Introduction
Colorectal cancer (CRC) is the second leading cause
of adult cancer-associated mortality in the USA and it is
associated with a low survival rate (1). Chemotherapeutic compounds that are
currently used to treat colorectal cancer include 5-flurouracil
(5-FU), oxaliplatin, and irinotecan (2). 5-FU is routinely used for the management
of patients with CRC (3). Treating
cells in vitro with 5-FU results in DNA damage, specifically
double-strand (and single-strand) breaks occur during S phase due
to the misincorporation of the metabolite of 5-FU, FdUTP, into the
DNA of the cell (4). However, the use
of 5-FU as a colorectal cancer chemotherapeutic agent has been
somewhat limited due to the toxicity, limited success and adverse
side effects associated with 5-FU treatment. As such identifying
and developing novel and safe treatment strategies that may enhance
the tumor cell response and overcome chemoresistance to antitumor
drugs.
The tumor suppressor candidate 4 (TUSC4), also
referred to as nitrogen permease regulator like 2 (NPRL2), is one
of the candidate tumor suppressor genes identified in human
chromosome 3p21.3 region in which genomic abnormalities, including
a loss of heterozygosity and homozygous deletion, are frequently
observed in the early stages of the development of various types of
human cancer (5–7). The overexpression of TUSC4 inhibits
proliferation and induces apoptosis in a variety of tumor cell
lines (8). Previous studies have
demonstrated that TUSC4 induces susceptibility to anticancer drugs
and apoptosis (9,10). Additional studies have indicated that
TUSC4 is involved in DNA mismatch repair, cell cycle checkpoint
signaling, and the regulation of apoptosis (5,11).
Previous studies have reported that TUSC4 is a
potential biomarker for predicting a patient's response to
cisplatin in addition to the prognosis of patients with lung and
other types of cancer; TUSC4 is also a molecular therapeutic agent
for enhancing and resensitizing the response of nonresponders to
cisplatin treatment (10,12). However, how TUSC4 suppresses tumor
proliferation and whether TUSC4 affects the sensitivity of CRC
cells to chemotherapy remains unknown. In the present study, the
colorectal cancer cell line HCT116 was used to determine the
effects of the TUSC4 signaling pathway on apoptosis induced by the
chemotherapeutic drug 5-FU to further elucidate the role of the
TUSC4 signaling pathway in increasing the 5-FU sensitivity in these
cells to contribute to the identification of an effective treatment
for CRC.
Materials and methods
Cell culture
The colon cancer cell line HCT116 was purchased from
the Chinese Academy of Sciences (Shanghai, China). The cells were
cultured in RPMI-1640 medium supplemented with 10% fetal bovine
serum (HyClone, Logan, UT, USA) and 1% penicillin/streptomycin
(Beyotime Institute of Biotechnology, Haimen, China) in a
humidified atmosphere of 5% CO2 at 37°C. Cells were
passaged every 2–3 days through digestion with 0.25% trypsin.
Logarithmically growing cells were prepared.
Transductions and assay
The full length human TUSC4 (NPRL2) gene
(GenBankaccession no. NM_006545) was purchased from Shanghai
Genechem Co. Ltd. (Shanghai, China) as a fusion with enhanced green
fluorescence protein (eGFP) in the GV208 vector. The lentiviral
vector system consisted of GV208 and the pHelper 1.0 and pHelper
2.0 packaging vectors. The three vectors were cotransfected into
293T cells in serum-free medium using Lipofectamine 2000
(Invitrogen Life Technologies, Carlsbad, CA, USA). The medium was
changed to complete medium after 8 h of incubation. High-titer
recombinant lentiviruses encoding TUSC4 were harvested 48 h after
transfection. HCT116 cells in the log phase were seeded at
5×105 cells/well in 96-well plates and transduced with
TUSC4-GFP or GFP lentiviruses in serum-free medium. Polybrene was
added to improve the transduction efficiency. After 8 h, the medium
was changed to complete medium. At 72 h after transduction, GFP
expression was examined by fluorescence microscopy (TE2000; Nikon
Corporation, Toyko, Japan) and a luciferase assay was performed in
HCT116 cells. The protein expression levels were analyzed 72 h
after transduction. All experiments were performed in triplicate,
and the representative results are reported.
Cell viability assay
Non-transduced and transduced cells were dispersed
and seeded at 5×103 cells/well in 96-well microplates.
After 24 h, freshly prepared 5-FU (Jinyao Amino Acid Co., Ltd.,
Tianjin, China) was used to determine the optimal concentration and
time course of the HCT116 cell response to 5-FU. Cell viability was
assessed with the cell counting kit-8 (CCK-8, Dojindo Molecular
Technologies, Inc., Kumamoto, Japan) assay following application of
various concentrations of 5-FU (1.25, 2.5, 5, 10 and 20 µM) and
incubating for a variety of time points (24, 48 and 72 h) in
culture. The absorbance value (A) at 450 nm was read using a
microplate reader (Thermo Fisher Scientific, Inc., Rockford, IL,
USA). At least 3 independent experiments were performed in
quadruplicate.
