Introduction
Ganglioneuromas are a type of benign peripheral
nerve tumor with an incidence of 1/100,000 (1,2).
Furthermore, women are more frequently affected than men (1–3).
Ganglioneuromas most frequently occur in the sympathetic ganglia
and adrenal medulla, exerting the majority of their effect on
spinal and sympathetic nerves, and causing neural dysfunction
(4). These benign tumors contain
well-differentiated ganglion cells, which are otherwise common in
the posterior mediastinum and retroperitoneal regions.
Ganglioneuromas located in the spinal cord are rare, and are
frequently dumbbell-shaped (5,6).
Ganglioneuromas typically present at a late stage due to their slow
growth. Recurrence and malignant transformation of the tumor are
rare, thus, complete surgical excision provides effective cure
(7). When present in uncommon
locations, such as the cauda equina, incorrect provisional
diagnoses of ganglioneuroma, as other more common neoplasms, are
likely, due to its analogous radiological features (8). For this reason, it is important to
maintain a broad list of differential diagnoses until
histopathological findings can confirm a diagnosis. To date, only
one mixed chemodectoma-ganglioneuroma of the conus medullaris
region has been reported (9). The
current study reports a case of ganglioneuroma located at the conus
medullaris. The patient underwent laminotomy surgery and a
favorable outcome was achieved. Written informed consent was
obtained from the patient.
Case report
A 38-year-old Chinese man presented to Beijing Tian
Tin Hospital (Beijing, China) in October 2012, with an 8-month
history of numbness in the lower limbs and muscle atrophy of the
right lower limb. The patient had also experienced dysuria over the
preceding month. Routine physical examination revealed no notable
abnormalities. Neurological examination confirmed hypoesthesia of
the hypogastrium region and the two lower limbs, grade IV
myodynamia of the lower limbs and grade V myodynamia of the upper
limbs (10). Family history revealed
no indication of a hereditary etiology. Routine blood, urine, blood
biochemistry and coagulation function tests were normal.
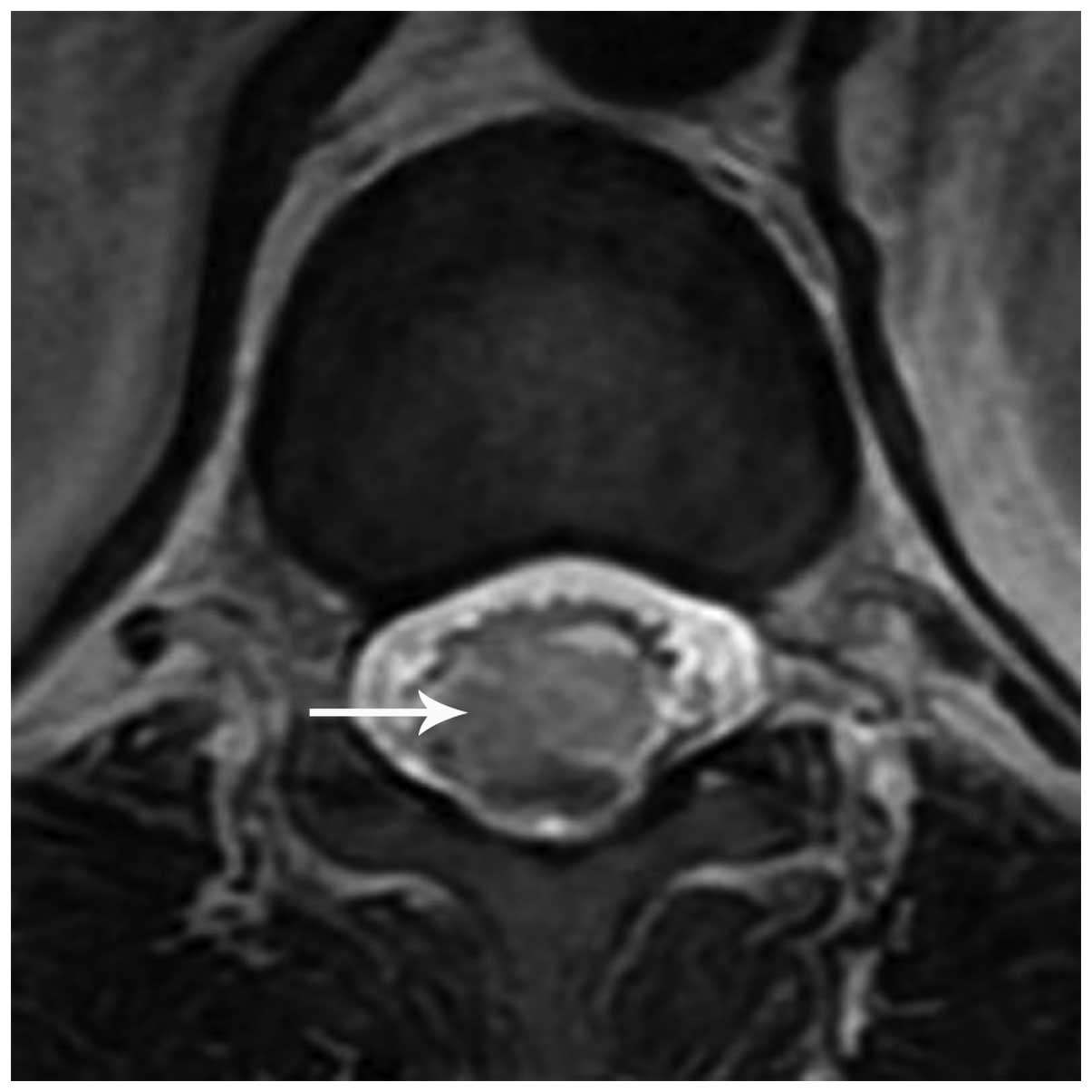
Spinal magnetic resonance imaging (MRI) revealed an
irregular-shaped mass (1.5×1.0×1.0 cm) located in the conus
medullaris (Fig. 1). The major
section of the mass appeared isointense on T1-weighted (T1WI) and
T2-weighted (T2WI) images. At the inferior pole of the lesion,
there was a nodular, low-intensity signal identified by T1WI and
T2WI scans. The conus medullaris surface revealed a discontinuous
punctiform or linear high-intensity signal on the T1WI. On the
contrast-enhanced T1WI, the major portion was homogeneously
enhanced and the conus medullaris surface showed a visible linear
