Introduction
Malignant glioma, which consists mainly of
anaplastic glioma and glioblastoma (GBM), represents up to 50% of
all primary brain gliomas (1) and is
the most common primary brain tumor in adults. The clinical outcome
in patients with malignant glioma is poor despite the improvements
in survival rate since the use of adjuvant chemoradiotherapy
post-operatively (2).
It has been reported that the prognosis of malignant
glioma may be affected by several factors, including not only
patient age, pre-operative Karnofsky performance status (KPS),
histological grade, pathological molecular markers (such as MGMT,
IDH1, 1p19q and PDGF-α) and temozolomide chemotherapy (3–7), but also
certain pre-operative magnetic resonance imaging (MRI) features on
routine scans, such as peritumoral edema (PTE) extent, degree of
necrosis, enhancement extent and the size of the cyst, among others
(5,8–11).
However, several studies have also shown that certain features of
pre-operative MRI, such as PTE, cysts and tumor size, are not
independent predictors of survival in patients with glioma
(12–14).
PTE is a significant contributor to morbidity and
mortality from glioma (15), but a
recent systematic review suggested that controversy remains with
regard to its prognostic value (14).
A requirement therefore exists to further evaluate the association
between PTE on MRI and patient survival, as such data from routine
imaging are irreplaceable during the formation of a pre-operative
diagnosis and are now the central basis of treatment decisions in
patients with glioma. Determination of clear associations between
these factors has a certain instructive significance for clinical
practice. In the present study, the prognostic value of MRI
features from pre-operative routine scans was assessed in patients
with malignant glioma in order to confirm which would be the most
valuable prognostic markers, as this data may provide aid in
everyday clinical activities.
Patients and methods
Patients and clinical data
The clinical and pre-operative MRI data of 109
patients treated by resection of a newly diagnosed supratentorial
malignant glioma at the First Affiliated Hospital of Fujian Medical
University (Fuzhou, China) between March 2006 and September 2012
were included in this retrospective study. Patients who succumbed
to non-glioma-based causes or those who received a biopsy were
excluded from the study. No patients received corticosteroids at
the time of the pre-operative MRI scan. For all patients, the tumor
was confirmed to be totally resected using post-operative enhanced
MRI within 3 days. According to the principles of the World Health
Organization classification (16),
the histological diagnosis and grading of each patient were
reaffirmed. In total, 65 cases were classified as GBM (grade 4) and
44 as anaplastic glioma (grade 3), consisting of anaplastic
astrocytoma, anaplastic oligodendroglioma and anaplastic
oligoastrocytoma. In the cohort, 65 patients were male and 44 were
female. The median age at diagnosis was 49 years (range, 18–78
years). The median pre-operative KPS of the patients was 80 (range,
70–100). Post-operatively, 53 patients were treated with standard
chemoradiotherapy (radiotherapy plus chemotherapy) and 56 were
treated with non-standard chemoradiotherapy (17) (radiotherapy alone, chemotherapy alone,
and no radiotherapy or chemotherapy). Oral temozolomide
chemotherapy (150–200 mg/m2/day) was administered for
4–6 cycles unless mortality or irreversible hematological toxicity
occurred. Radiotherapy was administered to the contrast-enhanced
lesion plus the area of the PTE and a 2-cm margin (60 Gy in 2-Gy
fractions). All patients were followed up via telephone
conversations or outpatient visits. Overall survival (OS) was
defined as the time (months) between the primary surgical resection
and mortality or the latest follow-up. This study was approved by
Ethics Committee of Fujian Medical University and conformed to the
principles outlined in the Declaration of Helsinki. Written
informed consent was provided by all patients.
