Introduction
Angioleiomyoma, or vascular leiomyoma, is an
uncommon benign tumor that consists of a mixture of
well-differentiated smooth muscle cells and prominent thick-walled
vessels. Angioleiomyoma usually occurs in the subcutis of
extremities, often developing in lower extremities, and presents as
a solitary, small, painful, cutaneous solid-type mass.
Angioleiomyoma located in the nasal cavity is uncommon, and studies
of nasal angioleiomyoma with >2 patients are extremely rare
(1–29). To the best of our knowledge, the cases
of ~50 patients with nasal angioleiomyoma have been reported in the
literature since the first case was reported by Maesaka et
al in 1966 (5).
Angioleiomyomas of the nasal cavity typically grow
slowly and the majority of these tumors measure <2 cm in size
(6–29). Patients may remain asymptomatic for a
long time, and may present with nasal obstruction, recurrent
epistaxis, nasal discharge, facial pain and headache (6–29). The
peak incidence of angioleiomyomas of the nasal cavity is between
the third and sixth decades of life and the tumor is more common in
women (6–29). Angioleiomyoma is rarely diagnosed
prior to surgery, as the physical and radiological findings are not
tumor-specific in the majority of cases (5–12).
Surgical excision is the recommended form of
treatment as has been documented in previous studies. The advent of
nasal endoscopes has opened new avenues for surgeons to deal with
these tumors. In general, the majority of tumors can be totally
excised through the transnasal endoscopic approach, while a smaller
number of cases may be removed with craniofacial resection
(2,11,26,29).
Hormone receptors, including the estrogen receptor
(ER) and progesterone receptor (PR), may be involved in the
tumorigenesis of extra-uterine smooth muscle tumors, including
uterine angioleiomyoma (6–15,30–34). To
date, only a small number of studies have demonstrated that hormone
receptors are expressed in angioleiomyoma of the nasal cavity and
nasal tip, and there are no studies reporting ER expression
(6–12).
Complete excision and pathological examination are
essential for a final diagnosis. A possibility of malignant
degeneration has been reported. If an angioleiomyoma recurs, it
should be treated as a low-grade malignancy (35).
The present study retrospectively reports the
clinical manifestations, imaging features, histological
characteristics, and management of nasal angioleiomyoma in 6
patients, and discusses the importance of ER and PR expression in
this tumor.
Patients and methods
Patients
Subsequent to obtaining approval from the
Institutional Review Board of Wuxi Second People's Hospital (Wuxi,
Jiangsu, China), the medical records of 6 patients that were
diagnosed with angioleiomyoma of the nasal cavity between January
2004 and December 2013 were reviewed. All clinical data are shown
in Table I. The data consisted of the
age, gender, presentation and duration of symptoms of the patients
and clinical features, methods of diagnosis, tumor location, tumor
size and subsequent management and surveillance of the patients
with angioleiomyoma. Paraffin-embedded sections of surgical
specimens from all patients were retrieved and reviewed by a
pathologist with 10 years of experience to confirm a diagnosis of
angioleiomyoma.
 | Table I.Characteristics of 6 patients with
nasal angioleiomyoma. |
Table I.
Characteristics of 6 patients with
nasal angioleiomyoma.
|
| Expression |
|
|---|
|
|
|
|
|---|
| Case | Age, years | Gender | Tumor site | Symptoms | Duration | Tumor size, cm | Image | Pre-operative
diagnosis | Treatment | ER | PR | Recurrence |
|---|
| 1 | 53 | M | Nasal septum | Recurrent
epistaxis | 1 year | 1.0×0.5×0.3 | CT | Hemangioma | Dissection | + | + | NR |
| 2 | 74 | F | Lateral wall of
nasal cavity | Mass, nasal
obstruction | 20 years | 2.0×1.0×1.0 | CT | Hemangioma | Dissection | + | + | NR |