enhancement compared with that of the T1WI. Furthermore, at the
upper end of the lesion, a large syringomyelia (T5–11) was
identified (Fig. 2). Based on the
provisional diagnosis of ependymoma, surgery was undertaken to
excise the mass. During the laminotomy, surgeons incised the dura
mater along the posterior midline to expose the lesion and the
tumor was visually confirmed to lie in the conus medullaris. The
tumor was gray-red and rubbery, with a blood supply and a draining
vein in the rostral pole. Complete resection was performed.
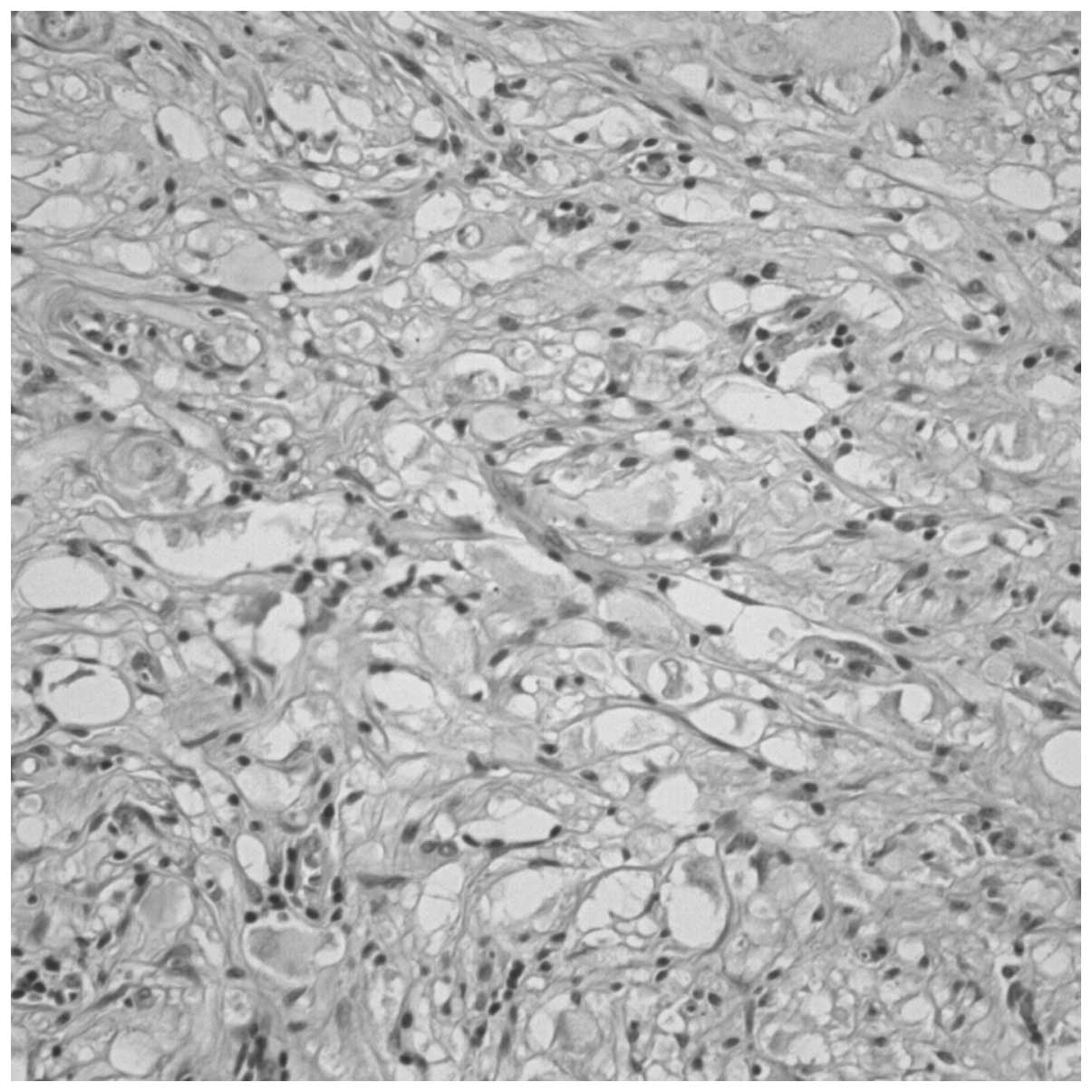
Histological examination revealed that the
neoplastic lesion consisted of spindle-shaped cells, between which,
large ganglion cells with vesicular nuclei and eosinophilic
nucleoli were identified (Fig. 3).
Immunohistochemical staining was positive for S-100, and the
neuronal markers synaptophysin (SYN), neuron-specific enolase
(NSE), microtubule-associated protein 2 (MAP-2) and neurofilament
(NF). Staining was negative for glial fibrillary acidic protein
(GFAP), NeuN and myelin basic protein (MBP). Furthermore, the
number of cells immunopositive for the cell proliferation marker
Ki-67 was <2%, confirming the benign nature of the mass.
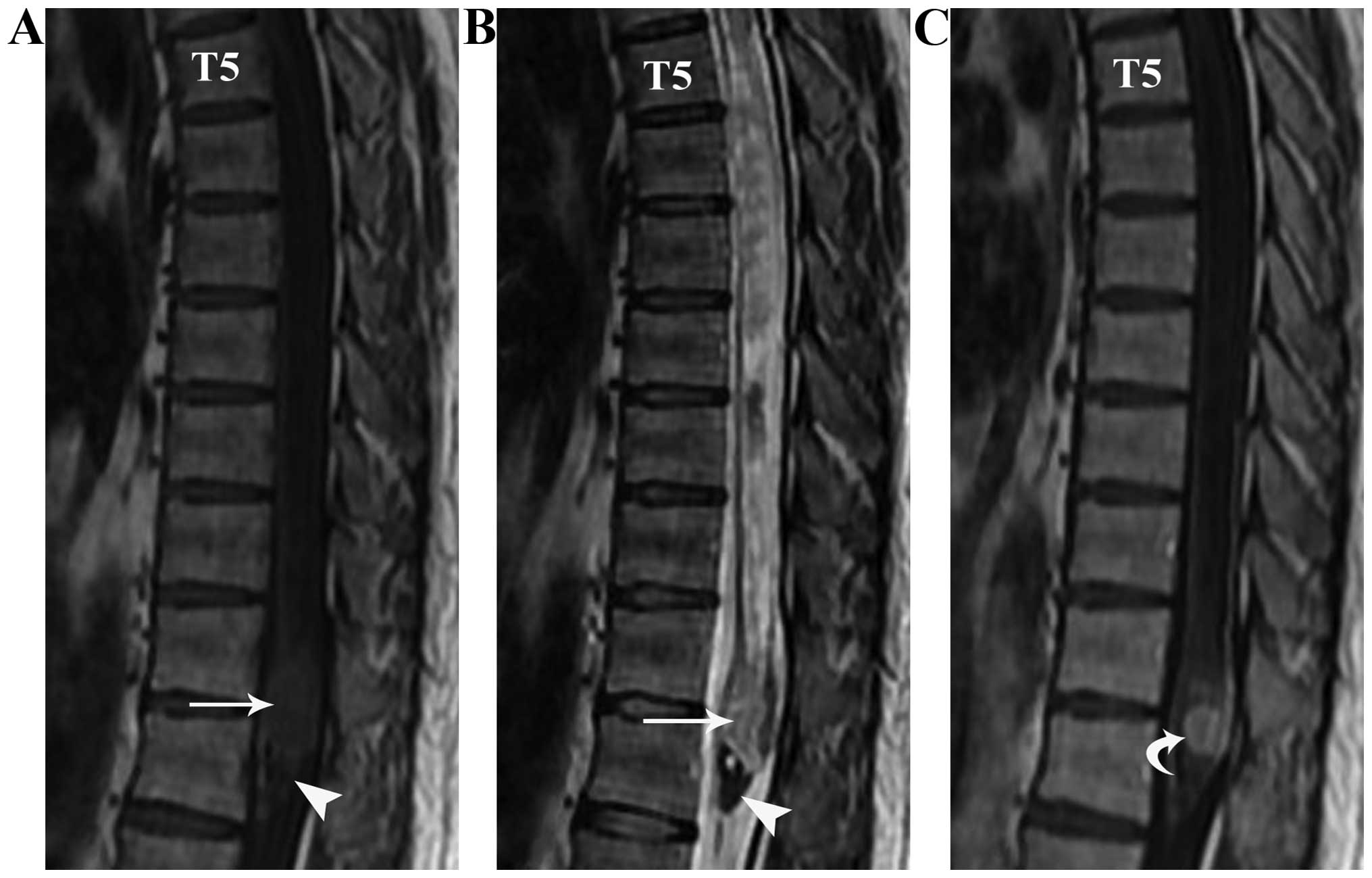
MRI examination 3 days subsequent to surgery
confirmed that the tumor was excised and there was no notable
