Introduction
Clinically, teratoma is a germ cell tumor derived
from primordial germ cells, which have the potential to
differentiate into somatic cells (1).
Mature teratoma usually contains two or three germ cell layers from
the endoderm, mesoderm and ectoderm (2). A typical mature teratoma often contains
components from each of the three germ layers, including lipid,
epithelium, bone, cartilage, hair, fat, muscle and nerve tissue.
Teratoma most commonly affects neonates and adolescents, and is
mainly prevalent in women. The most common sites of occurrence are
the gonads, namely the male testis and the female ovary, with
extragonadal teratomas accounting for only 15% of all teratomas and
often occurring in regions of the body axis, including the
mediastinal and sacrococcygeal regions (3,4).
Primary adrenal teratoma is extremely rare, and only
a few individual case studies and small series can be retrieved
from the clinical literature (5–7). To the
best of our knowledge, the largest reported series of patients with
primary adrenal teratoma described three cases in a study by Lam
and Lo (8). Between March 2009 and
February 2014, 3,901 patients with adrenal disease were treated in
the Peking Union Medical College Hospital (Chinese Academy of
Medical Sciences and Peking Union Medical College, Beijing, China),
of which, five patients presented with primary adrenal teratoma,
accounting for only 0.13%. Considering the limited information on
primary adrenal teratoma, a detailed analysis of the clinical
characteristics of the five patients was conducted in the present
study, and the literature on adrenal teratoma reported in the past
10 years was also briefly reviewed.
Patients and methods
General information and clinical
manifestations
Five female patients aged from 16 to 51 years (mean,
36.0±16.3 years) were treated for primary adrenal teratomas in the
Peking Union Medical College Hospital between March 2009 and
February 2014. All the adrenal teratomas were incidentally found by
ultrasonography during routine physical examinations, without
typical clinical symptoms and hypertension, with the exception of
abdominal discomfort in one patient and palpable masses in two. The
time period from the discovery of adrenal lesions to undergoing
surgery ranged from 2 weeks to 13 years. Informed consent was
obtained from all patients prior to collecting the clinical
data.
Imaging and laboratory tests
Imaging and laboratory tests were completed prior to
surgery. All five patients underwent color Doppler ultrasonography,
and four patients underwent normal and enhanced computed tomography
(CT) scanning. Three patients received somatostatin receptor
scintigraphy with
99mTc-hydrazinonicotinyl-Tyr3-octreotide
(99mTc-HTOC), and scintigraphy was performed at 1 and 4
h post-injection. Synthesis and labeling of 99mTc-HTOC
were conducted as previously described (9). ELISA kits (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) were used, according to the manufacturer's
instructions, to assess adrenal-related endocrine levels. This
included the following: Plasma adrenocorticotropic hormone (normal
range, 0–46 pg/ml), renin activity (normal range, 0.93–6.56
ng/ml/h), angiotensin II (normal range, 55.3–115.3 pg/ml),
aldosterone (normal range, 6.5–29.6 ng/dl), cortisol (normal range,
4.0–22.3 µg/dl), 24-h urinary free cortisol (normal range,
12.3–103.5 µg/24 h) and 24-h urinary catecholamines [normal ranges:
norepinephrine (NE), 16.69–40.65 µg/24 h; epinephrine (E),
1.74–6.42 µg/24 h; and dopamine (DA), 120.93–330.59 µg/24 h).
Treatment and follow-up
All patients were treated with retroperitoneal
laparoscopic resection. Patients with left-sided adrenal teratomas
were placed in the right lateral position for surgery, and patients
with right-sided adrenal teratomas were placed in the left lateral
position (Fig. 1A). Routinely three
trocars were placed during the procedure (Fig. 1B). One 10-mm trocar was placed at 2 cm
above the superior border of the iliac crest in the midaxillary
line (point 1), another 10-mm trocar was placed under the lower
edge of the twelfth rib in the posterior axillary line (point 2)
and one 5-mm trocar was placed in the anterior axillary line at the
level of point 1 (point 3). During the surgery to resect the
largest tumor (diameter, 9 cm), another 5-mm trocar was placed at
point iv (under the costal margin in the anterior axillary line),
in order to facilitate the procedure.
