Introduction
Although soft tissue sarcomas rarely metastasize,
they are locally invasive and have a high rate of recurrence
(1). Wide excision is the first
therapeutic choice; however, recurrence is common, even following
this approach. Therefore, the addition of therapies, including
radiation, chemotherapy or a combinations thereof, were reported to
have improved therapeutic benefits (2–5). Feline
vaccine-associated sarcoma (FVAS) in particular exhibits aggressive
local-infiltration tendencies and high recurrence rates. Therefore,
treatment of this type of sarcoma with a combination of these major
therapeutic modalities is advised (6–8).
Indocyanine green (ICG) generates heat when
stimulated by light at a wavelength of 808 nm (9,10) and
produces oxygen radicals when exposed to light at wavelengths of
600–800 nm (11). The use of ICG as a
photosensitizer with a broadband light source, instead of a diode
laser, in photodynamic therapy (PDT) has been established as a
combination therapy consisting of PDT and hyperthermia therapy
(HT), which was named photodynamic hyperthermal therapy (PHT)
(12,13). Previous studies by the current authors
have investigated the anticancer effects of PHT in experiments
in vitro (12) and in
vivo (13) and it has been
demonstrated that the effects of local chemotherapy were enhanced
by HT (14). Subsequently, a method
was developed using local chemotherapy in combination with PHT,
entitled photodynamic hyperthermal chemotherapy (PHCT), which has
previously demonstrated positive results in the treatment of
spontaneous soft tissue sarcomas in dogs and cats (15). The present study investigated the
therapeutic effects of PHCT in six cases of FVAS.
Materials and methods
Animals
Table I presents a
summary of the characteristics of the six cases of FVAS. The
animals were presented for examination at the Aino Animal Hospital
(Fukuroi, Japan) between August 2008 and May 2012. All six cats had
a history of repeated vaccination in the dorsal region of the neck,
interscapulum and scapular region. The ages of the animals ranged
from 9 to 13 years. The breeds included five Domestic Short-Hair
(DSH) cats and one American Short-Hair (ASH) cat. Tumors had
developed in the dorsal thoracic region and had a maximum diameter
range of 3–12 cm. No lung metastasis or bone resorption was