syrinx collapse or retraction of the spinal cord (Fig. 4). The patient was discharged on the
ninth post-operative day. At the follow-up consultation 18 months
later, the patient exhibited moderate difficulty walking and
urinating, but was otherwise well.
Discussion
Derived from the embryonic neural crest,
ganglioneuromas arise from sympathoblasts and mainly occur in the
sympathetic ganglion and adrenal medulla, common particularly in
the posterior mediastinum and retroperitoneal region (11). The explanation for the uncommon
location of ganglioneuroma is likely a result of the mechanism of
neural crest cell migration in the embryonic period (4). The neural crest consists of a band of
longitudinal cells between the neural tube and epidermis, when the
neural tube is completed (12).
Subsequently, sections of the neural crest cells migrate to the
ventral side, while certain cells remain in the neural tube (spinal
cord), when the neural crest is formed at the back of neural tube
(13,14). Therefore, the conus medullaris region
is a possible anatomical location for the origin of ganglioneuroma
(4,12–15).
Ganglioneuroma and ganglioneuroblastomas are types
of neuroblastoma (15,16). Ganglioneuromas are frequently
localized along the sympathetic chain in the posterior mediastinal,
retroperitoneal, adrenal gland and cervical spinal regions
(17,18). Other potential locations include the
presacrum, thoracolumbar region and bone (19,20).
