Introduction
Benign bone tumors mainly occur in children and
adolescents, and almost 42% of all bone lesions, including benign
and malignant etiologies, occur in the first two decades of life
(1,2).
However, this figure may be underestimated, as the majority of
benign tumors are not registered in clinical databases, due to the
asymptomatic clinical features that benign tumors present with
(1,2).
Surgical treatment is required when patients demonstrate
pathological fractures or locally aggressive benign tumors,
including giant cell tumors and chondroblastoma (1–3).
Additionally, symptomatic patients complaining of recurrent pain or
exhibiting a restricted range of motion in the affected joints are
considered for surgical treatment (1,2,4). There are various surgical treatment
strategies for benign bone tumors, consisting of curettage,
curettage with autologous/allogeneic bone grafting, and curettage
with artificial bone grafting (3–8). Calcium
phosphate cement (CPC) is an injectable biocompatible bone
substitute that has been used for various applications in
orthopedic surgery, due to its easy handling, high mechanical
strength and good osteoconductive biological properties (4,6–9). A previous study reported that CPC
offered a useful bone substitute for the treatment of bone and soft
tissue tumors; however, the follow-up time was short (median
follow-up, 18.5 months) (6).
Pediatric bone tumors remain a challenging field for
orthopedic tumor surgeons. Due to the active nature of
meta-epiphyseal tumors and iatrogenic damage to the growth plate,
surgery performed on this type of tumor may often lead to
progressive limb deformities (3,10–12). Special consideration must be provided
to pediatric tumor patients, not only for local tumor control, but
also for the long-term functional and developmental outcome of the
limb (3).
The aim of the present study was to investigate the
mid- to long-term clinical performance of CPC in the treatment of
benign bone tumors in pediatric patients with a follow-up of at
least 2-years. The present study was approved by the Ethics
Committee of Mie University Hospital (Tsu, Japan) and written
informed consent was obtained from all patients.
Patients and methods
Patient characteristics
The present study retrospectively reviewed the cases
of 33 patients with benign bone tumors treated by curettage and
subsequent implantation of CPC at the Mie University Hospital
between January 2001 and January 2011, with at least 2-years of
follow-up. This is more than the number of patients included in a
previous study that reported a mean follow-up time of 18.5 months
(6). Medical records were
predominantly used to review the cases. A total of 13 males and 20
females were reviewed in the present study (median age, 13 years;
range, 4–18 years) (Table I). The
median follow-up time was 79 months (mean, 79 months; range, 25–151
months). The bone tumors the patients presented with consisted of 9
solitary bone cysts, 6 enchondroma lesions, 5 aneurysmal bone
cysts, 4 giant cell tumors, 3 chondroblastoma lesions, 3
osteofibrous dysplasia lesions, 2 non-ossifying fibroma lesions,
and 1 fibrous dysplasia lesion. The location of the tumor lesions
were as follows: Femur (n=11); humerus (n=5); tibia (n=4); phalanx
(n=4); pelvis (n=3); calcaneus (n=3); and fibula, patella or ulna
(all n=1). In total 30 patients possessed primary bone tumors and 3
patients possessed recurrent tumors.
 | Table I.Patient characteristics and detail of
reconstruction for bone defect subsequent to curettage. |
Table I.
Patient characteristics and detail of
reconstruction for bone defect subsequent to curettage.