observed on radiography scans in any case. Histopathological
examination of preoperative biopsies or postoperative samples of
the tumors yielded a diagnosis of soft tissue sarcoma (STS) or
fibrosarcoma (FBS); in addition, all subjects were diagnosed with
FVAS on the basis of clinical history and histopathological
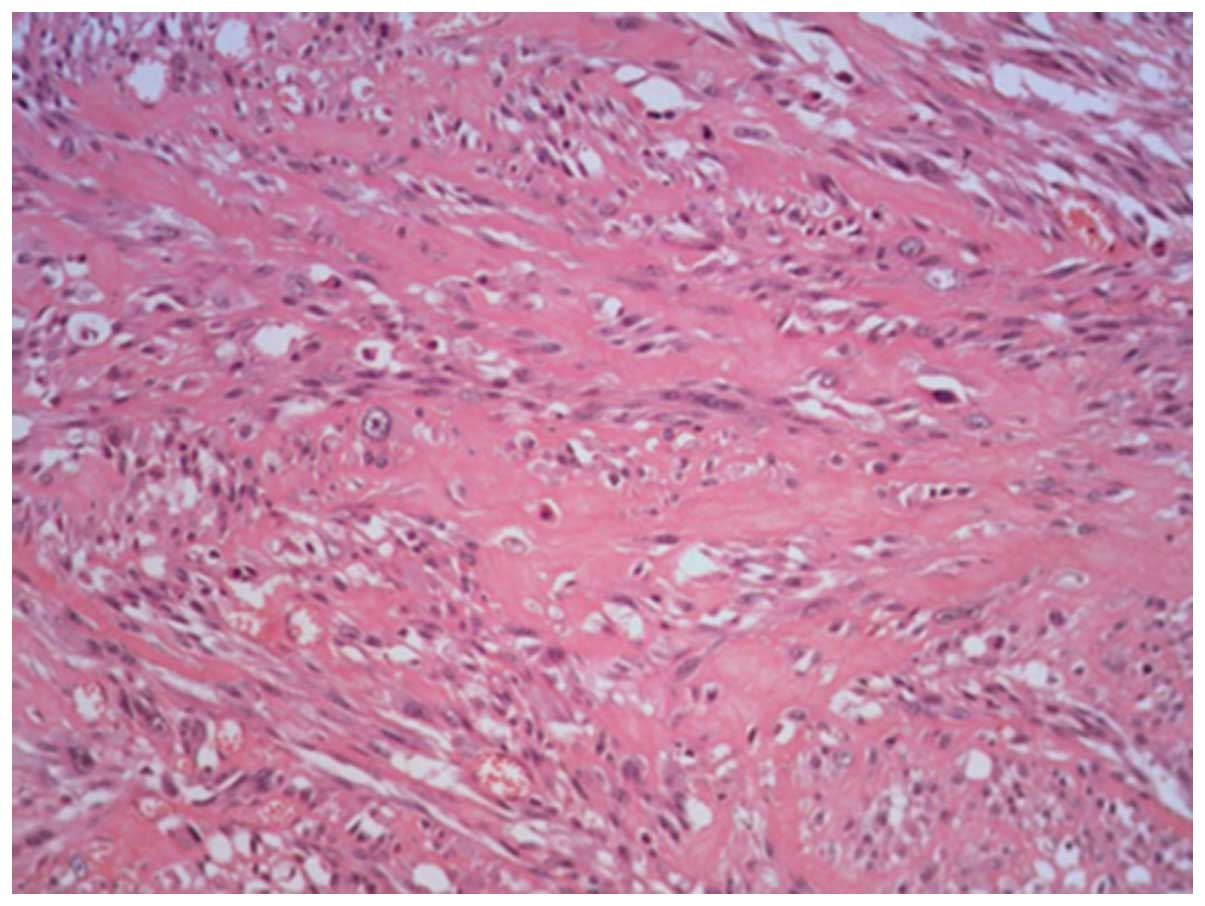
findings: Tumor tissues proliferate with collagen formation in
certain areas and exhibit a fibrosarcoma-like histology (Figs. 1 and 2).
In five out of the six cases, previous surgical resections had been
performed between one and four times at a different veterinary
hospital. Owners were informed about the risk of recurrence of this
sarcoma, necessity of a wide and radical excision, probability of a
functional illness, curative effect of combining surgery with other
therapies, prognosis and financial burden. A combination of
surgery, radiotherapy and chemotherapy performed at Azabu
University Veterinary Teaching Hospital (Sagamihara, Japan) was
proposed to the animal owners as the first therapeutic choice.
However, the owners, desiring to minimize the side effects,
invasiveness and stress of treatment, elected for treatment to take
place at their regular clinic. Other treatments were then discussed
with the owners, including the combination of PHCT and surgery. It
was explained that PHCT was an experimental therapy and all owners
of the pets enrolled in the present study provided written informed
consent.
 | Table I.Summary of six clinical cases of
feline vaccine-associated sarcoma. |
Table I.
Summary of six clinical cases of
feline vaccine-associated sarcoma.
| Case no. | Breed | Gender | Age | Size, cm | Histopathology | TNM |
|---|
| 1 | DSH | Male | 10 | 4.5×4 | STS |
T3N0M0 |
| 2 | DSH | Female | 16 | 9×4 | STS | T4N1bM0 |
| 3 | DSH | Female | 11 | 12×10 | STS |
T4N3M0 |
| 4 | DSH | Male | 9 | 6×6 | FBS |
T3N0M0 |
| 5 | ASH | Female | 12 | 4×4 | STS |
T3N0M0 |
| 6 | ASH | Male | 13 | Multiplea | STS |
T4N1M0 |
As the owners did not desire aggressive surgical
resection, conservative resection with 2–3 cm surgical margins was
performed. Excision involved regions of the neck and associated
muscles, without partial scapulectomy or removal of the dorsal
spinous processes; a representative image (Case 1), of the surgical
field is shown in Fig. 3.