Ganglioneuromas located in the conus medullaris, and associated
with syringomyelia, are rare. In the presence of a syringomyelia,
or when a low-intensity signal is present on T2WI scans (indicating
calcified foci or hemorrhage), an incorrect diagnosis of ependymoma
may be made. On occasion, the features of ganglioneuromas may mimic
those of myxopapillary ependymomas, which are often located in the
conus medullaris and the cauda equina, and are commonly associated
with hemorrhages that appear as low-intensity signals on T2WI
(9,21). Other spinal lesions, for example
hemangioblastomas, which are characterized by small lesions with
multi-sectional syringomyelia, are also difficult to differentiate
(22). Similarly, in the present
case, a contrast-enhanced vessel was identified above the lesion in
the T1WI. Differential diagnosis is also difficult with spinal
paragangliomas, where the tumor is always in the cauda equina,
showing intense homogeneous enhancement by MRI, whereas large
paragangliomas may show bony remodeling or erosion (23,24). For
these reasons a radiological diagnosis based on such analogous
imaging features is difficult and may result in misdiagnosis.
Pathologically, ganglioneuromas are
well-differentiated, benign tumors, which are populated by mature
sympathetic ganglion cells (17).
Typically, such features may aid the distinction of these tumors
from other spinal lesions, including ependymomas,
hemangioblastomas, paragangliomas, schwannomas and neurofibromas.
Further histological examination in the present study revealed that
the neoplastic lesion consisted of spindle-shaped cells, between
which, large ganglion cells with vesicular nuclei and eosinophilic
nucleoli were found. The ganglion cells were positively stained for
S-100, SYN, NSE, MAP-2 and NF.
During surgery, the tumor was completely resected
and the associated syringomyelia did not receive any specific
treatment. The tumor-associated cyst or syrinx is typically
expected to shrink, or even fully return to normal, following tumor
resection. However, post-operative MRI indicated no marked syrinx
collapse or retraction of the spinal cord. At the 18-month
follow-up, the patient still exhibited mild motor deficits. It was
therefore hypothesized that the pre-existing syringomyelia may have
been the reason underlying this long-term damage (25). Pressure on spinal cord parenchyma,
secondary to syringomyelia cavity expansion, may have contributed
to the spinal cord dysfunction, particularly since intra-syrinx
fluid is typically characterized by high viscosity and pressure
(26). While it has not been studied
extensively, it has been suggested that spinal cord ischemia may be
another significant contributor to spinal cord dysfunction.
Ischemia may arise from a marked increase in regional spinal cord
blood flow following decompression of the syrinx (27,28).
Damage sustained in this way is likely to contribute to compromised
post-operative spinal cord function and sub-optimal recovery from
surgical resection.
In conclusion, ganglioneuromas are benign,
well-differentiated tumors. When present in uncommon locations, for
example the cauda equina, incorrect provisional diagnoses of other,
more common neoplasms are likely due to the analogous radiological
features. For this reason, it is important to maintain a broad
range of differential diagnoses until histopathological findings
are able to confirm a diagnosis.
References
|
1
|
Mounasamy V, Thacker MM, Humble S, Azouz
ME, Pitcher JD, Scully SP, Temple HT and Eismont F: Ganglioneuromas
of the sacrum - a report of two cases with radiologic-pathologic
correlation. Skeletal Radiol. 35:117–121. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Modha A, Paty P and Bilsky MH: Presacral
ganglioneuromas. Report of five cases and review of the literature.
J Neurosurg Spine. 2:366–371. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
MacCarty CS, Waugh JM, Coventry MB and
Cope WF Jr: Surgical treatment of sacral and presacral tumors other
than sacrococcygeal chordoma. J Neurosurg. 22:458–464. 1965.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Spinelli C, Rossi L, Barbetta A, Ugolini C
and Strambi S: Incidental ganglioneuromas: A presentation of 14
surgical cases and literature review. J Endocrinol Invest.
38:547–554. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shephard RH and Sutton D: Dumb-bell
ganglioneuromata of the spine with a report of four cases. Br J
Surg. 45:305–317. 1958. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Oro JJ and Geise AW: Dumbbell
ganglioneuroma of the lumbar spine associated with a herniated
intervertebral disc: Case report. Neurosurgery. 13:711–714. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li L, Shao J, Gu J, Wang X and Qu L:
Adrenal ganglioneuromas: Experience from a retrospective study in a
Chinese population. Urol J. 11:1485–1490. 2014.PubMed/NCBI
|
|
8
|
Okudera Y, Miyakoshi N, Sugawara T, Hongo
M, Kasukawa Y, Ishikawa Y and Shimada Y: Ganglioneuroblastoma of
filum terminale: Case report. J Neurosurg Spine. 21:270–274. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schmitt HP, Wurster K, Bauer M and Parsch
K: Mixed chemodectoma-ganglioneuroma of the conus medullaris
region. Acta Neuropathol. 57:275–281. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu WH and Du YH: Therapeutic observation
of needling three hand yang meridian acupoints to treat
postapoplectic finger contracture. J Acupunct Tuina Sci. 5:301–303.