With regard to the post-operative follow-up scheme,
patients were followed up at 3, 6 and 12 months during the first
year, and at 18 and 24 months during the second year, then followed
up once a year thereafter.
Results
Each of the five patients presented with a solitary
adrenal teratoma, with a mean tumor diameter of ~6.0±2.7 cm (range,
2.4–9.0 cm) (Table I). Two lesions
were located in the left adrenal glands and three in the right.
Ultrasonography showed clear boundaries and regular shapes in all
patients. Lesions were of mixed echo in four patients and
hypoechoic in one. No marked blood flow signals were observed
within the masses of any patient upon color Doppler flow imaging
(Fig. 2A). CT showed mixed density
inside three lesions and soft tissue density inside one, including
irregular fat components, cystic areas and separation, as well as
nodular calcifications on the edge (Fig.
2B). The CT values of the soft tissues in the normal scan and
in the arterial phase were −92 to 43 HU and 58 to 85 HU,
respectively (Fig. 2C). Negative
results were found in the three patients who underwent somatostatin
receptor scintigraphy examination. Adrenal-related endocrine
laboratory results [range (mean ± SD)] were within normal limits in
all patients: Plasma adrenocorticotropic hormone, 7.7–26.7 pg/ml
(20.1±8.5 pg/ml); renin activity, 1.06–6.27 ng/ml/h (3.90±2.40
ng/ml/h); angiotensin II, 57.3–96.3 pg/ml (73.1±15.4 pg/ml);
aldosterone, 9.3–26.5 ng/dl (17.5±7.0 ng/dl); plasma cortisol,
6.5–20.3 µg/dl (13.5±6.1 µg/dl); 24-h urinary free cortisol,
30.4–63.6 µg/24 h (48.7±12.9 µg/24 h); and 24-h urinary
catecholamines [NE, 10.93–28.16 µg/24 h (19.66±6.27 µg/24 h); E,
0.87–2.20 µg/24 h (1.43±0.55 µg/24h); and DA, 72.00–282.66 µg/24 h
(162.12±77.97 µg/24 h)].
 | Table I.Demographic data and imaging
features. |
Table I.
Demographic data and imaging
features.
|
|
|
|
|
|
| Computed
tomography |
|---|
|
|
|
|
|
|
|
|
|---|
| Case no. | Gender/age,
years | Pre-operative
diagnosis | Tumor size, cm | Side | Ultrasonography
Echo/CDFI | Density | Calcification | Fat |
|---|
| 1 | F/21 | Adrenal teratoma | 8.5 | Right | Mixed/- | Mixed (mainly
cystic) | + | + |
| 2 | F/16 | Adrenal teratoma | 9.0 | Right | Mixed/- | Mixed (mainly
cystic) | + | + |
| 3 | F/43 | Adrenocortical
carcinoma | 4.9 | Left | Hypoechoic/- | Soft tissue
density | – | – |
| 4 | F/49 | Adrenal
myelolipoma | 5.3 | Left | Mixed/- | Mixed (mainly soft
tissue) | + | + |
| 5 | F/51 | Adrenal tumor
(unspecified) | 2.4 | Right | Mixed/- | NA | NA | NA |
The surgical duration (from skin incision to
suturing) was 70–135 min (mean=113.0±26.6 min) and the
intraoperative blood loss was 30–80 ml (mean, 56.0±19.5 ml). All
patients underwent surgery without any complications. All the
perirenal drainage tubes were removed at 2–4 days post-surgery, and
all the patients were discharged at 3–7 days post-surgery.