PHCT
The PHCT procedure was performed as previously
reported (15) under general
anesthesia with isoflulene (DS Pharma Animal Health Co., Ltd.,
Osaka, Japan). In brief, ICG (25 mg/vial; Giagnogreen; Daiich
Sankyo Co. Ltd, Tokyo, Japan) was dissolved in 9 ml saline (Otsuka
Pharmacy, Co., Ltd., Tokyo, Japan) with an adjusted pH of 5.0.
Anticancer drugs, including 1 ml carboplatin (50 mg/5 ml; Nippon
Kayaku Co. Ltd, Tokyo, Japan) and 0.1 ml paclitaxel (300 mg/5 ml;
Nippon Kayaku Co., Ltd). Tissue necrosis was observed in case 1 due
to the paclitaxel treatment; as such, the volume of paclitaxel was
reduced to 0.01–0.02 ml paclitaxel (30 mg/5 ml) in subsequent
procedures. The solution was prewarmed at 45°C. A broadband light
source (Super Lizer 5000; Tokyo Iken Co., Ltd, Tokyo, Japan),
emitting a wavelength spectrum from 600 to 1,600 nm with a 5,000 mW
maximum output power, was used. For each case, the tumors were
resected and the ICG solution was injected into the resected area
3-dimensionally, including the skin surgical margin (2–3 cm), at a
concentration of 1 ml per cm3 of the wound bed.
Irradiation was applied at a distance of 10 cm from the resected
area (irradiation area: 113 cm2, 40 mW/cm2)
for 20 min per 113 cm2 (48 J/cm2) immediately
following injection of the ICG solution, under general anesthesia
with isoflulene. A representative image (Case 2) of the surgical
procedure is shown in Fig. 4. The
temperature at the surface of the resected area was monitored with
a thermometer (Tokyo Iken Co., Ltd.) and was kept under 45°C by
altering the proximity of the light source to the skin surface in
order to maintain a uniform temperature at the radiation site; in
addition, the interstitial temperature was maintained at
39.5–42.5°C.
The first round of PHCT was performed immediately
following skin suturing post surgery. The treatment interval
between the second and fourth round of PHCT was generally 1 week;
treatment was then performed at intervals of 2–4 weeks. For the
second and subsequent rounds of PHCT, the treatments were performed
with all animals under either sedation or infiltration anesthesia
using 3–5 ml/head of lidocaine (Xylocaine; AstraZeneca, Inc.,
Osaka, Japan). In all cases, follow-up examinations for recurrence
and metastasis were performed at intervals of 2–3 months for one
year following the first round of the PHCT. Thereafter, follow-up
examinations were performed every 6 months.
Results
The results of the present study are summarized in
Table II. PHCT was performed between
6 and 20 times. The mean frequency of PHCT was 10.8 times (median,
10 times). The median disease-free survival (DFS) was 482 days
(range, 30–1797 days). In three out of six (50%) cases (Cases 1, 4
and 5), no recurrences were observed for 893–1797 days following
surgery; two of these cases had recurred following one previous
surgery. Recurrence was observed between 30 and 70 days post
surgery in the remaining three cases (Cases 2, 3 and 6); these
cases had all undergone more than three surgical resections prior
to PHCT. The three cats that exhibited cancer recurrence succumbed
to progression of the tumor.
 | Table II.Summary of treatment outcomes. |
Table II.