| 3 | 65 | F | Nasal septum | Recurrent
epistaxis, nasal obstruction | 2 years | 1.5×0.5×0.5 | CT | Hemangioma | Dissection | + | + | NR |
| 4 | 55 | M | Inferior
turbinate | Recurrent
epistaxis, nasal obstruction | 6 years | 1.0×0.8×0.5 | CT+MRI | Hemangioma | Dissection | + | + | NR |
| 5 | 62 | M | Middle
turbinate | Recurrent
epistaxis, nasal obstruction | 6 months | 1.5×1.0×1.0 | CT+MRI | Hemangioma | Dissection | + | + | NR |
| 6 | 54 | M | Nasal
vestibule | Mass, nasal
obstruction | 6 months | 1.0×0.5×0.5 | CT | Angiofibroma | Dissection | − | − | NR |
Immunohistochemical staining of
tissues
Immunohistochemical staining was performed on
4-µm-thick formalin-fixed (Xilong Chemical Co., Ltd., Shantou,
China) and paraffin-embedded (Shanghai Yiyang Medical Equipment
Co., Ltd., Shanghai, China) sections of surgical specimens from 6
patients, according to the manufacturer's protocol of the
Elivision™ plus Polyer HRP (Mouse/Rabbit) IHC kit (catalog no.,
KIT-9901; Maxim Biotech Inc., Fuzhou, China), which included
reagent A (polymer enhancer) and reagent B (enzyme-labeled goat
anti-mouse/rabbit polymer). All primary antibodies were
ready-to-use and purchased from Maxim Biotech Inc., and comprised
the following: mouse anti-human calponin monoclonal antibody
(catalog no., MAB-0335), mouse anti-human desmin monoclonal
antibody (catalog no., MAB-0055), mouse anti-human S-100 monoclonal
antibody (catalog no., MAB-0697), mouse anti-human melanoma
black-45 monoclonal antibody (catalog no., MAB-0098), mouse
anti-human progesterone receptor monoclonal antibody (catalog no.,
MAB-0675), mouse anti-human estrogen receptor monoclonal antibody
(catalog no., MAB-0062), mouse anti-human cluster of
differentiation (CD)31 monoclonal antibody (catalog no., MAB-0031),
mouse anti-human CD34 monoclonal antibody (catalog no.,
Kit-0004-2), mouse anti-human Ki-67 monoclonal antibody (catalog
no., MAB-0672) and mouse anti-human α-smooth muscle actin
monoclonal antibody (catalog no., Kit-0006).
Briefly, the slides were deparaffinized with xylene
(Xilong Chemical Co., Ltd.) and rehydrated with alcohol (Yang Xu
Ditch Chemical Plant, Yixing, China; immersed in 100% alcohol for 3
min 2 times, 95% alcohol for 3 min 2 times and 80% alcohol for 3
min, followed by washing with distilled water for 1 min), and
antigen retrieval was performed according to the requirements of
each primary antibody [CD31, 0.0001M EDTA antigen retrieval
solution (pH 9; Maxim Biotech Inc.); α-smooth muscle actin does not
require antigen retrieval; all other primary antibodies, 0.01M
citrate antigen retrieval solution (pH 6; Maxim Biotech Inc.)]; the
slides were incubated with 1 drop (~50 µl) of primary antibody at
4°C overnight, followed by 1 drop (~50 µl) of reagent A for 20 min
and reagent B (secondary antibody; from the Elivision™ plus Polyer
HRP (Mouse/Rabbit) IHC kit) for 30 min at room temperature. Color
reactions were visualized using Ultra Diaminobenzidine (Maxim
Biotech Inc.). At the end of each step mentioned above, phosphate
buffered saline (Maxim Biotech Inc.) was used to rinse the slides 3
times, for 3 min each time. Finally, the slides were counterstained
with hematoxylin and eosin (Maxim Biotech Inc.), washed, dehydrated
in alcohol, cleared in xylene, mounted with coverslips (Maxim
Biotech Inc.) and visualized using an optical microscope (BX-51,
Olympus, Japan).
Results
Patient characteristics
Of the 6 patients reviewed, 4 were men and 2 were
women. All patients were >50 years old, and the mean age of the
patients was 60.5 years. The primary site of the tumors were the
nasal septum, middle turbinate, inferior turbinate, lateral wall of
the nasal cavity and nasal vestibule. The clinical manifestations
reported by the patients consisted of a painless mass, recurrent
epistaxis and nasal obstruction, and the duration of disease was
between 6 months and 20 years.