| Case no. | Age | Gender | Location | Diagnosis | Type of CPC | Volume of CPC,
ml | Augmentation |
|---|
| 1 | 16 | F | Patella | CB | CPC-R | 5.0 |
|
| 2 | 7 | F | Pharanx | EC | CPC-R | 1.0 |
|
| 3 | 16 | M | Femur | CB | CPC-R | 4.0 |
|
| 4 | 18 | F | Pelvis | CB | CPC-R | 6.0 |
|
| 5 | 15 | M | Pelvis | ABC | CPC-R | 55.0 |
|
| 6 | 10 | M | Femur | SBC | CPC-R | 13.0 |
|
| 7 | 6 | F | Pharanx | EC | CPC-R | 0.5 |
|
| 8 | 8 | F | Humerus | SBC | CPC-R | 2.0 |
|
| 9 | 12 | M | Tibia | OFD | CPC-R | 6.0 |
|
| 10 | 16 | F | Pelvis | ABC | CPC | 10.0 |
|
| 11 | 18 | F | Ulna | GCT | CPC | 1.0 |
|
| 12 | 8 | F | Fibula | EC | CPC | 20.0 |
|
| 13 | 10 | M | Calcaneus | SBC | CPC-R | 12.0 |
|
| 14 | 16 | F | Humerus | SBC | CPC-R | 14.0 |
|
| 15 | 13 | M | Humerus | ABC | CPC-R | 24.0 |
|
| 16 | 12 | M | Humerus | SBC | CPC-R | 5.0 |
|
| 17 | 15 | F | Femur | FD | CPC-R | 54.0 | IM nail |
| 18 | 13 | F | Calcaneus | SBC | CPC-R | 10.0 |
|
| 19 | 15 | F | Femur | GCT (rec.) | CPC-R | 70.0 |
|
| 20 | 16 | F | Femur | NOF | CPC-R | 6.0 |
|
| 21 | 16 | F | Pharanx | EC | CPC-R | 3.0 |
|
| 22 | 4 | M | Femur | EC | CPC | 5.0 |
|
| 23 | 17 | M | Humerus | SBC (rec.) | CPC-R | 50.0 |
|
| 24 | 12 | F | Pharanx | EC | CPC-R |
2.0 |
|
| 25 | 15 | F | Tibia | OFD | CPC-R | 23.0 |
|
| 26 | 4 | M | Femur | SBC | CPC-R |
6.0 |
|
| 27 | 11 | F | Femur | ABC | CPC/CHA | 10.0 |
|
| 28 | 16 | F | Femur | GCT (rec.) | CPC/CHA | 30.0 | CHS |
| 29 | 11 | M | Tibia | OFD | CPC-R | 12.0 |
|
| 30 | 11 | F | Calcaneus | SBC | CPC-R | 12.0 |
|
| 31 | 18 | M | Femur | ABC | CPC-R | 12.0 | Plate |
| 32 | 12 | M | Femur | GCT | CPC-R/CHA | 18.0 |
|
| 33 | 12 | F | Tibia | NOF | CPC-R | 18.0 |
|
The present study was performed following ethical
principles of research.
CPC development
BIOPEX® or BIOPEX-R® (HOYA Technosurgical
Corporation, Tokyo, Japan) was the CPC used to fill intramedullary
bone defects. Briefly, CPC (6 ml) was made by mixing the powder
component of Biopex-R®, containing a-tricalcium phosphate,
tetracalcium phosphate and calcium hydrogen phosphate dehydrate,
with the liquid component of Biopex-R®, containing sodium succinate
and sodium chonroitin sulfate. The two components were mixed at an
appropriate powder-to-liquid ratio (P/L=2.0–4.5) for a few minutes
(typical quantities: 10 g powder and 2–4 ml liquid). To allow
reservation of the Biopex-R® cement at room temperature, magnesium
phosphate and sodium hydrogen sulfite were added to the powder
component (6). The compressive
strength of the cured materials was ~65 MPa 3 days following
mixing, reaching a final strength of >70 MPa 1 week following
mixing (13,14). Biopex-R® replaced Biopex® in 2002 at
Mie University Hospital.
Results
Surgical treatment
Surgery was performed using a tourniquet in 19
patients, and without in 14 patients. Tumor curettage was performed
using a curette (MIZUHO Corporation, Tokyo, Japan) and an airtome
(Zimmer K.K., Tokyo, Japan) until healthy cancellous bone was
visualized. CPC was injected subsequent to curettage (median
volume, 10 ml; range, 0.5–70 ml) for the reconstruction of bony
defects. Internal fixation was performed in 3 patients. The window
of the cortical bone at the tumor site must be large enough to
allow adequate curettage of the tumor until underlying normal bone
is exposed. After curettage, an internal fixation was inserted. CPC
was implanted in the bone defect. Intramedullary nail and
compression hip screw was inserted to prevent post-operative
fractures in 2 patients (case 17 and 28). Plate fixation was
performed in 1 patient, who presented with a pre-operative
pathological fracture (case 31). Calcium hydroxyapatite ceramic
(CHA) in granular form (BONECERAM®; Olympus Corporation, Tokyo,
Japan) was used in combination with CPC in 3 patients (case 27, 28
and 32) to reduce tumor spread in high-risk patients.