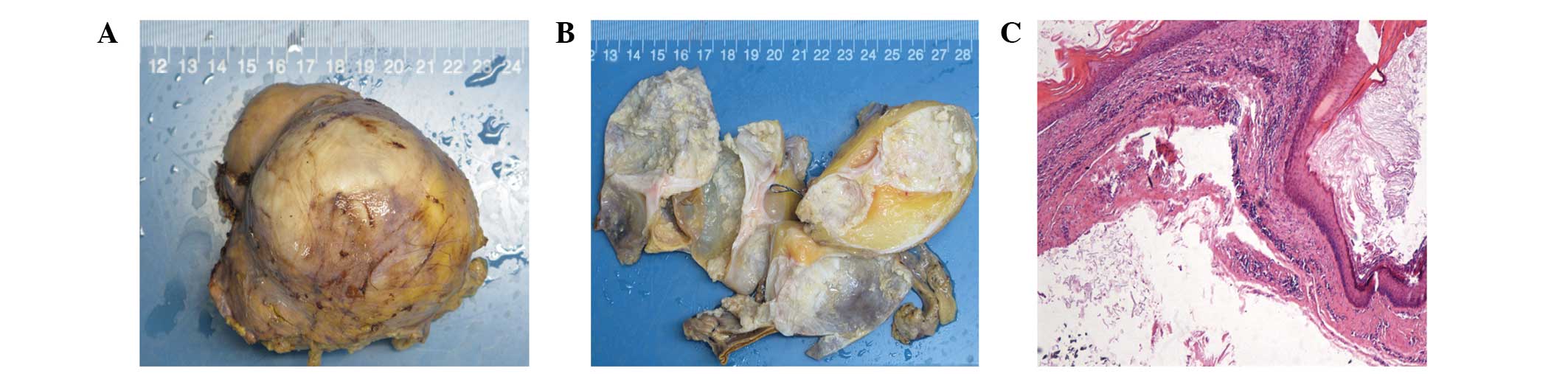
All the five specimens were identified as mature
cystic teratomas pathologically. The gross appearance showed sallow
or gray nodular masses, which were covered by smooth capsules
outside the surface (Fig. 3A), and
the cut surfaces were multilocular with diameters ranging from 2 to
5 cm, containing hair and cheese-like sebaceous material inside
(Fig. 3B). The inner surfaces of the
cyst walls were smooth and partially calcified. The wall thickness
was 0.1–0.4 cm, with certain cartilaginous components and adrenal
tissues. After draining the cystic fluid substances, the largest
specimen weighed ~200 g. The stratified squamous epithelium, hair
shafts, fat cells, gastrointestinal epithelium and respiratory
epithelium, were observed under a microscope (Fig. 3C).
The five patients have been followed up regularly
for 4–60 months post-surgery and are currently alive with no signs
of recurrence.
Discussion
Adrenal teratoma is so rare that a literature review
only retrieved a total of 11 cases from Pubmed in the past 10
years, with seven adult cases (5–7,10–12),
including one male and six females, aged from 21 to 64 years (mean
±SD, 40.0±17.0 years) (Table II).
This is consistent with the findings that adrenal teratomas only
accounted for 0.13% of adrenal lesions over the same period and
that reproductive females were susceptible, as indicated by the
present study.
 | Table II.Review of characteristics of adrenal
teratoma in the past 10 years. |
Table II.
Review of characteristics of adrenal
teratoma in the past 10 years.
|
|
|
|
|
|
| Imaging |
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| First author/s, year
(ref.) | Case no. | Gender/age,
years | Clinical signs | Tumor size, cm | Location | Texture | Calcification | Fat | Laboratory tests |
|---|
| Castillo et
al, 2006 (5) | 1 | F/61 | – | 8.0 | Left | Heterogeneous | + | NA | – |
| Li et al, 2011
(6) | 2 | F/38 | Soreness | 10.0 | Right | Heterogeneous | – | + | – |
| Huang and Wang, 2013
(7) | 3 | F/64 | – | 6.3 | Left | Heterogeneous | + | + | – |
| Bhatti et al,
2013 (10) | 4 | M/22 | Flank pain | 10.8 | Left | Heterogeneous | + | + | – |
| Tang et al,
2014 (11) | 5 | F/39 | – | 22.5 | Right | Heterogeneous | + | + | ↑CEA, ↑CA19-9 |
| Zhao et al,
2014 (12) | 6 | F/21 | Backache | 6.0 | Right | NA | + | + | NA |
|
| 7 | F/35 | – | 8.0 | Right | NA | + | + | NA |
Since patients with adrenal teratomas often have no
specific clinical manifestations, lesions cannot be found until the
diameters become large enough. The mean diameter (± SD) was 6.0±2.7
cm in the present study results and 10.2±5.7 cm in the review.