Flow cytometry (FCM) analysis of the
cell cycle and apoptosis
Cells transduced with TUSC4 were treated with 5 µM
of 5-FU for 48 h and harvested. After trypsinization, the cells
were washed with phosphate-buffered saline (PBS) and subsequently
fixed in 85% ethanol. Following fixation, the cells were washed
with PBS/1% fetal calf serum (FCS), resuspended in PBS/1% FCS
containing 5 µM PI and 250 µg/ml RNase A (Multisciences Biotech,
Hangzhou, China), and then incubated for 30 min at 37°C. Apoptosis
was evaluated using a FACS Calibur flow cytometer (Becton
Dickinson, Franklin Lakes, NJ, USA) with Annexin V-FITC and PI
(KeyGEN Biotech, Nanjing, China) staining.
Western blot analysis
Cellular protein extracts were separated by
electrophoresis on a 12 or 8% SDS-polyacrylamide gel and
electrophoretically transferred onto a PDVF membrane (Millipore,
Bedford, MA, USA). The membranes were blocked overnight with 5%
non-fat dried milk and then incubated overnight at 4°C with
antibodies (Abs) directed against GAPDH [rabbit monoclonal (m)Ab;
1:1,500 dilution; cat no. 2118], PDK1 (rabbit mAb; 1:1,000
dilution; cat no. 13037), p-Akt (mouse mAb; 1:1,000 dilution; cat
no. 4051), mTOR(p) (rabbit mAb; 1:1,000 dilution; cat no. 5536),
p70S6K(p) (mouse Ab; 1:1,000 dilution; cat no. 9209), and 4E-BP1
(rabbit mAb; 1:1,000 dilution; cat no. 9456) from Cell Signaling
Technology (Danvers, MA, USA) or TUSC4 (mouse Ab; 1:1,000 dilution;
cat no. sc-376986), PI3K(p) [rabbit polyclonal(Ab); 1:1,000
dilution; cat no. sc-134986], caspase-3 (rabbit pAb; 1:1,000
dilution; cat no. sc-7148), and caspase-9 (mouse mAb; 1:2,000
dilution; cat no. sc-56073) from Santa Cruz Biotechnology (Santa
Cruz, CA, USA). After washing with TBST, the membranes were
incubated with horseradish peroxidase-linked goat anti-rabbit
(1:1,000 dilution; cat no. A0208) and goat anti-mouse (1:1,000
dilution; cat no. A0216) IgG (heavy and light chain) secondary Abs
(Beyotime Institute of Biotechnology). The proteins were visualized
by ECL chemiluminescence using an integrated automatic
chemiluminescent imaging and analysis system (Sage Creation
Science, Beijing, China).
Statistical analyses
All experimental data are presented as the mean ±
standard error of the mean. Differences between samples were
analyzed using the two-tailed Student's t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
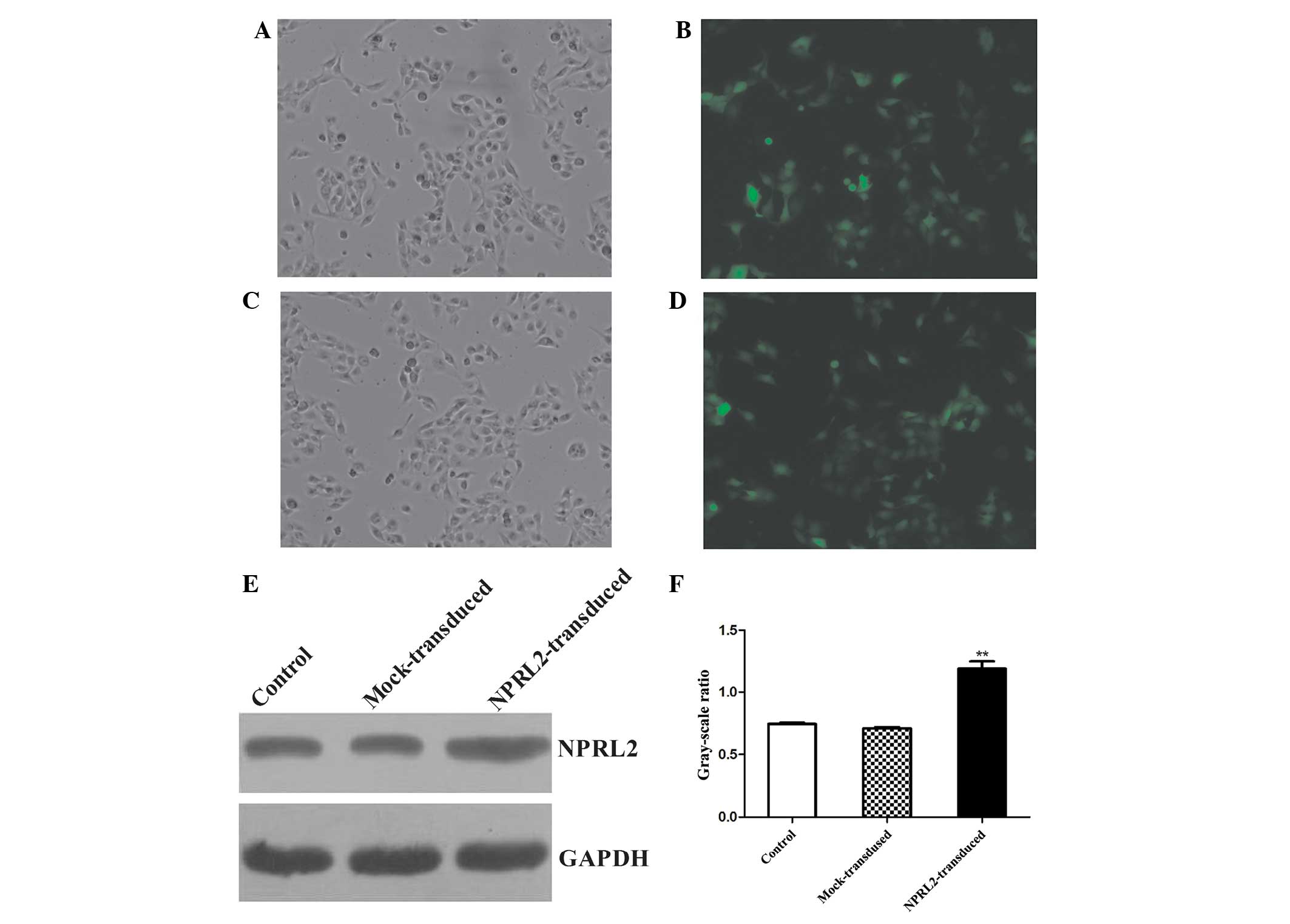
Lentiviral transduction of TUSC4
Transduction efficiency was evaluated 72 h after
TUSC4 transduction. eGFP was expressed in cells after lentiviral
transduction at different multiplicities of infection (MOIs). The
transduction efficiency (average proportion of GFP-expressing cells
compared with the total cell count) was >70% at an MOI of 10.