Summary of treatment outcomes.
| Case no. | Anti-cancer drug | No. of
treatments | No. of previous
surgeries | Recurrence | Disease-free survival
(days) |
|---|
| 1 | PTX, CBDCA | 6 | 1 | No |
893 |
| 2 | PTX, CBDCA | 11 | 3 | Yes |
34 |
| 3 | PTX, CBDCA | 10 | 3 | Yes |
70 |
| 4 | PTX, CBDCA | 10 | 1 | No | 1,526 |
| 5 | PTX, CBDCA | 8 | 0 | No | 1,797 |
| 6 | PTX, CBDCA | 20 | 4 | Yes |
30 |
According to the outcomes of the subjects in the
current study, the efficacy of the treatment was not suggested to
be affected by treatment frequency. Although slight skin redness
and minor skin burns occurred following PHCT, no severe side
effects, including severe skin burns and necrosis, were observed in
any of the animals except for Case 1. In Case 1, rupture of the
skin sutures occurred due to an excessive volume of paclitaxel. The
blood profile remained unchanged in all cases.
Discussion
The present study revealed that combination therapy
consisting of conservative surgery and PHCT in FVAS prevented
recurrence of the tumor in the cases that had undergone no or one
previous surgical resection. These results were comparable to those
of a previous study (15).
Reported rates of local recurrence of FVAS following
only conservative excision range from 35 to 59% (16–18).
Radical surgical resection, including two muscle planes and 5 cm
margins, resulted in clean margins in 97% of cases and a local
recurrence rate of 14% (19).
Recurrence rates of 26–52% have been reported for surgical excision
combined with adjuvant therapies, including pre- or postoperative
radiation and chemotherapy (6–8,16,20–24). A
previous study reported that the median DFSs for animals with
tumors treated with surgical resection by a general veterinarian
and a veterinary surgical specialist were 66 and 274 days,
respectively; in addition, the overall median reported DFS was 94
days (25). Furthermore, the median
DFS for radical excisions, including hemipelvectomy, partial
scapulectomy and removal of the dorsal spinous processes, was 325
days, whereas the median DFS with margins of <3 cm was 79 days
(25). These previous studies
indicated that it is difficult to prevent recurrence with routine
surgical excision alone. By contrast, the therapeutic effect of
chemotherapy alone is inadequate, as revealed by a previous study
which reported a 39% rate of effectiveness and a median DFS of 84
days (range, 21–240 days) (20).
Therefore, radiotherapy performed at the earliest possible time
following surgical resection has been recommended for the treatment
of FVAS (6–8,26), as
median DFSs of 661 days (23–1109 days) (7) and 584 days (37–2490 days) (8) have been obtained with this treatment.
Thus, this combined therapeutic approach is more effective compared
with surgery alone. However, radiotherapy is not readily accessible
to general practice veterinarians and owners due to the limited
availability of radiotherapy facilities. Therefore, few animals may
benefit from this treatment.
In the present study, the three cases (cases 2,3 and
6) that displayed cancer recurrence following PHCT had undergone
three or more previous surgical resections and recurrence was
observed earlier following PHCT in these cases. With regard to the
histology of the tumor tissues resected in the present study, no
difference in the characteristics of the malignancy was recognized
among the cases with multiple recurrences (Cases 2, 3 and 6) and
the cases with no recurrence with this treatment (Cases 1, 4 and
5). Of note, conservative surgeries were performed for all cases.
The present protocol of PHCT was unable to prevent recurrence in
all the present cases and cancer recurrences may have occurred due
to a more extensive tumor cell invasion in the cats with recurrence
compared with those without recurrence. Therefore, further
investigation of certain factors, including anticancer drugs and
the treatment interval, is necessary in order to prevent
recurrence.