Meanwhile, patients with adrenal teratomas usually have no
abnormalities in adrenal-related endocrine tests and nuclear
medicine examinations, resulting in difficulties in distinguishing
adrenal teratoma from other lipomatous tumors (12), such as myelolipoma, angiomyolipoma,
retroperitoneal lipoma and liposarcoma, as well as tumors with
massive necrotic liquefied components, such as pheochromocytoma. In
the present study, adrenal myelolipoma could not be pre-operatively
ruled out in four cases and pheochromocytoma could not be excluded
in one.
Myelolipoma is the most common lipomatous tumor of
the adrenal gland without endocrine function (12). Ultrasonography shows a hyperechoic
mass, and CT reveals a well-defined mass of mixed density with
mainly fat-containing components. Pheochromocytoma often has the
typical clinical symptoms of hypertension, headache, palpitation
and sweating, and the level of 24-h urinary catecholamines (NE, E
and DA) are always above the normal limits. Somatostatin receptor
scintigraphy and 131I-MIBG scintigraphy are mostly
positive, and the latter has a high specificity in diagnosis.
Features on CT include cystic masses or solid masses with necrosis
and significant enhancement. Due to the large impact on the
circulatory system, patients with a long history may suffer from
catecholamine cardiomyopathy (13).
Therefore, routine preparation with pre-operative oral
phenoxybenzamine should be conducted if pheochromocytoma cannot be
completely ruled out. Retroperitoneal liposarcoma (14), which originates from mesenchymal
tissue and can be classified into five types, mainly shows
expansive growth and results in a squeezing effect on the
surrounding tissues and organs, without any specific clinical
manifestations. The diagnosis also relies mainly on imaging. The CT
characteristics of well-differentiated liposarcoma are similar to
that of the fat content, while undifferentiated tumors appear dense
and heterogenous, and can be markedly enhanced with intravenous
contrast (15).
As for a typical mature cystic teratoma, the
radiographic characteristics are usually as follows: i)
Ultrasonography shows a mixed echo in the mass, hyperechoic
fat-rich ingredients and hypoechoic cystic areas, with a clear
boundary and regular morphology (16). ii) CT scan shows mixed density
elements, such as fat, bone and other soft tissue densities with
separations and calcifications. Mild enhancement can be seen in the
soft-tissue components or cystic walls (17). It has been reported that 93% of
lesions contain fat components and 56% contain calcifications
(18). iii) Magnetic resonance
imaging often reveals equal signals on T1-weighted imaging (T1WI)
and slightly higher signals on T2WI, with a nodular focus and clear
boundary (19). Among the 12 patients
in the present study and review, 75% (9/12) presented with
heterogeneous lesions, as well as fat compositions and
calcifications.
Currently, laparoscopic surgery remains the primary
option for adrenal tumors. Laparoscopic technology, which had been
used in adrenal surgery since 1992 (20), has been developed rapidly and used
increasingly in urology cases. Today, laparoscopic surgery is the
gold standard for adrenal lesion removal (21). Upon consideration of our limited
clinical experience, we believe that laparoscopic surgery is
superior to open surgery for the following reasons: i) Anatomical
factors: Adrenal glands are deeply and covertly located inside the
perirenal fat medially above the kidney, which means that it is too
difficult to complete surgical procedures by open surgery due to
the limited vision, particularly when dealing with the central vein
of the adrenal gland. However, the zoom effect of the laparoscopic
camera system has just solved these problems. ii) Nature of lesion:
The majority of benign adrenal tumors are regularly shaped.
Although closely associated with the surrounding tissues and
organs, local infiltration and a rich blood supply are rare.
Therefore, a laparoscopic resection with less bleeding and
surrounding damage is more suitable. iii) Advanced laparoscopic
devices and skilled surgeons: Compared with open surgery, in
addition to its equal safety and effectiveness, laparoscopic
surgery is less invasive so patients experience a shorter
hospitalization period.
In contrast to previous studies, which conducted
open surgery in 71.4% (5/7) of patients (Table III), patients in the Peking Union
Medical College Hospital, for whom the maximum diameter was ~9 cm,
were all treated successfully with a retroperitoneal laparoscopic
resection. This proves that laparoscopic surgery is suitable for
even those adrenal tumors with large diameters and can be
successfully conducted by skilled surgeons.
 | Table III.Review of management of adrenal
teratoma in the past 10 years. |
Table III.