Classification of MRI features
For all patients, pre-operative MRI data from
routine scans, including T1-weighted (W), T2-W and
contrast-enhanced T1-W sequences were available. The unidimensional
maximum diameter (cm) was used for measuring the tumor size on the
T1-W images, and the median tumor size was recorded as 5.0 cm
(range, 2.3–9.0 cm). The bright T2-W signal region surrounding the
tumor was defined as PTE, which was estimated based on the maximum
distance from the tumor margin to the outer edge of the edema, and
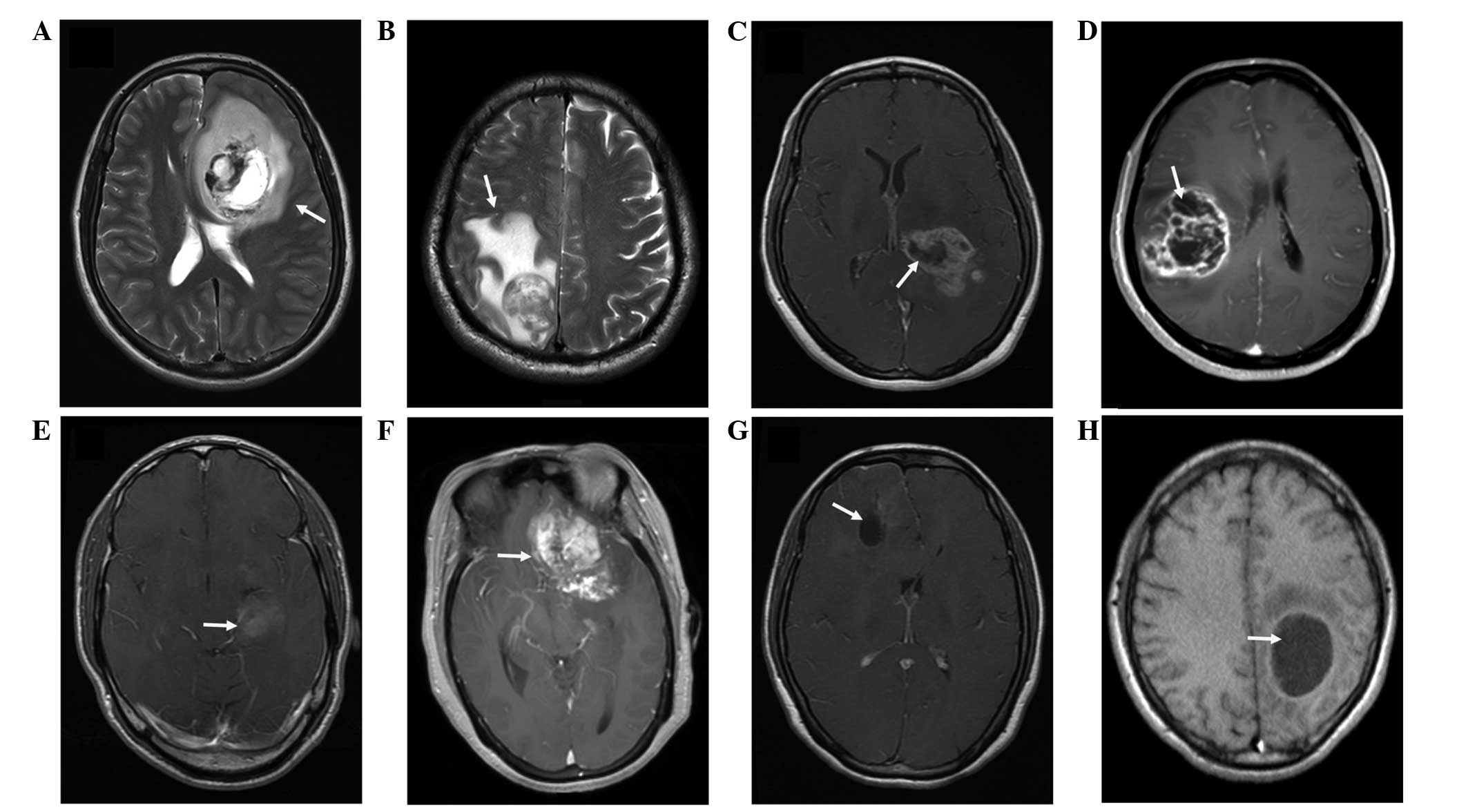
was graded into minor (Fig. 1A) and
major (Fig. 1B) types (5). According to the method reported by
Hartmann et al (18), the
morphological classification of PTE was performed on the basis of
the T2-W images (Fig. 1A and B).
Necrosis, which was estimated on the axial contrast-enhanced T1-W
images (19), was demonstrated when a
region had a high signal intensity on T2-W images, but a low signal
intensity on T1-W images, and an irregular enhancing border on
contrast-enhanced images (Fig. 1C and
D). Enhancement was defined as not marked or marked when the
enhancement signal was less than (Fig.