Diagnosis and treatment
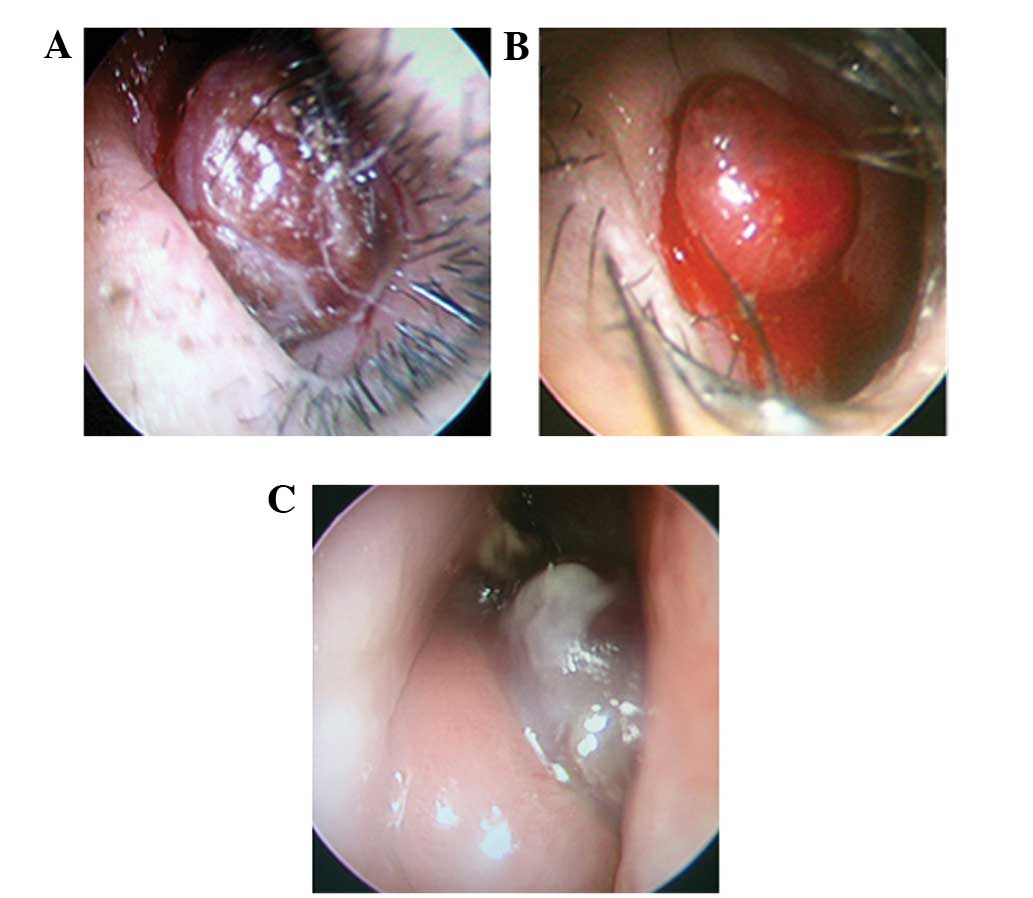
Endoscopic examination revealed that all the tumors
were solitary, mobile, well-circumscribed, ovoid or round in shape,
with a rough surface. Patient 6 presented with a tumor that
exhibited a medium texture, while other patients possessed soft
tumors. In total, 4 tumors were dark red, with hemorrhagic areas on
the surface, and 2 tumors were bright red, which is a similar color
to the nasal mucosa (Fig. 1). All
patients underwent a plain, spiral computed tomography (CT) scan.
The CT scan revealed solitary nasal lesions with well-defined
margins, and the CT values were 46–54 Hounsfield units, which was
similar to the CT values of the surrounding non-tumorous soft
tissue. No tumors involved the paranasal sinus, orbit or skull
base. There were no evident bony erosions observed in any of the
patients. Magnetic resonance imaging (MRI) examination was
performed in 2 patients, using fast-spin-echo sequence T1-weighted
imaging (T1WI) and T2-weighted imaging (T2WI) with fat suppression.
The tumor signals in the examination were hypointensive or
isointensive to muscle on T1WI, and heterogeneously hyperintensive
on T2WI. The tumors exhibited clear delayed enhancement following
an intravenous injection of
gadolinium-diethylenetriaminepentaacetic acid (Beijing BeiLu
Pharmaceutical Co., Ltd., Beijing, China; Fig. 2). Pre-operatively, 5 patients were
misdiagnosed with hemangioma and 1 patient was misdiagnosed with
angiofibroma.
All patients were treated by tumor resection using a
nasal endoscope. Intra-operatively, tumors were easy to completely
separate from non-tumorous tissues. The size of the tumors ranged
between 1.0×0.5×0.3 cm and 2.0×1.0×1.0 cm. There was no evident
bleeding during surgery in any of the patients. Macroscopically,
all lesions had complete capsules and gray-white, brown or dark red
sections. All patients recovered without recurrence or malignancy
post-operatively, and were followed-up for 1–10 years without
evidence of recurrence or malignancy.
Pathological examination
Hematoxylin-eosin staining revealed that the tumors
were formed by bundles of spindle-shape smooth muscle cells
circumscribing numerous slit-like blood vessels (Fig. 3). The tumors were in close contact
with the mucosa, without evidence of ulceration. No mitosis,
necrosis or significant nuclear atypia was observed in the tumor
cells.