The protein expression levels were analyzed at 72 h
post-transduction. TUSC4 expression was increased in the transduced
cells compared with the negative control (NC) and mock cells
(unloaded lentivirus) (P<0.05, Fig.
1).
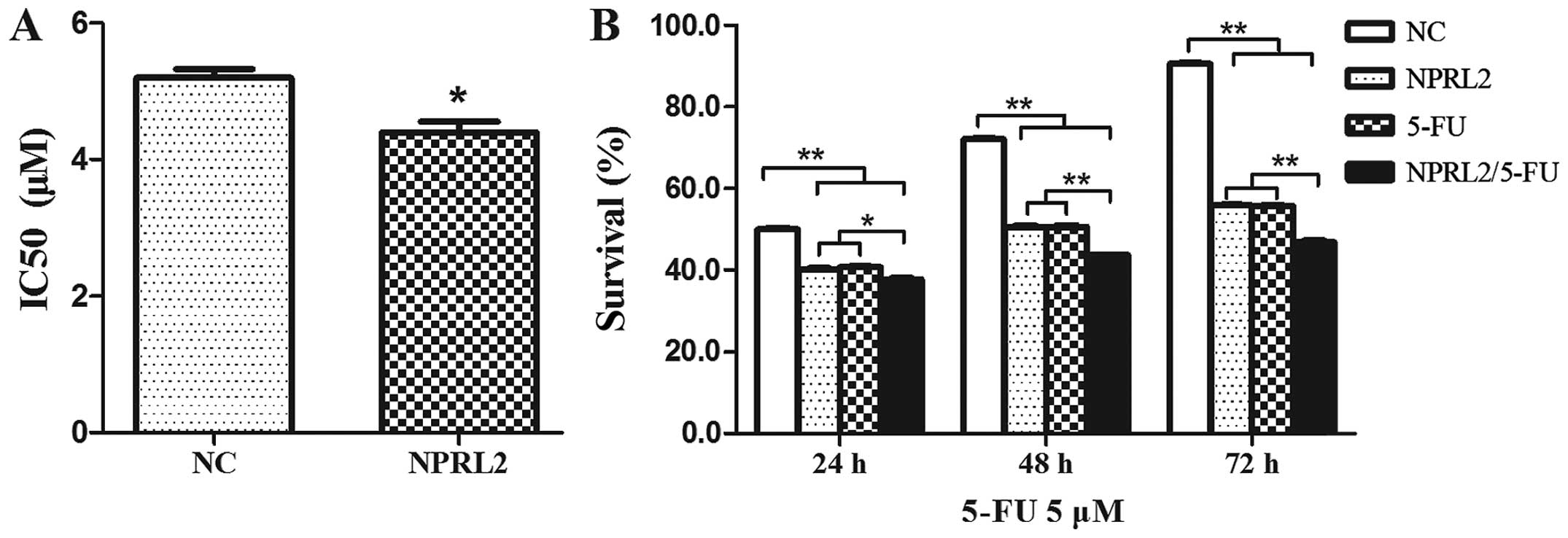
TUSC4 overexpression increases the
sensitivity of HCT116 cells to 5-FU
To investigate the role of TUSC4 in 5-FU-induced
cytotoxicity, TUSC4 was transduced into HCT116 cells. The
IC50 of 5-FU was reduced in cells transduced with TUSC4
compared with in NC cells (P<0.05), indicating that
overexpression of TUSC4 markedly increased the 5-FU sensitivity of
HCT116 cells (Fig. 2A). Cell survival
was assayed following TUSC4 transduction and treatment with 5 µM
5-FU for an additional 24, 48, and 72 h, which demonstrated that
the effects of TUSC4 on 5-FU sensitivity were time dependent
(Fig. 2B): Cell survival in
5-FU-treated TUSC4-transduced cells reduced over time
(P<0.01).