1E) or similar to (Fig. 1F) the
signal intensity of fat, respectively. Cysts (Fig. 1G and H) were defined as rounded
regions that exhibited low intensity T1-W signals and extremely
high intensity T2-W signals matching the cerebrospinal fluid
signal. Additionally, the regions presented with a thin, smooth,
regular, slightly enhancing or non-enhancing wall (9). The specific classification of the
imaging features is listed in Table I
and an example of this classification is presented in Fig. 2. According to these aforementioned
classification methods, the imaging data of all the patients were
analyzed independently by two experienced radiologists who were
blinded to the patient's clinical information.
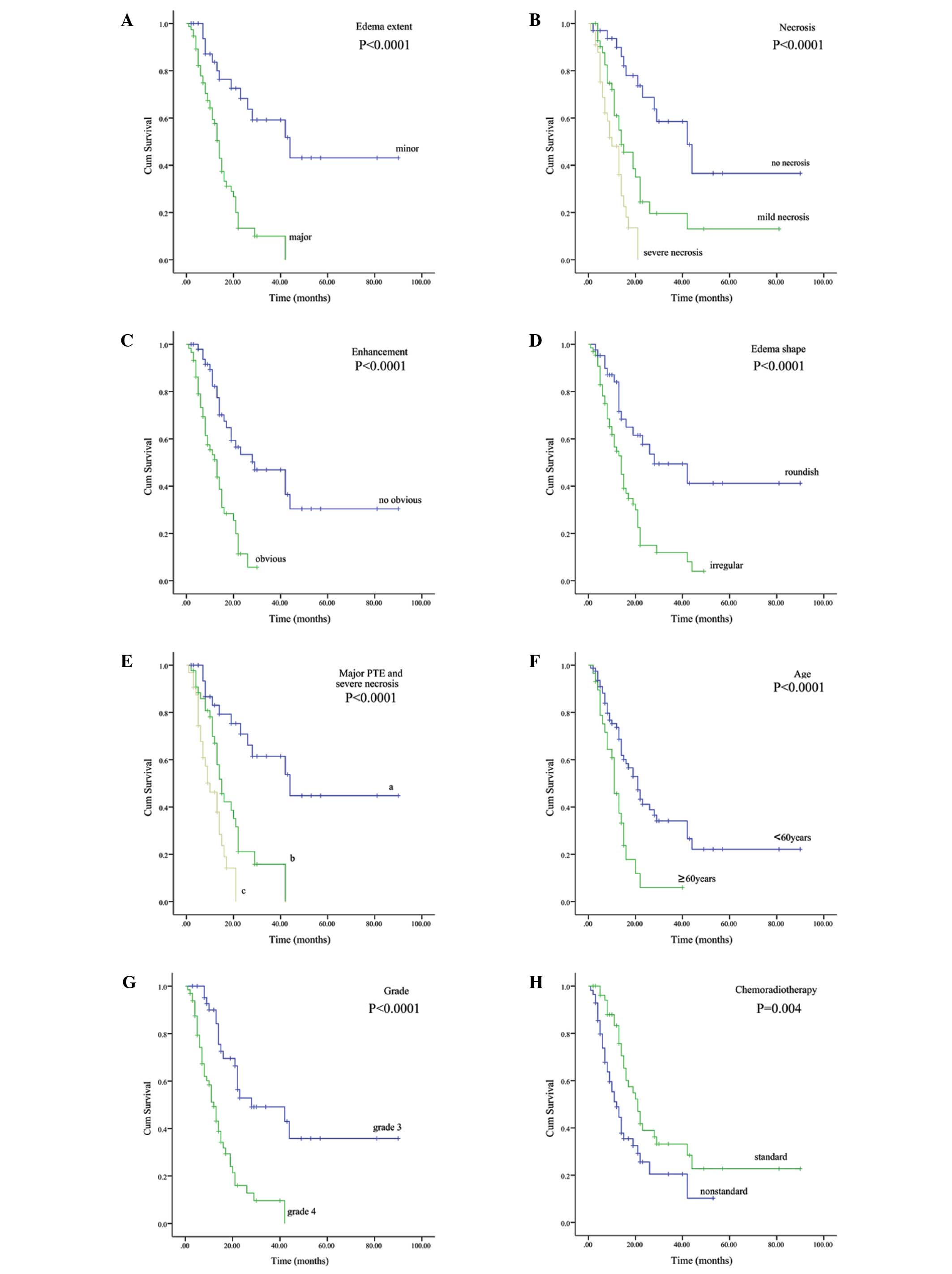 | Figure 2.Correlations between (A) PTE, (B)
necrosis, (C) enhancement, (D) edema shape, (E) major PTE and
severe necrosis, (F) age, (G) grade and (H) chemoradiotherapy, and
overall survival in the entire cohort (Kaplan-Meier curves). (E) a,
the group with two unfavorable factors (major PTE and severe
necrosis); b, the group with only one unfavorable factor (major PTE
or severe necrosis); c, the group without major PTE and severe
necrosis. PTE, peritumoral edema. |
 | Table I.Specific classification of imaging
features. |
Table I.