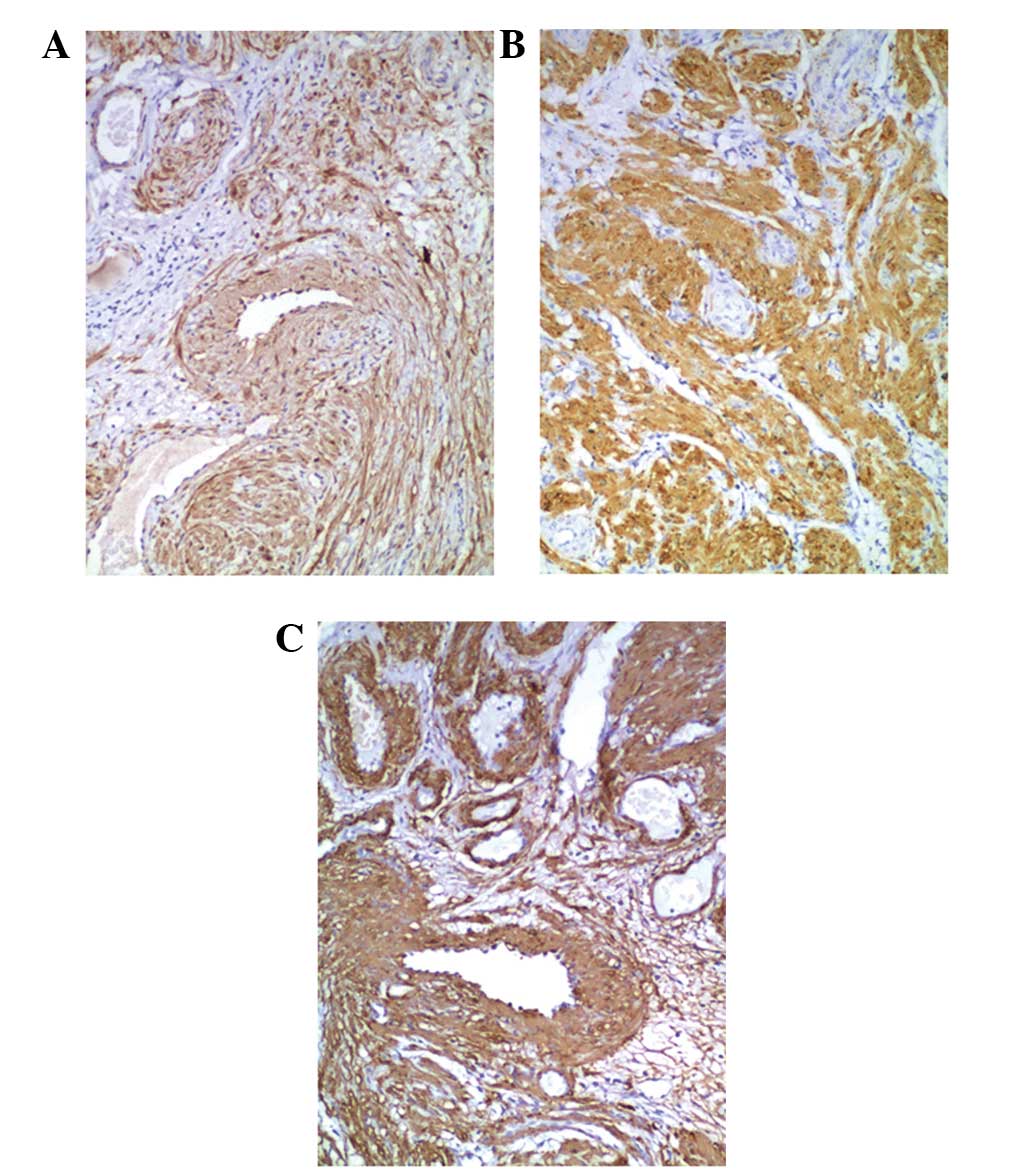
Immunohistochemical staining revealed that the tumor
cells exhibited high expression of calponin, desmin and α-smooth
muscle actin in the cytoplasm of the smooth muscle cells (Fig. 4). Cluster of differentiation (CD)-31
and CD34 were expressed in the endothelial cells that lined the
vascular spaces. The tumor cells did not express CD31, CD34, human
melanoma black-45 or S100. The population of cells expressing Ki-67
was evaluated as a marker for cell proliferation, and the level of
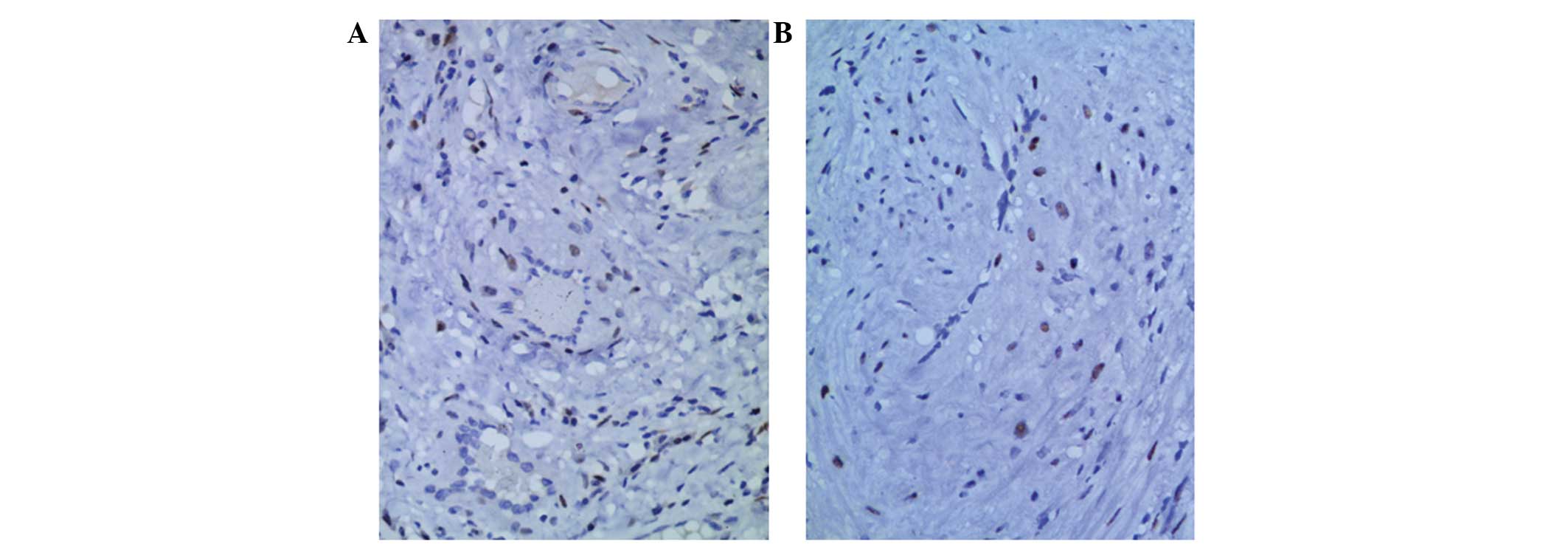
positive expression of Ki-67 was <3% in all patients. ER and PR
were partially expressed in the nuclei of the smooth muscle tumor
cells of patients, with the exception of patient 6 (Fig. 5).
Discussion
The tissue origin of angioleiomyoma is uncertain
(2–4);
however, hypotheses include angioleiomyomas forming from aberrant
undifferentiated mesenchyme and smooth muscle in the wall of blood
vessels (7,8). In addition, if the tumor has developed
in the nasal vestibule, which is covered with skin, it may have
originated from the hair erecting muscles (7). Angioleiomyomas are an extremely rare
occurrence in the nasal cavity, which is possibly due to the
paucity of smooth muscle in this region (14). Since nasal angioleiomyoma was first
identified by Maesaka et al (5) in 1966, this entity has been reported in
a small number of papers with 1 or 2 patients. To the best of our
knowledge, the current data may be one of the largest groups of
patients with nasal angioleiomyoma reported (1–29).
In the 49 cases of nasal angioleiomyoma reported
since 1966, the gender of the patients was known in 42 cases and