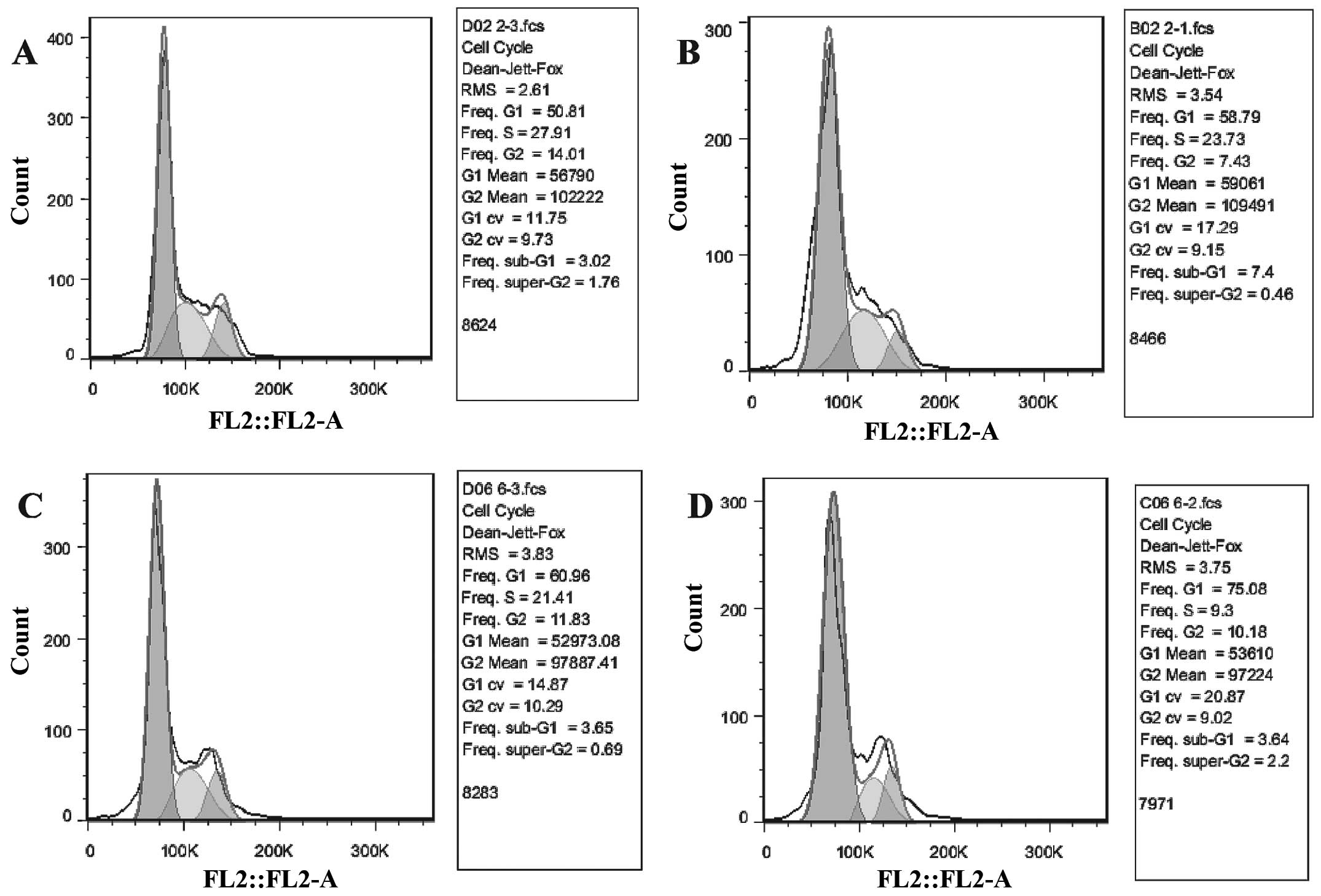
TUSC4 overexpression increases 5-FU
sensitivity by inhibiting cell growth
Cell survival assays demonstrated that a proportion
of cells were was arrested in the G1 phase and that there was a
reduction in the S phase population following TUSC4 transduction of
HCT116 cells (P<0.05). In addition, 5-FU significantly inhibited
the growth of TUSC4-transduced cells compared to NC cells and
further promoted this effect (P<0.01; Fig. 3).