Specific classification of imaging
features.
| Imaging features | Classification
criteria |
|---|
| PTE |
|
|
Minor | PTE extending <1
cm from tumor margin |
|
Major | PTE extending ≥1 cm
from tumor margin |
| Edema shape |
|
|
Rounded | The edema shape is
rounded and is not radial |
|
Irregular | The edema shape tends
to be irregular, e.g., finger-like or radial shapes |
| Necrosis |
|
| No | No necrosis within
the tumor |
| Mild | Necrosis affecting
≤50% of the tumor |
|
Severe | Necrosis affecting
>50% of the tumor |
| Cyst |
|
| No | No cyst in the
tumor |
|
Small | Cyst cavity in ≤50%
of the total tumor |
|
Large | Cyst cavity in
>50% of the total tumor |
| Enhancement |
|
| No
marked | Enhancement signal
is less than the signal intensity of fat |
|
Marked | Enhancement signal
is similar to the signal intensity of fat |
| Tumor crosses
midline |
|
| No | Tumor is limited to
the unilateral cerebral hemisphere |
|
Yes | Tumor crosses the
brain midline and extends into the other side of the cerebral
hemisphere |
| Edema crosses
midline |
|
| No | PTE extent is
limited to the unilateral cerebral hemisphere |
|
Yes | PTE extent crosses
the brain midline and is not confined to the unilateral cerebral
hemisphere |
| Size, cm |
|
|
<5 | The maximum
diameter of the tumor is <5 cm |
| ≥5 | The maximum
diameter of the tumor is ≥5 cm |
Statistical analysis
SPSS 19.0 (IBM SPSS, Armonk, NY, USA) was applied
for the statistical analysis. For the univariate analysis, the
Kaplan-Meier method was used to calculate survival rates, which
were compared by the log-rank test. Factors that were statistically
significant on the univariate analysis were analyzed on
multivariate analysis. COX proportional hazards model and stepwise
regression analysis were applied to estimate the effect of
pre-operative MRI features on survival in the multivariate
analysis. P≤0.05 (two-sided) was considered to indicate a
statistically significant difference.
Results
PTE
Univariate analysis (Table II) showed that patients with major
PTE survived a significantly shorter amount of time than those with
minor PTE (P<0.0001; Fig. 2A); the
median OS time was 14.0 and 44.0 months, respectively. Multivariate
analysis (Table III) indicated that
the PTE extent shown by pre-operative MRI was an independent
prognostic factor in patients with malignant glioma [P=0.029;
hazard ratio (HR), 2.337].
 | Table II.Variables associated with OS in the
entire cohort: Univariate analysis. |
Table II.
Variables associated with OS in the
entire cohort: Univariate analysis.