there was a male to female ratio of 5:9; this ratio is similar to
that of subcutaneous angioleiomyoma, which has a female
predominance (1–29). By contrast, the present results
observed a higher male to female ratio. The three most common sites
of angioleiomyoma in the nasal cavity are, in order of prevalence,
the inferior turbinate, nasal septum and nasal vestibule (1–29). The
site of angioleiomyoma development was the superior turbinate and
middle turbinate; other sites in the nasal cavity are more rarely
observed (8,21,24).
The main clinical symptoms that patients present
with are recurrent epistaxis, nasal obstruction and the development
of a painless mass, which are non-specific. Occasionally,
angioleiomyoma may involve the paranasal sinus, which leads to the
destruction of the medial orbit wall (20), a breach of the orbital periosteum
(21) and infrequently an extension
into the anterior cranial fossa (23). As Figs.1
and 2 reveal, all angioleiomyomas in
the present study were confined to the nasal cavity and the tumors
were not extremely large in size, and were confirmed by endoscope
examination, imaging or surgery.
CT and MRI are the most common imaging methods used
for the examination of nasal tumors (3,36), while
angiography is only employed in rare cases (24,37). There
were no specific features observed during imaging in the present
study, but post-contrast images were easily misdiagnosed
pre-operatively as hemangioma. However, imaging may aid in
determining the extent of tumor invasion and formulating the most
appropriate surgical approach, but they may occasionally rule out
multi-centric lesions accidentally (38,39).