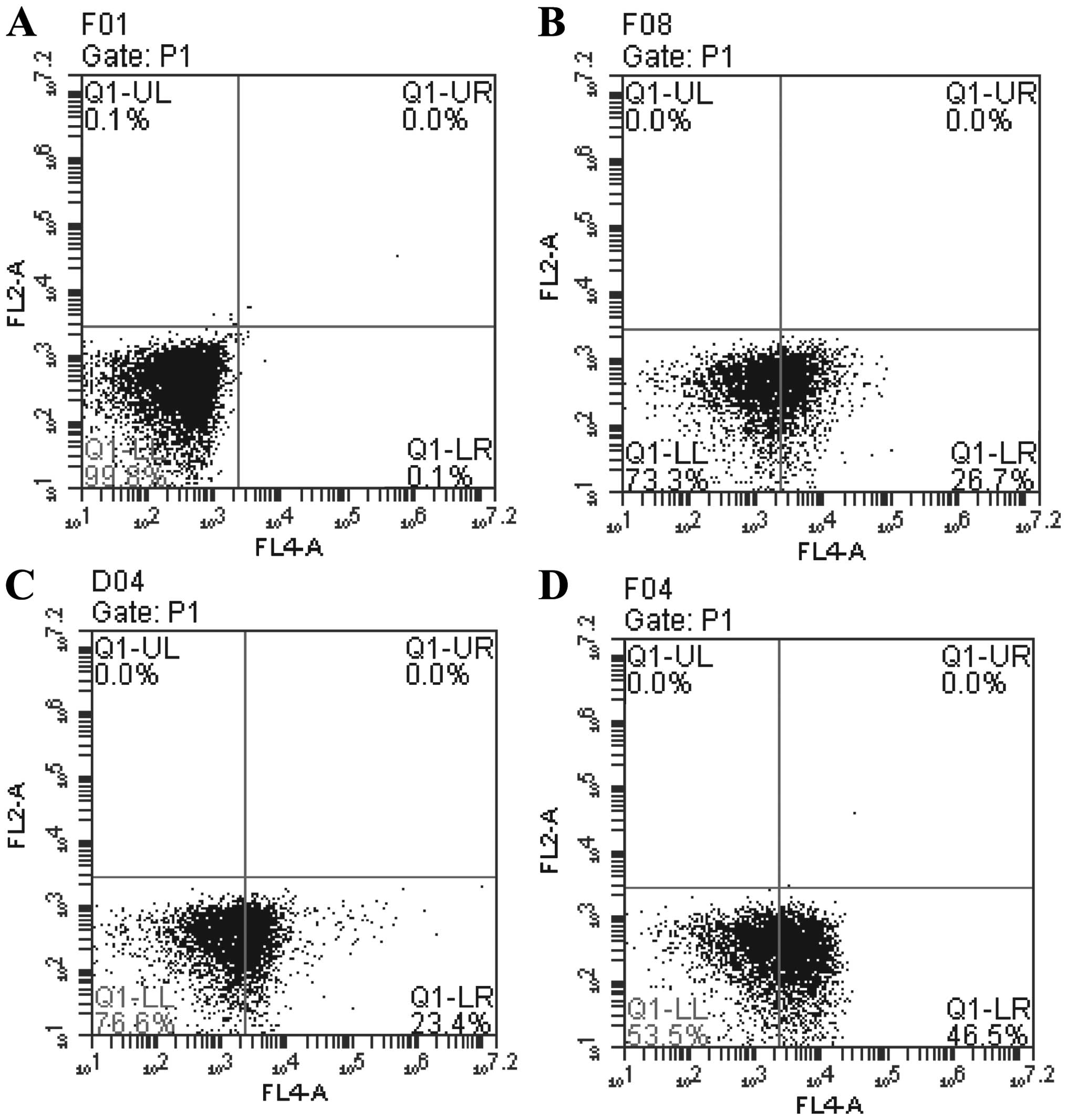
TUSC4 overexpression increases 5-FU
sensitivity by promoting apoptosis
Flow cytometry was performed to investigate the role
of TUSC4 in 5-FU-induced apoptosis. The number of apoptotic cells
was greater in TUSC4-transduced and 5-FU cells compared with NC
cells (P<0.05). In addition, the combination of TUSC4
overexpression and 5-FU treatment promoted apoptosis more
significantly than either perturbation alone (P<0.01) (Fig. 4).
TUSC4 promotes HCT116 cell sensitivity
to 5-FU by inhibiting the PI3K/Akt/mTOR signaling pathway
The protein expression in the following 4 groups of
cells was evaluated by western blot analysis: negative control
cells, TUSC4-transduced cells, HCT116 cells treated with 5 µM 5-FU
and cultured for 48 h, and TUSC4-transduced HCT116 cells treated
with 5 µM 5-FU and cultured for 48 h. Overexpression of TUSC4 and
5-FU treatment downregulated PDK1, 4E-BP1, phosphorylated PI3K,
Akt, mTOR, and p70S6K in HCT116 cells. In addition, the combination
of TUSC4 overexpression and 5-FU treatment resulted in
significantly greater downregulation of these genes compared to
either perturbation alone (P<0.01). Furthermore, 5-FU
upregulated caspase-3 and caspase-9, promoting apoptosis in TUSC4
overexpressing cells compared with cells subjected to either
perturbation alone or NC cells (P<0.01; Fig. 5).