|
|
| OS, months |
|
|---|
|
|
|
|
|
|---|
| Variable | Number of
cases | Median | 95% CI | P-value |
|---|
| Total | 109 | 15.0 | 11.2–18.8 |
| Gender |
|
|
| 0.357 |
|
Male | 65 | 15.0 | 10.2–19.8 |
|
Female | 44 | 16.0 |
9.8–22.2 |
| Age, years |
|
|
| <0.0001 |
|
≥60 | 29 | 11.0 |
8.6–13.4 |
|
<60 | 80 | 21.0 | 16.2–25.8 |
| KPS |
|
|
| <0.0001 |
|
≤80 | 42 | 13.0 | 10.6–16.8 |
|
>80 | 67 | 26.0 | 16.2–30.8 |
|
Chemoradiotherapy |
|
|
| 0.004 |
|
Standard | 53 | 21.0 | 16.1–26.0 |
|
Non-standard | 56 | 12.0 |
8.1–15.9 |
| Pathological
grade |
|
|
| <0.0001 |
| Grade
3 | 44 | 28.0 |
8.0–54.0 |
| Grade
4 | 65 | 12.0 |
9.5–14.5 |
| PTE |
|
|
| <0.0001 |
|
Minor | 34 | 44.0 | 20.4–67.6 |
|
Major | 75 | 14.0 | 12.3–15.7 |
| Edema shape |
|
|
| <0.0001 |
|
Rounded | 43 | 28.0 |
7.0–49.0 |
|
Irregular | 66 | 14.0 | 10.7–17.3 |
| Necrosis |
|
|
| <0.0001 |
|
None | 33 | 42.0 | 25.3–58.7 |
|
Mild | 43 | 14.0 |
8.2–19.8 |
|
Severe | 33 | 10.0 |
6.1–14.0 |
| Cyst |
|
|
| 0.593 |
|
None | 71 | 16.0 | 10.1–21.9 |
|
Small | 22 | 15.0 | 11.6–18.4 |
|
Large | 16 | 15.0 |
7.3–22.7 |
| Enhancement |
|
|
| <0.0001 |
| No
marked | 50 | 29.0 | 12.1–45.9 |
|
Marked | 59 | 13.0 |
9.4–16.6 |
| Tumor crosses
midline |
|
|
| 0.762 |
| No | 89 | 15.0 | 11.6–18.4 |
|
Yes | 20 | 21.0 |
9.0–33.0 |
| Edema crosses
midline |
|
|
| 0.220 |
| No | 77 | 17.0 | 12.7–21.3 |
|
Yes | 32 | 13.0 |
9.2–16.8 |
| Size, cm |
|
|
| 0.467 |
|
<5 | 65 | 15.0 | 12.4–17.6 |
| ≥5 | 44 | 19.0 | 10.7–27.3 |
 | Table III.Statistically significant prognosis
indicators evaluated by multivariate analysis in the entire
cohort. |
Table III.
Statistically significant prognosis
indicators evaluated by multivariate analysis in the entire
cohort.
| Variables | Hazard ratio | 95% CI | P-value |
|---|
| PTE | 2.337 | 1.089–5.015 | 0.029 |
| Necrosis | 2.218 | 1.447–3.401 | <0.0001 |
| Pathological
grade | 2.066 | 1.150–3.713 | 0.015 |
| Age, years | 1.954 | 1.137–3.358 | 0.015 |
| KPS | 1.892 | 1.230–3.371 | 0.023 |
|
Chemoradiotherapy | 0.481 | 0.287–0.806 | 0.005 |
Necrosis
The degree of necrosis on pre-operative MRI was also
an independent prognostic factor in malignant glioma (P<0.0001;
HR, 2.218), with a median OS time of 42.0, 14.0 and 10.0 months for
patients with no, mild and severe necrosis, respectively
(P<0.0001; Fig. 2B).
Additionally, the prognosis in the patients with two
unfavorable factors (major edema and severe necrosis) was markedly
poor compared with those with only one unfavorable factor and those
without unfavorable factors (P<0.0001; Fig 2E), with median OS times of 10.0, 15.0
and 44.0 months, respectively.
Other imaging features
On univariate analysis, a significant difference
(P<0.0001; Fig. 2C) was indicated
between the patients with no marked enhancement and those with
marked enhancement, with median OS times of 29.0 and 13.0 months,
respectively. However, multivariate analysis showed that the extent
of enhancement was not an independent prognostic factor. Similarly,
a significant difference (P<0.0001; Fig. 2D) existed among patients with edema of
rounded and irregular shapes, with median OS times of 28.0 and 14.0
months, respectively However, multivariate analysis failed to
confirm this significance. In addition, univariate analysis showed
that cysts (P=0.593), tumor crossing the midline (P=0.762), edema
crossing the midline (P=0.220) and tumor size (P=0.467) were not
significantly associated with the prognosis in malignant
glioma.
Association between gender, age, KPS
or pathological grade and patient prognosis
Gender did not affect the clinical outcome
(P=0.357), but age (P<0.0001; Fig.
2F), KPS (P<0.0001), pathological grade (P<0.0001;
Fig. 2G) and post-operative
chemoradiotherapy (P=0.004; Fig. 2H)
were correlated with patient survival. Multivariate analysis
indicated that age (P=0.015), KPS (P=0.023), pathological grade
(P=0.015) and chemoradiotherapy (P=0.005) were all independent
prognostic factors in the patients with malignant glioma.