Sex hormone receptors may play a role in the
tumorigenesis of extra-uterine smooth muscle tumors, such as
uterine angioleiomyoma. To the best of our knowledge, the presence
of the PR and ER have only been evaluated in 20 patients with
angioleiomyoma located in various regions of the body, as exhibited
in Table II (6,7,9–12,30–34), and
these results were inconsistent. Among the 20 patients, 5 patients
possessed angioleiomyoma that originated in the nasal cavity, 1
patient possessed angioleiomyoma of the nasal tip, and PR was
expressed in 3 patients and ER was not expressed in any of the
patients. The present study also evaluated the expression of ER and
PR in 6 patients; the two receptors were clearly expressed in 5
patients. To the best of our knowledge, the present study is the
first study to demonstrate PR and ER expression in angioleiomyoma
of the nasal cavity. The present result is corroborated with ER and
PR expression in 2 female patients with angioleiomyoma of the
extra-peritoneal cavity and angioleiomyoma of the bilateral broad
ligaments, reported by Hayashi et al in 2009 (33) and Chen et al in 2010 (32), respectively. Chen et al
(32) suggested that sex hormones may
be associated with the growth and degeneration of certain
angioleiomyomas, since those receptors are clearly expressed in the
nucleus of tumor cells. Additional large-scale studies with more
patients are required to elucidate the effect of the PR and ER,
particularly the ER, in nasal angioleiomyoma.
 | Table II.Literature review of studies
reporting ER and PR expression in various patients with
angioleiomyoma. |
Table II.
Literature review of studies
reporting ER and PR expression in various patients with
angioleiomyoma.
|
|
|
| Expression |
|---|
|
|
|
|
|
|---|
| First author, year
(Ref) | No. of
patients | Site of tumor | ER | PR |
|---|
| Onesti et
al, 2012 (6) | 1 | Nasal tip | − | − |
| He et al,
2009 (7) | 1 | Nasal cavity | − | + |
| Kim et al,
2004 (9) | 1 | Nasal cavity | − | − |
| Marioni et
al, 2002 (10) | 1 | Nasal cavity | − | + |
| Tseng et al,
2014 (11) | 1 | Nasal cavity | − | + |
| Chen et al,
2007 (12) | 1 | Nasal cavity | − | − |
| Inaba et al,
2015 (30) | 1 | Buccal space | − | + |
| Terada, 2013
(31) | 1 | Lung | − | − |
| Chen et al,
2010 (32) | 1 | Broad
ligaments | + | + |
| Hayashi et
al, 2009 (33) | 1 | Extra-peritoneal
cavity | + | + |
| Di Tommaso et
al, 2000 (34) | 10 | Subcutaneous | −, 100% | +, 60% |
Complete surgical excision is the recommended
treatment for nasal angioleiomyoma, as reported by previous studies
(13,16–29). The
surgical approach depends on the size, location and extension of
the tumor, and the experience of the surgeon. In the majority of
cases, transnasal endoscopic excision is performed successfully,
since the majority of the tumors are limited to the nasal cavity,
which is detected by radiological examination pre-operatively. When
tumors are large or have developed to various locations, a
transnasal endoscopic approach is insufficient. Instead, the
Caldwell-Luc surgical procedure, local excision, lateral rhinotomy,
external ethmoidetomy, medial maxillectomy or craniofacial
resection is required (16,20–23).
Although angioleiomyomas may be easily detached from the
surrounding tissue in the majority of cases, certain studies
suggest that a pre-operative endovascular embolism may be an
effective method to minimize intra-operative bleeding, particularly
in certain lesions with an abundance of vascular structure or a
prominent vascular pedicle at the base (24). Singh et al (27) reported that a KTP 532 laser-assisted
transnasal endoscopic approach was successfully used in a patient
with leiomyoma, to prevent excessive bleeding of the nasal septum.
When surgery for intracranial angioleiomyomas is too dangerous,
radiotherapy becomes an alternative treatment (40). In the present study, all
angioleiomyomas were located in the nasal cavity, so an endoscopic
approach with controlled hypotension was sufficient to provide
adequate exposure and control over hemostasis without a
pre-operative embolism or laser-assisted surgery.
Recurrence and malignant transformation of
angioleiomyomas are extremely rare. In 1977, Neviaser et al
(41) documented a female patient
with angioleiomyoma of the forearm that recurred as leiomyosarcoma
7 years post-operatively. In 1995, Herren et al (42) reported the malignant transformation of
angioleiomyoma in the index finger of a 17-year-old patient; the
patient relapsed twice and the tumor underwent a malignant
alteration. Wide local resection proved to be curative. In 2009,
Hayashi et al (33) reported 1
angioleiomyoma that possibly possessed a low malignant potential
due to its large size, marked degeneration and abundant expression
of mast cells. In 2011, Mahima et al (43) reported an unusual angioleiomyoma of
the retromolar region, which possessed short-term recurrence and a
rapid growth rate.