2007. View Article : Google Scholar
|
|
11
|
Kyoshima K, Sakai K, Kanaji M, Oikawa S,
Kobayashi S, Sato A and Nakayama J: Symmetric dumbbell
ganglioneuromas of bilateral C2 and C3 roots with intradural
extension associated with von Recklinghausen's disease: Case
report. Surg Neurol. 61:468–473. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Geoerger B, Hero B, Harms D, Grebe J,
Scheidhauer K and Berthold F: Metabolic activity and clinical
features of primary ganglioneuromas. Cancer. 91:1905–1913. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dimou J, Russell JH, Jithoo R and Pitcher
M: Sacral ganglioneuroma in a 19-year-old woman. J Clin Neurosci.
16:1692–1694. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ma J, Liang L and Liu H: Multiple cervical
ganglioneuroma: A case report and review of the literature. Oncol
Lett. 4:509–512. 2012.PubMed/NCBI
|
|
15
|
Hayes FA, Green AA and Rao BN: Clinical
manifestations of ganglioneuroma. Cancer. 63:1211–1214. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Esiri M: Russell and Rubinstein's
pathology of tumors of the nervous system. Sixth edition. J Neurol
Neurosurg Psychiatry. 68:538D2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ghali VS, Gold JE, Vincent RA and Cosgrove
JM: Malignant peripheral nerve sheath tumor arising spontaneously
from retroperitoneal ganglioneuroma: A case report, review of the
literature and immunohistochemical study. Hum Pathol. 23:72–75.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Andersen HJ, Hansen LG, Lange P and
Teglbjaerg PS: Presacral ganglioneuroma. Case report. Acta Chir
Scand. 152:777–778. 1986.PubMed/NCBI
|
|
19
|
Levy DI, Bucci MN, Weatherbee L and
Chandler WF: Intradural extramedullary ganglioneuroma: Case report
and review of the literature. Surg Neurol. 37:216–218. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cerullo G, Marrelli D, Rampone B, Miracco
C, Caruso S, Di Martino M, Mazzei MA and Roviello F: Presacral
ganglioneuroma: A case report and review of literature. World J
Gastroenterol. 13:2129–2131. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kariev GM, Halikulov ES and Rasulov SO:
Unspecific clinical manifestation of cauda equina myxopapillary
ependymoma. Asian J Neurosurg. 10:256–258. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Deng X, Wang K, Wu L, Yang C, Yang T, Zhao
L, Yang J, Wang G, Fang J and Xu Y: Intraspinal hemangioblastomas:
analysis of 92 cases in a single institution: Clinical article. J
Neurosurg Spine. 21:260–269. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lmejjati M, Parker F, Lacroix C and Tadie
M: Paraganglioma of the sacral spinal canal. Neurosciences
(Riyadh). 16:270–272. 2011.PubMed/NCBI
|
|
24
|
Midi A, Yener AN, Sav A and Cubuk R: Cauda
equina paraganglioma with ependymoma-like histology: A case report.
Turk Neurosurg. 22:353–359. 2012.PubMed/NCBI
|
|
25
|
Young WF, Tuma R and O'Grady T:
Intraoperative measurement of spinal cord blood flow in
syringomyelia. Clin Neurol Neurosurg. 102:119–123. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Klekamp J: The pathophysiology of
syringomyelia - historical overview and current concept. Acta
Neurochir (Wien). 144:649–664. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Levine DN: The pathogenesis of
syringomyelia associated with lesions at the foramen magnum: A
critical review of existing theories and proposal of a new
hypothesis. J Neurol Sci. 220:3–21. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schaller B, Mindermann T and Gratzl O:
Treatment of syringomyelia after posttraumatic paraparesis or
tetraparesis. J Spinal Disord. 12:485–488. 1999. View Article : Google Scholar : PubMed/NCBI
|