Review of management of adrenal
teratoma in the past 10 years.
| First author/s, year
(ref.) | Case no. | Surgery | Pathology | Follow-up,
months |
|---|
| Castillo et
al, 2006 (5) | 1 | Laparoscopic | MCT | 12 |
| Li et al,
2011 (6) | 2 | Open | MCT | 24 |
| Huang and Wang,
2013 (7) | 3 | NA | MCT | 6 |
| Bhatti et
al, 2013 (10) | 4 | Open | MCT | 6 |
| Tang et al,
2014 (11) | 5 | Open | MCT | 18 |
| Zhao et al,
2014 (12) | 6 | Open | MCT | 80 |
|
| 7 | Open | MCT | 57 |
The pathological characteristics of typical mature
teratomas are mostly cystic with two or three germ layers, an
intact capsule, a smooth appearance, cheese-like sebaceous
material, hair, bone and fat compositions. Immature teratomas are
mostly solid and contain various differentiations of immature
embryonic tissues (22). In the
present study and the review of the previous 10 years, a total of
12 cases were identified with mature cystic teratomas, which have
been followed up for 4 to 80 months post-surgery with no recurrence
(Table III). Although a prior study
has stated that 1.46% of mature cystic teratomas develop malignant
transformation (23), a good
prognosis should be obtained as long as the lesion is removed
completely. However, regardless of whether the tumor is a mature or
immature teratoma, regular post-operative follow-up is necessary to
detect and treat the recurrence or metastasis in a timely manner
(24).
In conclusion, reproductive females are susceptible
to these rare adrenal teratomas. Without typical clinical
manifestations and adrenal-related laboratory abnormalities, the
lesions are found relatively late when they become larger. The
pre-operative diagnosis mainly relies on the imaging
characteristics of a mixed echo on ultrasonography, and a
heterogeneous density containing fat components with scattered and
marginal calcifications on CT. The combination of a variety of
screening methods may improve the accuracy of diagnosis
pre-operatively to distinguish the mass from other lipomatous
tumors. Retroperitoneal laparoscopic surgery is the preferred
treatment for adrenal teratoma, and the prognosis is good. Patients
should be closely followed up after surgery whether the tumor is a
mature or immature teratoma. However, the present results are not
comprehensive due to the limited number of cases. Further studies
and long-term follow-up data are required in the future.
Acknowledgements
The authors would like to thank Dr Yu Xiao
(Department of Pathology, Peking Union Medical College Hospital)
for providing support in analyzing the pathological results.
References
|
1
|
Mikuz G and Colecchia M: Teratoma with
somatic-type malignant components of the testis. A review and an
update. Virchows Arch. 461:27–32. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim MJ, Kim NY, Lee DY, Yoon BK and Choi
D: Clinical characteristics of ovarian teratoma: Age-focused
retrospective analysis of 580 cases. Am J Obstet Gynecol.
205:32.e1–32.e4. 2011. View Article : Google Scholar
|
|
3
|
Shintani Y, Funaki S, Nakagiri T, Inoue M,
Sawabata N, Minami M, Kadota Y and Okumura M: Experience with
thoracoscopic resection for mediastinal mature teratoma: A
retrospective analysis of 15 patients. Interact Cardiovasc Thorac
Surg. 16:441–444. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Simpson PJ, Wise KB, Merchea A, Cheville
JC, Moir C, Larson DW and Dozois EJ: Surgical outcomes in adults
with benign and malignant sacrococcygeal teratoma: A
single-institution experience of 26 cases. Dis Colon Rectum.
57:851–857. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Castillo OA, Vitagliano G, Villeta M,
Arellano L and Santis O: Laparoscopic resection of adrenal
teratoma. JSLS. 10:522–524. 2006.PubMed/NCBI
|
|
6
|
Li Y, Wu H, Yao G and Zhao X: Diagnosis
and treatment of mature adrenal teratoma. Zhong Nan Da Xue Xue Bao
Yi Xue Ban. 36:174–177. 2011.(In Chinese). PubMed/NCBI
|
|
7
|
Huang D and Wang Y: Left cystic mature
adrenal teratoma: A case report. Nan Fang Yi Ke Da Xue Xue Bao.