In conclusion, in the present study, the overall
recurrence rate was 50% and the median DFS was 482 days; however,
the recurrence rate was 0% in the incipient cases or those with
only one previous recurrence. Compared with the results of previous
reports, these results suggested that PHCT may have an equivalent
effect to that of advanced treatments, including radiotherapy. In
the present study, conservative excision was performed in order to
preserve the basal region of the muscular tissue and spinous
processes with 2–3 cm surgical margins. Therefore, it was suggested
that the risk of recurrence may be equivalent or increased compared
with previous studies. However, the median DFS of the animals
treated with PHCT was greater than that previously reported for
conservative surgical resection. As a result, PHCT may be
considered a useful adjuvant therapeutic modality for the treatment
of FVAS.
References
|
1
|
Kuntz CA, Dernell WS, Powers BE, Devitt C,
Straw RC and Withrow SJ: Prognostic factors for surgical treatment
of soft-tissue sarcomas in dogs: 75 cases (1986–1996). J Am Vet Med
Assoc. 211:1147–1151. 1997.PubMed/NCBI
|
|
2
|
Ettinger SN: Principles of treatment for
soft-tissue sarcomas in the dog. Clin Tech Small Anim Pract.
18:118–122. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McChesney SL, Gillette EL, Dewhirst MW and
Withrow SJ: Influence of WR 2721 on radiation response of canine
soft-tissue sarcomas. Int J Radiat Oncol Biol Phys. 12:1957–1963.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ogilvie GK, Reynolds HA, Richardson RC,
Withrow SJ, Norris AM, Henderson RA, Klausner JS, Fowler JD and
McCaw D: Phase II evaluation of doxorubicin for treatment of
various canine neoplasms. J Am Vet Med Assoc. 195:1580–1583.
1989.PubMed/NCBI
|
|
5
|
Ogilvie GK, Obradovich JE, Elmslie RE,
Vail DM, Moore AS, Straw RC, Dickinson K, Cooper MF and Withrow SJ:
Efficacy of mitoxantrone against various neoplasms in dogs. J Am
Vet Med Assoc. 198:1618–1621. 1991.PubMed/NCBI
|
|
6
|
Cronin K, Page RL, Spodnick G, Dodge R,
Hardie EN, Price GS, Ruslander D and Thrall DE: Radiation therapy
and surgery for fibrosarcoma in 33 cats. Vet Radiol Ultrasound.
39:51–56. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bregazzi VS, LaRue SM, McNiel E, Macy DW,
Dernell WS, Powers BE and Withrow SJ: Treatment with a combination
of doxorubicin, surgery, and radiation versus surgery and radiation
alone for cats with vaccine-associated sarcomas: 25 cases
(1995–2000). J Am Vet Med Assoc. 218:547–550. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kobayashi T, Hauck ML, Dodge R, Page RL,
Price GS, Williams LE, Hardie EM, Mathews KG and Thrall DE:
Preoperative radiotherapy for vaccine associated sarcoma in 92
cats. Vet Radiol Ultrasound. 43:473–479. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen WR, Adams RL, Bartels KE and
Nordquist RE: Chromophore-enhanced in vivo tumor cell destruction
using an 808-nm diode laser. Cancer Lett. 94:125–131. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen WR, Adams RL, Higgins AK, Bartels KE
and Nordquist RE: Photothermal effects on murine mammary tumors
using indocyanine green and an 808-nm diode laser: An in vivo
efficacy study. Cancer Lett. 98:169–173. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hirano T, Kohno E, Gohto Y and Obana A:
Singlet oxygen generation by irradiation of Indocyanine green (ICG)
and its effect to tissues. J Jpn Soc Laser Surg Med. 28:122–128.
2007.
|
|
12
|
Radzi R, Osaki T, Tsuka T, Imagawa T,
Minami S, Nakayama Y and Okamoto Y: Photodynamic hyperthermal
therapy with indocyanine green (ICG) induces apoptosis and cell
cycle arrest in B16F10 murine melanoma cells. J Vet Med Sci.