A differential diagnosis of angioleiomyoma of the
nasal cavity may consist of hemangioma, angiomyolipoma,
myopericytoma, angiofibroma, leiomyosarcoma and neurofibroma
(7,10,44,45). In
hemangioma, the intervascular stroma does not have smooth muscle
bundles, which are observed in angioleiomyomas. Angiomyolipomas are
composed of blood vessels, smooth muscle cells and adipose cells,
which express HMB-45. Myopericytomas are formed of thin-walled
vascular channels with round or ovoid myopericytes, which express
smooth muscle actin (SMA) and focally express, or do not express,
desmin. Angiofibromas usually develop in male teenagers and are
tumors with proliferated thin-walled vascular channels in a stroma
containing round, stellate or spindle-shaped fibroblasts, but
without smooth muscle. They express CD34 and do not express SMA. A
leiomyosarcoma is a malignant smooth muscle tumor with nuclear
atypia and mitosis. A tumor that does not express S100 protein
cannot be a neurogenic tumor, such as neurofibroma, neurilemmoma
and neuroma, since the S100 protein is only expressed by neurogenic
tumors.
In summary, to the best of our knowledge, the
present study is the first study to report the expression of the
two hormone receptors ER and PR in angioleiomyoma of the nasal
cavity. The present study suggests that sex hormones are possibly
associated with the growth of angioleiomyoma.
References
|
1
|
Woo KS, Kim SH, Kim HS and Cho PD:
Clinical experience with treatment of angioleiomyoma. Arch Plast
Surg. 41:374–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yoon TM, Yang HC, Choi YD, Lee DH, Lee JK
and Lim SC: Vascular leiomyoma in the head and neck region: 11
years experience in one institution. Clin Exp Otorhinolaryngol.
6:171–175. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu Y, Li B, Li L, Liu Y, Wang C and Zha
L: Angioleiomyomas in the head and neck: A retrospective clinical
and immunohistochemical analysis. Oncol Lett. 8:241–247.
2014.PubMed/NCBI
|
|
4
|
Wang CP, Chang YL and Sheen TS: Vascular
leiomyoma of the head and neck. Laryngoscope. 114:661–665. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maesaka A, Keyaki Y and Nakahashi T: Nasal
angioleiomyoma and leiomyosarcoma: Report of 2 cases. Otologia
(Fukuoka). 12:42–47. 1966.
|
|
6
|
Onesti MG, Maruccia M, Carella S, Rossi A,
Soda G and Scuderi N: Subcutaneous angioleiomyoma of the nasal tip.
Report of a rare case. In Vivo. 26:1091–1094. 2012.PubMed/NCBI
|
|
7
|
He J, Zhao LN, Jiang ZN and Zhang SZ:
Angioleiomyoma of the nasal cavity: A rare cause of epistaxis.
Otolaryngol Head Neck Surg. 141:663–664. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Meher R and Varshney S: Leiomyoma of the
nose. Singapore Med J. 48:e275–e276. 2007.PubMed/NCBI
|
|
9
|
Kim SJ, Hong SH and Roh MS: Angioleiomyoma
of the nasal cavity: A case report. Korean J Pathol. 38:181–183.
2004.
|
|
10
|
Marioni G, Marchese-Ragona R, Fernandez S,
Bruzon J, Marino F and Staffieri A: Progesterone receptor
expression in angioleiomyoma of the nasal cavity. Acta Otolaryngol.
122:408–412. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tseng PY, Lai YS, Chen MK and Shen KH:
Progesterone receptor expression in sinonasal leiomyoma: A case
report and review of the literature. Int J Clin Exp Pathol.
7:1224–1228. 2014.PubMed/NCBI
|
|
12
|
Chen CJ, Lai MT, Chen CY and Fang CL:
Vascular leiomyoma of the nasal cavity: Case report. Chin Med J
(Engl). 120:350–352. 2007.PubMed/NCBI
|
|
13
|
Purohit GN, Agarwal N and Agarwal R:
Leiomyoma arising from septum of nose. Indian J Otolaryngol Head
Neck Surg. 63(Suppl 1): 64–67. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Barr GD, More IA and McCallum HM:
Leiomyoma of the nasal septum. J Laryngol Otol. 104:891–893. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Trott MS, Gewirtz A, Lavertu P, Wood BG
and Sebek BA: Sinonasal leiomyomas. Otolaryngol Head Neck Surg.
111:660–664. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schwartzman J and Schwartzman J:
Leiomyoangioma of paranasal sinuses: Case report. Laryngoscope.
83:1856–1858. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wolfowitz BL and Schmaman A: Smooth-muscle
tumours of the upper respiratory tract. S Afr Med J. 47:1189–1191.