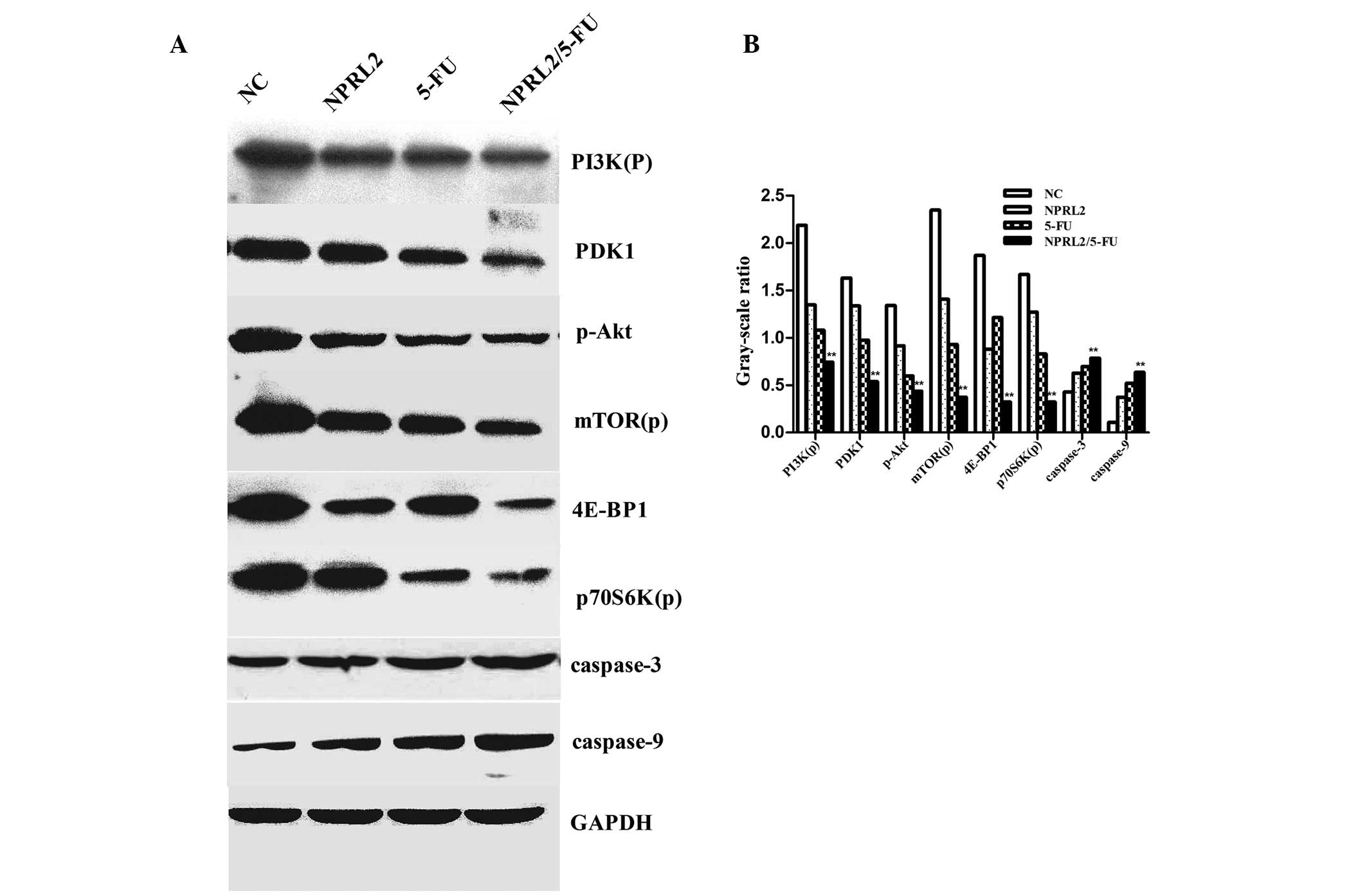 | Figure 5.Western blot analysis of the protein
levels of PI3K(p), PDK1, p-Akt, mTOR(p), p70S6K(p), 4E-BP1,
caspase-3, caspase-9, and GAPDH. (A) The down-regulation of the
expression of PDK1, 4E-BP1, phosphorylated PI3K, Akt, mTOR(p), and
p70S6K(p) in cells overexpressing NPRL2 and treated with 5-FU
compared with NC cells, NPRL2-transduced cells, and cells treated
with 5 µM 5-FU. NPRL2 overexpression resulted in increased levels
of caspase-3 and caspase-9 compared with NC cells. (B) In addition,
combined NPRL2 over-expression and L-OHP treatment significantly
upregulated apoptosis compared with either perturbation alone.
Western blot analysis was performed using GAPDH as a loading
control, **P<0.01 vs. NC, NPRL2 and 5-FU. |
Discussion
5-FU has been the first-choice chemotherapy drug for
colorectal cancer for a number of years. Although the combination
of 5-FU with other chemotherapeutic agents improves response rates
and survival in breast and head and neck cancers, 5-FU has the
greatest impact in the treatment of colorectal cancer (13). Nonetheless, the response rates for
5-FU-based chemotherapy as a first-line treatment for advanced
colorectal cancer are only ~10–15% (14). Additionally, the use of 5-FU as a
colorectal cancer chemotherapeutic agent has been somewhat limited
due to the toxicity, limited success and associated adverse side
effects. Although the exact mechanism involved in the inactivation
of TUSC4 in human cancers has not been determined, dysfunctional
alterations in the TUSC4 gene and its products, including aberrant
splicing transcripts and intragenic homozygous deletions, have been
observed in various types of human cancer and cancer cell lines
(5,11). Recently, it was demonstrated that
TUSC4 has tumor suppressing potential in vitro and in
vivo, and TUSC4 may be involved in DNA mismatch repair, cell
cycle checkpoint signaling, and the regulation of the apoptotic
pathway (9,11,15).
Previous reports also demonstrated that NPRL2 is inactivated in
various types of human cancer and cancer cell lines by aberrant
splicing of its transcripts and/or intragenic homozygous deletions
(5,11). Additionally, exogenous NPRL2
expression in NPRL2-negative tumor cells activates the DNA damage
pathway in lung cancer cells following NPRL2 treatment (12).