Clinical outcome
All patients were alive at the time of review
(Table II). No toxicity was detected
in routine blood tests, including white blood cell (normal range,
3,900–6,600 cells/mm2), C-reactive protein (normal
range, 0.3 mg/dl) and creatinine (normal range, 0.60–1.40 mg/dl)
tests. Radiography confirmed that CPC was well adapted to the
surrounding host bone in all patients, an example of which is shown
in Fig. 1. However, the resorbability
of the CPC had not been determined in all cases at the final
follow-up. Local tumor recurrence occurred in 4 patients (cases 8,
9, 28 and 32). None of the patients reported post-operative
fractures. A total of 20 patients, in whom tumors were located in
the lower extremities, required between 1–16 weeks to bear the full
weight subsequent to surgery. There was no requirement for intense
physical therapy in the majority of patients. In total, 1 patient
with large recurrent giant cell tumor of the bone (case 28)
required 8 weeks of cast fixation followed by physical therapy for
1 month, as the residual bone stock was too scarce to allow early
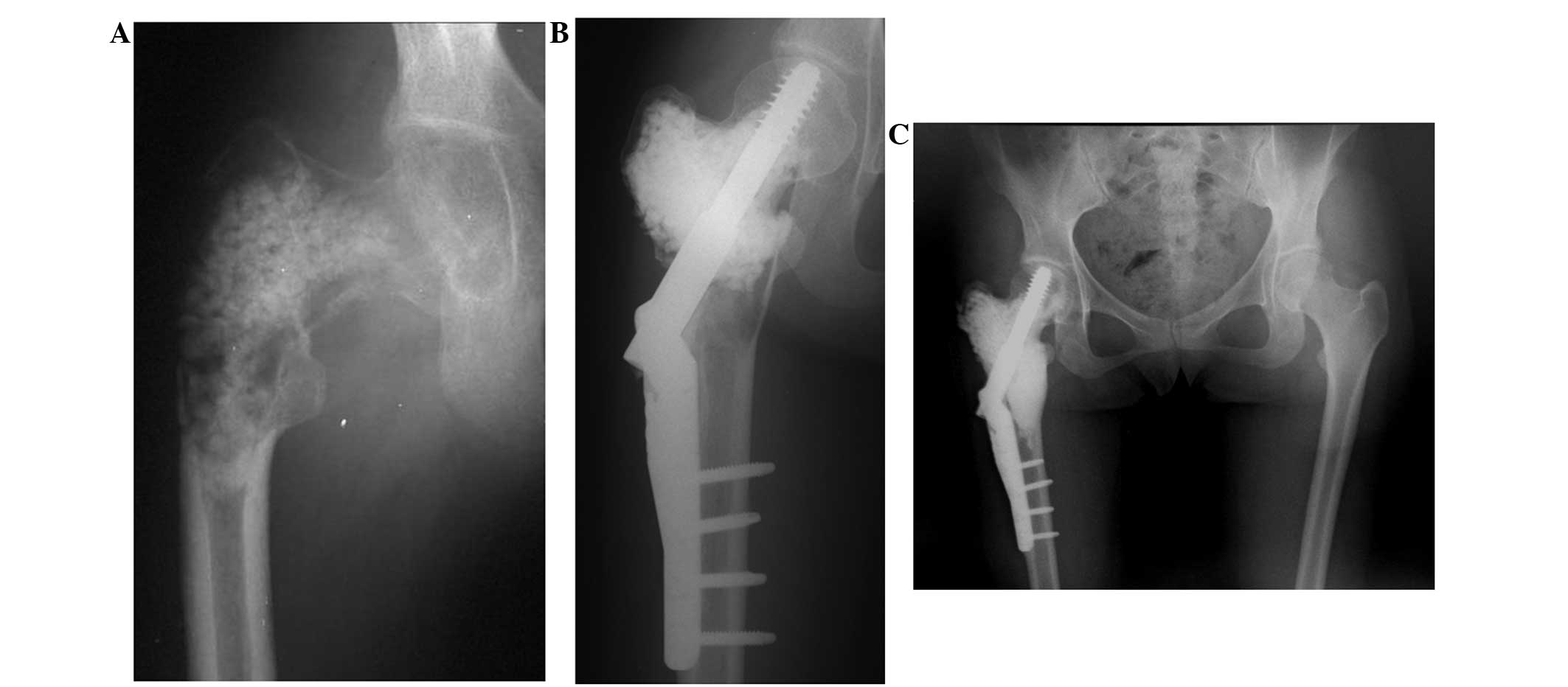
weight bearing following curettage of the bone tumor (Fig. 2), and 1 patient (case 32), in whom CPC
was implanted at a fracture site, required physical therapy for 2
months subsequent to 6 weeks of cast fixation (Fig. 3).