33:159–161. 2013.(In Chinese). PubMed/NCBI
|
|
8
|
Lam KY and Lo CY: Adrenal lipomatous
tumours: A 30 year clinicopathological experience at a single
institution. J Clin Pathol. 54:707–712. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Decristoforo C, Melendez-Alafort L,
Sosabowski JK and Mather SJ: 99mTc-HYNIC-Tyr3-octreotide for
imaging somatostatin-receptor-positive tumors: Preclinical
evaluation and comparison with 111 In-octreotide. J Nucl Med.
41:1114–1119. 2000.PubMed/NCBI
|
|
10
|
Bhatti A, Al-Hindi H, Azzam A, Amin T and
Abu-Zaid A: Mature (benign) cystic retroperitoneal teratoma
involving the left adrenal gland in a 22-year-old male: A case
report and literature review. Case Rep Oncol Med.
2013:6102802013.PubMed/NCBI
|
|
11
|
Tang DD, Zhang XS, Hao ZY, Zhou J and
Liang CZ: A giant primary retroperitoneal mature cystic teratoma in
right adrenal region in a 39-year-old female. Int J Clin Exp Med.
7:1611–1613. 2014.PubMed/NCBI
|
|
12
|
Zhao J, Sun F, Jing X, Zhou W, Huang X,
Wang H, Zhu Y, Yuan F and Shen Z: The diagnosis and treatment of
primary adrenal lipomatous tumours in Chinese patients: A 31-year
follow-up study. Can Urol Assoc J. 8:E132–E136. 2014.PubMed/NCBI
|
|
13
|
Hodin R, Lubitz C, Phitayakorn R and
Stephen A: Diagnosis and management of pheochromocytoma. Curr Probl
Surg. 51:151–187. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nagy V, Bober J, Zavacky P, Brandebur O Jr
and Svajdler M: The recurrent primary retroperitoneal liposarcoma.
Bratisl Lek Listy. 114:662–667. 2013.PubMed/NCBI
|
|
15
|
Vijay A and Ram L: Retroperitoneal
liposarcoma: A comprehensive review. Am J Clin Oncol. 38:213–219.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Resnick EL, Talmadge JM and Winn SS:
Mediastinal teratoma diagnosed via ultrasound-guided biopsy.
Ultrasound Q. 29:245–246. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park SB, Cho KS and Kim JK: CT findings of
mature cystic teratoma with malignant transformation: Comparison
with mature cystic teratoma. Clin Imaging. 35:294–300. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamashita Y, Torashima M, Hatanaka Y,
Harada M, Sakamoto Y, Takahashi M, Miyazaki K and Okamura H: Value
of phase-shift gradient-echo MR imaging in the differentiation of
pelvic lesions with high signal intensity at T1-weighted imaging.
Radiology. 191:759–764. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Manchali MM, Sharabu C, Latha M and Kumar
L: A rare case of oropharyngeal teratoma diagnosed antenatally with
MRI. J Clin Imaging Sci. 4:152014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gagner M, Lacroix A and Bolté E:
Laparoscopic adrenalectomy in Cushing's syndrome and
pheochromocytoma. N Engl J Med. 327:10331992. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chuan-Yu S, Yat-Faat H, Wei-Hong D,
Yuan-Cheng G, Qing-Feng H, Ke X, Bin G and Guo-Wei X: Laparoscopic
adrenalectomy for adrenal tumors. Int J Endocrinol.
2014:2418542014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schmidt D and Kommoss F: Teratoma of the
ovary. Clinical and pathological differences between mature and
immature teratomas. Pathologe. 28:203–208. 2007.(In German).
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Oranratanaphan S and Khemapech N:
Characteristics and treatment outcomes of patients with malignant
transformation arising from mature cystic teratoma of the ovary:
Experience at a single institution. Asian Pac J Cancer Prev.
14:4693–4697. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nishioka K, Furukawa N, Noguchi T,
Kajihara H and Horie K: Immature teratoma after three laparoscopic
resections for mature cystic teratomas. Case Rep Obstet Gynecol.
2014:2649592014.PubMed/NCBI
|