutation in cisplatin-treated lung cancer
cells. Biochem Biophys Res Commun. 458:856–861. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tong J, Taylor P, Peterman SM, Prakash A
and Moran MF: Epidermal growth factor receptor phosphorylation
sites Ser991 and Tyr998 are implicated in the regulation of
receptor endocytosis and phosphorylations at Ser1039 and Thr1041.
Mol Cell Proteomics. 8:2131–2144. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Refaat A, Zhou Y, Suzuki S, Takasaki I,
Koizumi K, Yamaoka S, Tabuchi Y, Saiki I and Sakurai H: Distinct
roles of transforming growth factor-beta-activated kinase 1
(TAK1)-c-Rel and interferon regulatory factor 4 (IRF4) pathways in
human T cell lymphotropic virus 1-transformed T helper 17 cells
producing interleukin-9. J Biol Chem. 286:21092–21099. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee SJ, Noh HJ, Sung EG, Song IH, Kim JY,
Kwon TK and Lee TJ: Berberine sensitizes TRAIL-induced apoptosis
through proteasome-mediated downregulation of c-FLIP and Mcl-1
proteins. Int J Oncol. 38:485–492. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zaidi SF, Refaat A, Zhou Y, Sualeh
Muhammad J, Shin MS, Saiki I, Sakurai H and Sugiyama T:
Helicobacter pylori induces serine phosphorylation of EGFR via
novel TAK1-p38 activation pathway in an HB-EGF-independent manner.
Helicobacter. Feb 23–2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou Y, Tanaka T, Sugiyama N, Yokoyama S,
Kawasaki Y, Sakuma T, Ishihama Y, Saiki I and Sakurai H:
p38-Mediated phosphorylation of Eps15 endocytic adaptor protein.
FEBS Lett. 588:131–137. 2014. View Article : Google Scholar : PubMed/NCBI
|

