 | Table II.Clinical outcome and complications in
patients. |
Table II.
Clinical outcome and complications in
patients.
| Case no. | Age, years | Gender | Location | Diagnosis | Volume of CPC,
ml | Augmentation | Time to FWB,
weeks | Complication | F/U, months |
|---|
| 1 | 16 | F | Patella | CB |
5.0 |
| 1 |
| 114 |
| 2 | 7 | F | Pharanx | EC |
1.0 |
|
|
| 96 |
| 3 | 16 | M | Femur (prox) | CB |
4.0 |
| 1 |
| 32 |
| 4 | 18 | F | Pelvis | CB |
6.0 |
|
|
| 34 |
| 5 | 15 | M | Pelvis | ABC | 55.0 |
|
| Superficial
infection | 107 |
| 6 | 10 | M | Femur (prox) | SBC | 13.0 |
| 2 |
| 86 |
| 7 | 6 | F | Pharanx | EC |
0.5 |
|
|
| 99 |
| 8 | 8 | F | Humerus (mid) | SBC |
2.0 |
|
| Local rec. | 78 |
| 9 | 12 | M | Tibia (mid) | OFD |
6.0 |
| 1 | Local rec. | 119 |
| 10 | 16 | F | Pelvis | ABC | 10.0 |
|
|
| 25 |
| 11 | 18 | F | Ulna (distal) | GCT |
1.0 |
|
|
| 129 |
| 12 | 8 | F | Fibula (prox) | EC | 20.0 |
| 2 |
| 142 |
| 13 | 10 | M | Calcaneus | SBC | 12.0 |
| 1 |
| 82 |
| 14 | 16 | F | Humerus (prox) | SBC | 14.0 |
|
|
| 79 |
| 15 | 13 | M | Humerus (prox) | ABC | 24.0 |
|
|
| 81 |
| 16 | 12 | M | Humerus (prox) | SBC |
5.0 |
|
|
| 85 |
| 17 | 15 | F | Femur (prox) | FD | 54.0 | IM nail | 2 |
| 68 |
| 18 | 13 | F | Calcaneus | SBC | 10.0 |
| 1 |
| 34 |
| 19 | 15 | F | Femur (prox) | GCT (rec.) | 70.0 |
| 8 |
| 67 |
| 20 | 16 | F | Femur (prox) | NOF |
6.0 |
| 2 |
| 127 |
| 21 | 16 | F | Pharanx | EC |
3.0 |
|
|
| 63 |
| 22 | 4 | M | Femur (prox) | EC |
5.0 |
| 4 |
| 74 |
| 23 | 17 | M | Humerus (prox) | SBC (rec.) | 50.0 |
|
| Deep infection | 110 |
| 24 | 12 | F | Pharanx | EC |
2.0 |
|
|
| 40 |
| 25 | 15 | F | Tibia (mid) | OFD | 23.0 |
| 6 |
| 54 |
| 26 | 4 | M | Femur (prox) | SBC |
6.0 |
| 8 |
| 100 |
| 27 | 11 | F | Femur (mid) | ABC | 10.0 |
| 6 |
| 137 |
| 28 | 16 | F | Femur (prox) | GCT (rec.) | 30.0 | CHS | 5 | Local rec./1.5 cm
short. | 151 |
| 29 | 11 | M | Tibia (mid) | OFD | 12.0 |
| 4 |
| 49 |
| 30 | 11 | F | Calcaneus | SBC | 12.0 |
| 1 |
| 35 |
| 31 | 18 | M | Femur (distal) | ABC | 12.0 | Plate | 12 |
| 35 |
| 32 | 12 | M | Femur (distal) | GCT | 18.0 |
| 16 | Local rec./2.0 cm
short., VD | 50 |
| 33 | 12 | F | Tibia (mid) | NOF | 18.0 |
| 8 |
| 37 |
In total, 6 patients required a second surgery, as
follows: 4 patients with local tumor recurrence (cases 8, 9, 28 and
32); 1 patient with post-operative superficial wound infection
(case 5) that underwent wound debridement; and 1 patient with a
simple bone cyst at the proximal humerus that required the removal
of CPC and the implantation of antibiotic-impregnated (vancomycin)
CHA due to deep infection (case 23).