Discussion
In the clinic, MRI is a routine examination for
central nervous system diseases, and in particular, for the
pre-operative diagnosis of glioma. Moreover, different signs shown
by pre-operative MRI reflect the different biological behaviors of
glioma. An improved understanding of the correlation between
pre-operative MRI features and survival is therefore critical to
clinical practice. The present study retrospectively analyzed
several pre-operative MRI features, and found that PTE and necrosis
were statistically significant unfavorable prognosis indicators
affecting OS in patients with newly diagnosed supratentorial
malignant glioma.
PTE, one of the main characteristics of malignant
glioma, is a significant contributor to morbidity and mortality
from glioma (15). The present study
found that patients with major PTE exhibited a significantly worse
OS time, and that PTE was an independent prognosis indicator. This
may be associated with the fact that the glioma cells infiltrate
the peritumoral area (20). Moreover,
a recent study discovered a population of glioma stem cells
infiltrating the area of PTE (21),
and it has been found that these cells, which exhibit resistance to
therapy, are the source of tumor recurrence (22–24). These
data have provided convincing evidence that PTE is an indicator of
an adverse prognosis in malignant glioma. However, a recent
systematic review (14) suggested
that the association between pre-operative PTE and survival in
patients with glioma remains a controversial topic; one explanation
may be that considerable heterogeneity exists in terms of patient
clinical characteristics and the MRI technology used in these
studies. Another factor that is inconsistent between the studies is
the discrepancy in the measurement and classification of PTE. The
present study also showed that patients with a rounded edema shape
survived longer than those with an irregular edema shape, but
multivariate analysis indicated that edema shape was not an
independent predictor of prognosis. We speculate that edema extent
may contribute to the result that edema shape affects patient
survival, as this is consistent with the phenomenon observed in the
clinic where PTE extent in patients with an irregular edema shape
(such as a radial or finger-like shape) tends to be severe.
Necrosis is one of the pathological characteristics
of malignant glioma. Previous studies have suggested that tumor
necrosis extent is associated with a poor clinical outcome in
malignant glioma (8,25,26). The
present study also found that tumor necrosis shown by pre-operative
MRI was an independent unfavorable prognosis factor. This may be
associated with the malignant biological behaviors of glioma cells
in necrotic areas. The rapid cellular proliferation of malignant
glioma causes nutrient imbalance, which leads to hypoxia and
necrosis in tumor tissue. Necrotic areas are typically surrounded
by pseudopalisading cells, which are relatively unique to malignant
glioma and have long been considered as an unfavorable prognostic
indicator (27). Moreover, the
pseudopalisading cells show a more hypoxic nature (28), which not only is suspected to
contribute to glioma development and progress (29), but also would promote tumor
recurrence, and improve the invasion ability and resistance to
radiochemotherapy (30).
It was previously believed that enhancement on
pre-operative MRI was an independent predictor of survival in
malignant glioma (8,25). However, in the present study, the
enhancement extent was associated with the OS of the patients with
malignant glioma on univariate analysis, while it failed to retain
its significance on multivariate analysis. One proposed explanation
is that the enhancement of brain tumors mainly reflects the
breakdown of the blood brain barrier and is affected by all
processes that increase or decrease the abnormal permeability,
regardless of the size and activity of tumor (31). Previous studies showed that cysts were
associated with a better prognosis in low-grade glioma (32,33). It is
likely that this is since cyst formation represents more indolent
neoplasm growth and therefore, improved survival (12). Nevertheless, the present study found
that cysts did not affect the clinical outcome in patients with
malignant glioma, which is in accordance with a previous
large-scale study (12). The proposed
hypothesis holds that cyst formation is associated with a disturbed
blood brain barrier (34) or
malignant transformation of a glioma (35). However, the exact mechanism of cyst
formation remains unknown and requires further research.
Additionally, it has been reported that age and
pathological grade are independent predictors of prognosis in
malignant glioma (3,4), which is confirmed by the present
results. We speculate that it may be due to the biological
characteristics of patient at different age groups and the
biological behaviors of glioma cells with different pathological
grades. In the present study, post-operative standard chemotherapy
plus radiotherapy was able to prolong the survival time of the
malignant glioma patients, in accordance with the results of
previous studies (2,36). Therefore, we advocate that standard
chemoradiotherapy for patients with malignant glioma should be
actively pursued post-operatively.
However, a number of limitations that should be
acknowledged exist in the present study. Firstly, this is a
retrospective study, which may inevitably be subject to bias for
information collection and patient selection. Moreover, the sample
size is small in the study. Therefore, the association between
pre-operative MRI features and prognosis in patients with
supratentorial malignant glioma requires further analysis through
large-scale and prospective studies, and particularly, the key
molecular mechanisms of those independent predictors of survival
require identification.