The present study demonstrated that TUSC4
over-expression increases 5-FU sensitivity in HCT116 cells. It was
initially determined that the IC50 of 5-FU was reduced
in cells transduced with TUSC4 than in NC cells (P<0.05). The
effects of TUSC4 on 5-FU sensitivity were determined to be time
dependent. Following TUSC4 transduction in HCT116 cells, the cell
cycle was arrested in G1 phase, and there was a reduction in cells
in S phase (P<0.05). In addition, 5-FU significantly inhibited
cell growth in TUSC4-transduced cells compared with NC cells
(P<0.01). These data further confirm that TUSC4 overexpression
increases 5-FU sensitivity by inhibiting cell growth. Flow
cytometry analysis revealed an increase in apoptotic cells due to
TUSC4 transduction and 5-FU treatment compared to NC cells
(P<0.05). Moreover, combined TUSC4 over-expression and 5-FU
treatment promoted apoptosis more significantly than either
perturbation alone. These findings indicate that there is a
significant correlation between TUSC4 protein expression and 5-FU
sensitivity in colorectal cancer cells, which is consistent with
the hypothesis that TUSC4 serves a role in regulating the
DNA-damage repair pathway and that inactivation of TUSC4 in tumor
cells may promote drug resistance, which is likely to be due to
interrupting DNA-damage repair and apoptotic signaling.
TUSC4 may enhance 5-FU sensitivity via additional
mechanisms, which should be examined in further studies. In the
present study, it was demonstrated that TUSC4 is a novel potential
therapeutic molecule. Compared with traditional drugs, TUSC4 serves
a more modulatory role. Combined TUSC4 overexpression and 5-FU
treatment effectively downregulated the phosphorylation of
phosphoinositide-3 kinase (PI3K), Akt, and mTOR in addition to the
mTOR downstream target proteins phospho-p70S6K (Thr389) and 4E-BP1
(Thr37/46). The PI3K/Akt/mTOR signaling axis is critical for
proliferation, apoptosis resistance, angiogenesis, and metastasis
and is central to the development and maintenance of CRC (16). Previous studies have reported the
potential for the PI3K/Akt/mTOR network to be therapeutically
targeted at multiple molecular levels (17,18).
Activated PI3K generates a second messenger
phosphatidylinositol(3–5)-triphosphate (PIP3); PIP3 then binds to
and activates phosphoinositide-dependent kinase-1 (PDK1), which
phosphorylates and activates Akt (19,20). Akt
activates several downstream targets, including mTOR. Deregulation
of mTOR signaling occurs in several types of human tumor, including
colon cancer (16,20). mTOR resides in 2 distinct multiprotein
complexes, mTORC1 and mTORC2 (21).
mTORC1 directly phosphorylates ribosomal protein S6 kinase 1 (S6K1)
and the eukaryotic translation initiation factor eIF4E-binding
protein 1 (4EBP1), which are involved in protein translation
(22,23). Phospho-p70S6K is usually located in
the cytoplasm, but it is often observed in the nucleus in tumors.
Phospho-p70S6K stimulates ribosome rearrangement into active
polysomes and increases the capacity of the translational events
that are essential for the G1/S transition of the cell cycle
(24). 4E-BP1 is considered a
funneling factor through which transforming signals converge,
channeling oncogenic proliferative signals regardless of the
specific upstream oncogenic alteration (25). These findings indicate that TUSC4
overexpression enhances 5-FU sensitivity by downregulating the
functions of the PI3K/Akt/mTOR network, leading to the inhibition
of cell proliferation and G1 cell cycle arrest. Furthermore, 5-FU
upregulates caspase-3 and caspase-9 to promote apoptosis in TUSC4
overexpressing cells compared with either perturbation alone and NC
cells (P<0.01). In addition, the present study demonstrated that
the TUSC4-mediated increase in 5-FU sensitivity that induces
apoptosis in HCT116 cells is associated with significant activation
of caspase-3 and caspase-9.
In summary, the present study demonstrates for the
first time that the expression of endogenous TUSC4 significantly
increased sensitivity to 5-FU in colorectal cancer cells. The
results provide novel evidence and previously unrecognized effects
of TUSC4, demonstrating that targeting TUSC4 in combination with
the conventional colorectal cancer chemotherapeutic agent 5-FU may
serve as an effective therapeutic strategy, resulting in the
significant inhibition of colorectal cancer cell growth by
downregulating the function of the PI3K/Akt/mTOR network. These
mechanisms are likely active in other cancers and may be exploited
for the development of novel cancer therapies.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Patel BB and Majumdar AP: Synergistic role
of curcumin with current therapeutics in colorectal cancer:
Minireview. Nutr Cancer. 61:842–6. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Borralho PM, da Silva Moreira IB, Aranha
MM, Albuquerque C and Nobre Leitao C: Inhibition of Fas expression
by RNAi modulates 5-fluorouracilinduced apoptosis in HCT116 cells
expressing wild-type p53. Biochim Biophys Acta. 1772:40–47. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Peters GJ, van Triest B, Backus HH, Kuiper
CM, van der Wilt CL and Pinedo HM: Molecular downstream events and
induction of thymidylate synthase in mutant and wild-type p53 colon
cancer cell lines after treatment with 5-fluorouracil and the
thymidylate synthase inhibitor raltitrexed. Eur J Cancer.
36:916–924. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lerman MI and Minna JD: The 630-kb lung
cancer homozygous deletion region on human chromosome 3p21.3:
Identification and evaluation of the resident candidate tumor
suppressor genes. The International lung cancer chromosome 3p21.3
tumor suppressor gene consortium. Cancer Res. 60:6116–6133.