74:545–551. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Onoyama M, Azuma K, Tsuka T, Imagawa T,
Osaki T, Minami S, Ogawa N and Okamoto Y: Effects of photodynamic
hyperthermal therapy with indocyanine green on tumor growth in a
colon 26 tumor-bearing mouse model. Oncol Lett. 7:1147–1150.
2014.PubMed/NCBI
|
|
14
|
Ohguri T, Imada H, Narisada H and Korogi
Y: Clinical results of systemic chemotherapy combined with regional
hyperthermia. Thermal Med (Japanese Journal of Hyperthermic
Oncology). 23:49–61. 2007.(In Japanese). View Article : Google Scholar
|
|
15
|
Onoyama M, Tsuka T, Imagawa T, Osaki T,
Minami S, Azuma K, Kawashima K, Ishi H, Ogawa N and Okamoto Y:
Photodynamic hyperthermal chemotherapy with indocyanine green: A
novel cancer therapy for 16 cases of malignant soft tissue sarcoma.
J Vet Sci. 15:117–123. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Martano M, Morello E, Ughetto M, Iussich
S, Petterino C, Cascio P and Buracco P: Surgery alone versus
surgery and doxorubicin for the treatment of feline injection-site
sarcomas: A report on 69 cases. Vet J. 170:84–90. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Banerji N and Kanjilal S: Somatic
alterations of the p53 tumor suppressor gene in vaccine-associated
feline sarcoma. Am J Vet Res. 67:1766–1772. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Giudice C, Stefanello D, Sala M, Cantatore
M, Russo F, Romussi S, Travetti O, Di Giancamillo M and Grieco V:
Feline injection-site sarcoma: Recurrence, tumour grading and
surgical margin status evaluated using the three-dimensional
histological technique. Vet J. 186:84–88. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Phelps HA, Kuntz CA, Milner RJ, Powers BE
and Bacon NJ: Radical excision with five-centimeter margins for
treatment of feline injection-site sarcomas: 91 cases (1998–2002).
J Am Vet Med Assoc. 239:97–106. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Poirier VJ, Thamm DH, Kurzman ID, Jeglum
KA, Chun R, Obradovich JE, O'Brien M, Fred RM III, Phillips BS and
Vail DM: Liposome-encapsulated doxorubicin (Doxil) and doxorubicin
in the treatment of vaccine-associated sarcoma in cats. J Vet
Intern Med. 16:726–731. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Davidson EB, Gregory CR and Kass PH:
Surgical excision of soft tissue fibrosarcomas in cats. Vet Surg.
26:265–269. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cohen M, Wright JC, Brawner WR, Smith AN,
Henderson R and Behrend EN: Use of surgery and electron beam
irradiation, with or without chemotherapy, for treatment of
vaccine-associated sarcomas in cats: 78 cases (1996–2000). J Am Vet
Med Assoc. 219:1582–1589. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hahn KA, Endicott MM, King GK and
Harris-King FD: Evaluation of radiotherapy alone or in combination
with doxorubicin chemotherapy for the treatment of cats with
incompletely excised soft tissue sarcomas: 71 cases (1989–1999). J
Am Vet Med Assoc. 231:742–745. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Romanelli G, Marconato L, Olivero D,
Massari F and Zini E: Analysis of prognostic factors associated
with injection-site sarcomas in cats: 57 cases (2001–2007). J Am
Vet Med Assoc. 232:1193–1199. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hershey AE, Sorenmo KU, Hendrick MJ,
Shofer FS and Vail DM: Prognosis for presumed feline
vaccine-associated sarcoma after excision: 61 cases (1986–1996). J
Am Vet Med Assoc. 216:58–61. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Eckstein C, Guscetti F, Roos M, Martín de
las Mulas J, KaserHotz B and Rohrer Bley C: A retrospective
analysis of radiation therapy for the treatment of feline
vaccine-associated sarcoma. Vet Comp Oncol. 7:54–68. 2009.
View Article : Google Scholar : PubMed/NCBI
|