In conclusion, PTE and necrosis shown by MRI on
pre-operative routine scans are independent indicators of an
unfavorable prognosis, and patients with major edema plus severe
necrosis exhibit a poorer prognosis, thereby indicating that PTE
and extent of necrosis on routine MRI scans can be used to predict
OS in patients with newly diagnosed supratentorial malignant
glioma.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation of China (grant no. 30973083)
and the Key Clinical Special Discipline Construction Program of
Fujian (grant no. 2010-149).
References
|
1
|
Zhang X, Zhang W, Cao WD, Cheng G and
Zhang YQ: Glioblastoma multiforme: Molecular characterization and
current treatment strategy (Review). Exp Ther Med. 3:9–14.
2012.PubMed/NCBI
|
|
2
|
Yang LJ, Zhou CF and Lin ZX: Temozolomide
and radiotherapy for newly diagnosed glioblastoma multiforme: A
systematic review. Cancer Invest. 32:31–36. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Buckner JC: Factors influencing survival
in high-grade gliomas. Semin Oncol. 30:10–14. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang LS, Huang FP, Zheng K, et al: Factors
affecting prognosis of patients with intracranial anaplastic
oligodendrogliomas: A single institutional review of 70 patients. J
Neurooncol. 100:113–120. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schoenegger K, Oberndorfer S, Wuschitz B,
et al: Peritumoral edema on MRI at initial diagnosis: An
independent prognostic factor for glioblastoma? Eur J Neurol.
16:874–878. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Das P, Puri T, Jha P, Pathak P, Joshi N,
Suri V, Sharma MC, Sharma BS, Mahapatra AK, Suri A and Sarkar C: A
clinicopathological and molecular analysis of glioblastoma
multiforme with long-term survival. J Clin Neurosci. 18:66–70.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Arshad H, Ahmad Z and Hasan SH: Gliomas:
Correlation of histologic grade, Ki67 and p53 expression with
patient survival. Asian Pac J Cancer Prev. 11:1637–1640.
2010.PubMed/NCBI
|
|
8
|
Hammoud MA, Sawaya R, Shi W, Thall PF and
Leeds NE: Prognostic significance of preoperative MRI scans in
glioblastoma multiforme. J Neurooncol. 27:65–73. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pope WB, Sayre J, Perlina A, Villablanca
JP, Mischel PS and Cloughesy TF: MR imaging correlates of survival
in patients with high-grade gliomas. AJNR Am J Neuroradiol.
26:2466–2474. 2005.PubMed/NCBI
|
|
10
|
Maldaun MV, Suki D, Lang FF, Prabhu S, Shi
W, Fuller GN, Wildrick DM and Sawaya R: Cystic glioblastoma
multiforme: Survival outcomes in 22 cases. J Neurosurg. 100:61–67.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li WB, Tang K, Chen Q, Li S, Qiu G, Li SW
and Jiang T: MRI manifestions correlate with survival of
glioblastoma multiforme patients. Cancer Biol Med. 9:120–123.
2012.PubMed/NCBI
|
|
12
|
Kaur G, Bloch O, Jian BJ, Kaur R, Sughrue
ME, Aghi MK, McDermott MW, Berger MS, Chang SM and Parsa AT: A
critical evaluation of cystic features in primary glioblastoma as a
prognostic factor for survival. J Neurosurg. 115:754–759. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pierallini A, Bonamini M, Osti MF, Pantano
P, Palmeggiani F, Santoro A, Enrici R Maurizi and Bozzao L:
Supratentorial glioblastoma: Neuroradiological findings and
survival after surgery and radiotherapy. Neuroradiology. 38(Suppl
1): S26–S30. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu SY, Mei WZ and Lin ZX: Pre-operative
peritumoral edema and survival rate in glioblastoma multiforme.