The limb length discrepancy was 1.5 cm in 1 patient
with recurrent giant cell tumor (case 28; Fig. 2) and 2.0 cm in 1 patient with giant
cell tumor accompanying a varus deformity of the femur (case 32;
Fig. 3). Neither patient was
restricted in daily life. All patients had regained full physical
function without any pain at the final follow-up.
Discussion
Autologous bone grafting for a bone defect following
curettage of a bone tumor is the gold standard as a reconstruction
method, as it guarantees rapid graft incorporation and bone
remodeling (4,15). The advantages of CPC include a fast
setting time, excellent moldability and good osteoconductivity
(4,6–9). The early
structural support that CPC offers is beneficial for large bone
defects at risk of fracture. The compressive strength of CPC ranges
between 26 and 70 MPa, which is comparable to that of cancellous
bone (16,17). Studies have indicated that the
injection of CPC can increase the strength of a fractured vertebral
body to that of an intact vertebral body (18,19).
Thordarson et al reported the superior compressive strength
of a calcaneal fracture construct when it was augmented with CPC
(20). Therefore, the present study
suggests that full weight bearing can be allowed several days
subsequent to surgery when CPC is injected into a cavity subsequent
to curettage of a bone tumor of flat bone, including tumors of the
calcaneus and patella. However, the results of an experimental
study using rabbits indicate that, although CPC increases the
torsional strength of the shaft of long bones in the early phase,
the resultant strength is not adequate to preclude the requirement
for external fixation (14). When CPC
is implanted into a cavity that occupies more than one-half of the
medullary cavity of long bones at lower extremities, the present
study recommends waiting for ≥3 weeks following surgery prior to
full weight bearing. Furthermore, when a tumor is localized in
metaphyseal or diaphyseal bone, metal augmentation, including plate
fixation, should be considered; unless the metal augumentation is a
hindrance to growth, as pediatric patients are active and so there
is a risk of post-operative fracture.
The presence of an active physis within the zone of
resection and iatrogenic damage to the growth plate has been linked
to growth associated complications and progressive limb deformities
(3,10–12). In
total, 2 patients (cases 28 and 32) reported in the present study
demonstrated limb length discrepancy. However, this may be
associated with pre-operative pathological fractures resulting in
leg shortening and deformity in the 2 patients. Furthermore, the
patient that presented with a giant cell tumor at the distal region
of the femur (case 32), did not undergo correction osteotomy for
the angulated deformity due to pathological fracture, as metal
reinforcement may be a cause of growth arrest of the femur.
Although post-operative deformity of the bone was not observed in
the remaining 31 patients in the present study, additional
follow-up is essential until the closure of the growth plate is
observed.
The present study described how CPC may demonstrate
a therapeutic potential as a useful bone substitute in pediatric
patients that present with benign bone tumors. However, there are
several disadvantages of CPC. First, CPC remains expensive despite
efforts to reduce the cost. Second, even at the median 6 years
follow-up described in the present study, the resorbability of CPC
was far from complete. Graft material, such as CPC, may impede
future surgical reconstructive alternatives, including joint
replacement. Therefore the properties of the CPC must be taken into
consideration and applied to the reconstruction of bone defects
after curettage of bone tumors.