2000.PubMed/NCBI
|
|
6
|
Wistuba II, Behrens C, Virmani AK, Mele G,
Milchgrub S, Girard L, Fondon JW III, Garner HR, McKay B, Latif F,
et al: High resolution chromosome 3p allelotyping of human lung
cancer and preneoplastic/preinvasive bronchial epithelium reveals
multiple, discontinuous sites of 3p allele loss and three regions
of frequent breakpoints. Cancer Res. 60:1949–1960. 2000.PubMed/NCBI
|
|
7
|
Zabarovsky ER, Lerman MI and Minna JD:
Tumor suppressor genes on chromosome 3p involved in the
pathogenesis of lung and other cancers. Oncogene. 21:6915–6935.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ji L, Nishizaki M, Gao B, Burbee D, Kondo
M, Kamibayashi C, Xu K, Yen N, Atkinson EN, Fang B, et al:
Expression of several genes in the human chromosome 3p21.3
homozygous deletion region by an adenovirus vector results in tumor
suppressor activities in vitro and in vivo. Cancer Res.
62:2715–2720. 2002.PubMed/NCBI
|
|
9
|
Schenk PW, Brok M, Boersma AW, Brandsma
JA, Den Dulk H, Burger H, Stoter G, Brouwer J and Nooter K:
Anticancer drug resistance induced by disruption of the
Saccharomyces cerevisiae NPR2 gene: A novel component involved in
cisplatin- and doxorubicin-provoked cell kill. Mol Pharmacol.
64:259–268. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ueda K, Kawashima H, Ohtani S, Deng WG,
Ravoori M, Bankson J, Gao B, Girard L, Minna JD and Roth JA: The
3p21.3 tumor suppressor NPRL2 plays an important role in
cisplatin-induced resistance in human non-small-cell lung cancer
cells. Cancer Res. 66:9682–9690. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li J, Wang F, Haraldson K, Protopopov A,
Duh FM, Geil L, Kuzmin I, Minna JD, Stanbridge E, Braga E, et al:
Functional characterization of the candidate tumor suppressor gene
NPRL2/G21 located in 3p21.3C. Cancer Res. 64:6438–6443. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jayachandran G, Ueda K, Wang B, Roth JA
and Ji L: NPRL2 sensitizes human non-small cell lung cancer (NSCLC)
cells to cisplatin treatment by regulating key components in the
DNA repair pathway. PLoS One. 8:e119942010. View Article : Google Scholar
|
|
13
|
Efficacy of adjuvant fluorouracil and
folinic acid in colon cancer. International multicentre pooled
analysis of colon cancer trials (IMPACT) investigators. Lancet.
345:939–944. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Johnston PG and Kaye S: Capecitabine: A
novel agent for the treatment of solid tumors. Anticancer Drugs.
12:639–646. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou BB and Elledge SJ: The DNA damage
response: Putting checkpoints in perspective. Nature. 408:433–439.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Johnson SM, Gulhati P, Rampy BA, Han Y,
Rychahou PG, Doan HQ, Weiss HL and Evers BM: Novel expression
patterns of PI3K/Akt/mTOR signaling pathway components in
colorectal cancer. J Am Coll Surg. 210:767–776. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Maira SM, Voliva C and Garcia-Echeverria
C: Class IA phosphatidylinositol 3-kinase: From their biologic
implication in human cancers to drug discovery. Exp Opin Ther
Targets. 12:223–238. 2008. View Article : Google Scholar
|
|
18
|
Ekstrand AI, Jönsson M, Lindblom A, Borg A
and Nilbert M: Frequent alterations of the PI3K/AKT/mTOR pathways
in hereditary nonpolyposis colorectal cancer. Fam Cancer.
9:125–129. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Samuels Y and Ericson K: Oncogenic PI3K
and its role in cancer. Curr Opin Oncol. 18:77–82. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vignot S, Faivre S, Aguirre D and Raymond
E: mTOR-targeted therapy of cancer with rapamycin derivatives. Ann
Oncol. 16:525–537. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guertin DA and Sabatini DM: Defining the
role of mTOR in cancer. Cancer Cell. 12:9–22. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tabernero J, Rojo F, Calvo E, Burris H,
Judson I, Hazell K, Martinelli E, Cajal Ramony S, Jones S, Vidal L,
et al: Doseand-and schedule-dependent inhibition of the mammalian
target of rapamycin pathway with everolimus: A phase I tumor
pharmacodynamic study in patients with advanced solid tumors. J
Clin Oncol. 26:1603–1610. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Proud CG: mTORC1 signalling and mRNA
translation. Biochem Soc Trans. 37:227–231. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu G, Zhang W, Bertram P, Zheng XF and
McLeod H: Pharmacogenomic profiling of the PI3K/PTEN-AKT-mTOR
pathway in common human tumors. Int J Oncol. 24:893–900.
2004.PubMed/NCBI
|
|
25
|
Armegnol G, Rojo F, CastellviĹ J, Iglesias
C, Cuatrecasas M, Pons B, Baselga J, Ramón Y and Cajal S:
4E-binding protein 1: A key molecular ‘funnel factor’ in human
cancer with clinical implications. Cancer Res. 67:7551–7555. 2007.
View Article : Google Scholar : PubMed/NCBI
|