Onkologie. 36:679–684. 2013.PubMed/NCBI
|
|
15
|
Lin ZX: Glioma-related edema: New insight
into molecular mechanisms and their clinical implications. Chin J
Cancer. 32:49–52. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lin GS, Yang LJ, Wang XF, Chen YP, Tang
WL, Chen L and Lin ZX: STAT3 Tyr705 phosphorylation affects
clinical outcome in patients with newly diagnosed supratentorial
glioblastoma. Med Oncol. 31:9242014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hartmann M, Jansen O, Egelhof T, Forsting
M, Albert FK and Sartor K: Effect of brain edema on the recurrence
pattern of malignant gliomas. Radiologe. 38:948–953. 1998.(In
German). View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Seidel C, Dörner N, Osswald M, Wick A,
Platten M, Bendszus M and Wick W: Does age matter? A MRI study on
peritumoral edema in newly diagnosed primary glioblastoma. BMC
Cancer. 11:1272011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yamahara T, Numa Y, Oishi T, Kawaguchi T,
Seno T, Asai A and Kawamoto K: Morphological and flow cytometric
analysis of cell infiltration in glioblastoma: A comparison of
autopsy brain and neuroimaging. Brain Tumor Pathol. 27:81–87. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ruiz-Ontañon P, Orgaz JL, Aldaz B,
Elosegui-Artola A, Martino J, Berciano MT, Montero JA, Grande L,
Nogueira L, Diaz-Moralli S, et al: Cellular plasticity confers
migratory and invasive advantages to a population of
glioblastoma-initiating cells that infiltrate peritumoral tissue.
Stem Cells. 31:1075–1085. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang X, Zhang W, Mao XG, Zhen HN, Cao WD
and Hu SJ: Targeting role of glioma stem cells for glioblastoma
multiforme. Curr Med Chem. 20:1974–1984. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen J, Li Y, Yu TS, McKay RM, Burns DK,
Kernie SG and Parada LF: A restricted cell population propagates
glioblastoma growth after chemotherapy. Nature. 488:522–526. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mangiola A, de Bonis P, Maira G, Balducci
M, Sica G, Lama G, Lauriola L and Anile C: Invasive tumor cells and
prognosis in a selected population of patients with glioblastoma
multiforme. Cancer. 113:841–846. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lacroix M, Abi-Said D, Fourney DR,
Gokaslan ZL, Shi W, DeMonte F, Lang FF, McCutcheon IE, Hassenbusch
SJ, Holland E, et al: A multivariate analysis of 416 patients with
glioblastoma multiforme: Prognosis, extent of resection and
survival. J Neurosurg. 95:190–198. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pierallini A, Bonamini M, Pantano P, et
al: Radiological assessment of necrosis in glioblastoma:
Variability and prognostic value. Neuroradiology. 40:150–153. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rong Y, Durden DL, Van Meir EG and Brat
DJ: 'Pseudopalisading' necrosis in glioblastoma: A familiar
morphologic feature that links vascular pathology, hypoxia and
angiogenesis. J Neuropathol Exp Neurol. 65:529–539. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Brat DJ, Castellano-Sanchez AA, Hunter SB,
Pecot M, Cohen C, Hammond EH, Devi SN, Kaur B and Van Meir EG:
Pseudopalisades in glioblastoma are hypoxic, express extracellular
matrix proteases and are formed by an actively migrating cell
population. Cancer Res. 64:920–927. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang XD, Wang ZF, Dai LM and Li ZQ:
Microarray analysis of the hypoxia-induced gene expression profile
in malignant C6 glioma cells. Asian Pac J Cancer Prev.
13:4793–4799. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Oliver L, Olivier C, Marhuenda FB, et al:
Hypoxia and the malignant glioma microenvironment: Regulation and
implications for therapy. Curr Mol Pharmacol. 2:263–284. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Brandsma D and van den Bent MJ:
Pseudoprogression and pseudoresponse in the treatment of gliomas.
Curr Opin Neurol. 22:633–638. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jyothirmayi R, Madhavan J, Nair MK and
Rajan B: Conservative surgery and radiotherapy in the treatment of
spinal cord astrocytoma. J Neurooncol. 33:205–211. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shibamoto Y, Kitakabu Y, Takahashi M,
Yamashita J, Oda Y, Kikuchi H and Abe M: Supratentorial low-grade
astrocytoma. Correlation of computed tomography findings with
effect of radiation therapy and prognostic variables. Cancer.
72:190–195. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Adn M, Saikali S, Guegan Y and Hamlat A:
Pathophysiology of glioma cyst formation. Med Hypotheses.
66:801–804. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Utsuki S, Oka H, Suzuki S, Shimizu S,
Tanizaki Y, Kondo K, Tanaka S, Kawano N and Fujii K: Pathological
and clinical features of cystic and noncystic glioblastomas. Brain
Tumor Pathol. 23:29–34. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|