References
|
1
|
Wyers MR: Evaluation of pediatric bone
lesions. Pediatr Radiol. 40:468–473. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vlychou M and Athanasou NA: Radiological
and pathological diagnosis of pediatric bone tumors and tumor-like
lesions. Pathology. 40:196–216. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wallace MT and Henshaw RM: Results of
cement versus bone graft reconstruction after intralesional
curettage of bone tumors in the skeletally immature patient. J
Pediatr Orthop. 34:92–100. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kirschner HJ, Obermary F, Schaefer J and
Lieber J: Treatment of benign bone defects in children with
silicate-substituted calcium phosphate (SiCaP). Eur J Pediatr Surg.
22:143–147. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
George B, Abudu A, Grimer RJ, Carter SR
and Tillman RM: The treatment of benign lesions of the proximal
femur with non-vascularised autologous fibular strut grafts. J Bone
Joint Surg Br. 90:648–651. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Matsumine A, Kusuzaki K, Matsubara T,
Okamura A, Okuyama N, Miyazaki S, Shintani K and Uchida A: Calcium
phosphate cement in musculoskeletal tumor surgery. J Surg Oncol.
93:212–220. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yasuda M, Masada K and Takeuchi E:
Treatment of enchondroma of the hand with injectable calcium
phosphate bone cement. J Hand Surg. 31:98–102. 2006. View Article : Google Scholar
|
|
8
|
Strauss EJ, Pahk B, Kummer FJ and Egol K:
Calcium phosphate cement augmentation of the femoral neck defect
created after dynamic hip screw removal. J Orthop Trauma.
21:295–300. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ambard AJ and Mueninghoff L: Calcium
phosphate cement: Review of mechanical and biological properties. J
Prosthodont. 15:321–328. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ozaki T, Hillmann A, Linder N and
Winkelmann W: Cementation of primary aneurismal bone cysts. Clin
Orthop Relat Res. 337:240–248. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin PP, Thenappan A, Deavers MT, Lewis VO
and Yasko AW: Treatment and prognosis of chondroblastoma. Clin
Orthop Relat Res. 438:103–109. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hajdu S, Schwendenwein E, Kaltenecker G,
László I, Lang S, Vécsei V and Sarahrudi K: The effect of drilling
and screw fixation of the growth plate - an experimental study in
rabbits. J Orthop Res. 29:1834–1839. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ikeuchi M, Yamamoto H, Shibata T and Otani
M: Mechanical augmentation of the vertebral body by calcium
phosphate cement injection. J Orthop Sci. 6:39–45. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mizobuchi H, Tani T, Takemasa R, Yamamoto
H and Sonobe H: Mechanical properties of the femur filled with
calcium phosphate cement under torsional loading: A model in
rabbits. J Orthop Sci. 7:562–569. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Matsumine A, Myoui A, Kusuzaki K, Araki K,
Seto M, Yoshikawa H and Uchida A: Calcium hydroxyapatite ceramic
implants in bone tumour surgery. J Bone Joint Surg. 86:719·7252004.
View Article : Google Scholar
|
|
16
|
Welch RD, Zhang H and Bronson DG:
Experimental tibial plateau fractures augumented with calcium
phosphate cement or autologous bone graft. J Bone Joint Surg Am.
85-A:222–231. 2003.PubMed/NCBI
|
|
17
|
Larsson S and Bauer TW: Use of injectable
calcium phosphate cement for fracture fixation: A review. Clin
Orthop Relat Res. 395:23–32. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lim TH, Brebach GT, Renner SM, Kim WJ, Kim
JG, Lee RE, Andersson GB and An HS: Biomechanical evaluation of an
injectable calcium phosphate cement for vertebroplasty. Spine
(Phila Pa 1976). 27:1297–1302. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tomita S, Kin A, Yazu M and Abe M:
Biomechanical evaluation of kyphoplasty and vertebroplasty with
calcium phosphate cement in a simulated osteoporotic compression
fracture. J Orthop Sci. 8:192–197. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Thordarson DB, Hedman TP, Yetkinler DN,
Eskander E, Lawrence TN and Poser RD: Superior compressive strength
of a calcaneal fracture construct augmented with remodelable
cancellous bone cement. J Bone Joint Surg Am. 81:239–246.
1999.PubMed/NCBI
|