1973.PubMed/NCBI
|
|
18
|
Ardekian L, Samet N, Talmi YP, Roth Y,
Bendet E and Kronenberg J: Vascular leiomyoma of the nasal septum.
Otolaryngol Head Neck Surg. 114:798–8001996. View Article : Google Scholar
|
|
19
|
Hanna GS, Akosa AB and Ali MH: Vascular
leiomyoma of the inferior turbinate-report of a case and review of
the literature. J Laryngol Otol. 102:1159–1160. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zijlker TD and Visser R: A vascular
leiomyoma of the ethmoid. Report of case. Rhinology. 27:129–135.
1989.PubMed/NCBI
|
|
21
|
Harcourt JP and Gallimore AP: Leiomyoma of
the paranasal sinuses. J Laryngol Otol. 107:740–741. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Khan MHZ, Jones AS and Haqqani MT:
Angioleiomyoma of the nasal cavity - report of a case and review of
the literature. J Laryngol Otol. 108:244–246. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nicolai P Redaelli, de Zinis LO, Facchetti
F, Maroldi R and Antonelli AR: Craniofacial resection for vascular
leiomyoma of the nasal cavity. Am J Otolaryngol. 17:340–344. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nall AV, Stringer SP and Baughman RA:
Vascular leiomyoma of the superior turbinate: First reported case.
Head Neck. 19:63–67. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Murono S, Ohmura T, Sugimori S and
Furukawa M: Vascular leiomyoma with abundant adipose cells of the
nasal cavity. Am J Otolaryngol. 19:50–53. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bloom DC, Finley JC Jr, Broberg TG and
Cueva RA: Leiomyoma of the nasal septum. Rhinology. 39:233–235.
2001.PubMed/NCBI
|
|
27
|
Singh R, Hazarika P, Balakrishnan R,
Gangwar N and Pujary P: Leiomyoma of the nasal septum. Indian J
Cancer. 45:173–175. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Campelo VE, Neves MC, Nakanishi M and
Voegels RL: Nasal cavity vascular leiomyoma: Case report and
literature review. Rev Bras Otorrinolaringol (Engl Ed). 74:147–150.
2008. View Article : Google Scholar
|
|
29
|
Navarro Júnior CR, Fonseca AS, Mattos JR
and Andrade NA: Angioleiomyoma of the nasal septum. Braz J
Otorhinolaryngol. 76:6752010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Inaba T, Adachi M and Yagisita H: A case
of angioleiomyoma in the buccal space. Odontology. 103:109–111.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Terada T: Vascular leiomyoma of the lung
arising from pulmonary artery. Int J Clin Exp Pathol. 6:97–99.
2013.PubMed/NCBI
|
|
32
|
Chen X, Zhang X, Zhang S and Lü B:
Angioleiomyomas in the bilateral broad ligaments. Int J Gynecol
Pathol. 29:39–43. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hayashi M, Tomita S, Fukasawa I and Inaba
N: Large angioleiomyoma, rich of mast cell and sex hormone receptor
expression. Arch Gynecol Obstet. 279:193–197. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Di Tommaso L, Scarpellini F, Salvi F,
Ragazzini T and Foschini MP and Foschini MP: Progesterone receptor
expression in orbital cavernous hemangiomas. Virchows Arch.
436:284–288. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sawada Y: Angioleiomyoma of the nasal
cavity. J Oral Maxillofac Surg. 48:1100–1101. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gupte C, Butt SH, Tirabosco R and
Saifuddin A: Angioleiomyoma: Magnetic resonance imaging features in
ten cases. Skeletal Radiol. 37:1003–1009. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ranjan S and Singh KT: Gingival
angioleiomyoma-infrequent lesion of oral cavity at a rare site. J
Oral Maxillofac Pathol. 18:107–110. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ravikumar C, Veerendrakumar M, Hegde T,
Nagaraja D, Jayakumar PN and Shankar SK: Basal ganglionic
angioleiomyoma. Clin Neurol Neurosurg. 98:253–257. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shinde SV, Shah AB, Baviskar RB and
Deshpande JR: Primary intracranial multicentric angioleiomyomas.
Neurol India. 60:115–117. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sun L, Zhu Y and Wang H: Angioleiomyoma, a
rare intracranial tumor: 3 case report and a literature review.
World J Surg Oncol. 12:216–222. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Neviaser RJ and Newman W: Dermal
angiomyoma of the upper extremity. J Hand Surg Am. 2:271–274. 1977.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Herren DB, Zimmermann A and Büchler U:
Vascular leiomyoma in an index finger undergoing malignant
transformation. J Hand Surg [Br]. 20:484–487. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mahima VG, Patil K and Srikanth HS:
Recurrent oral angioleiomyoma. Contemp Clin Dent. 2:102–105. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Matsuyama A, Hisaoka M and Hashimoto H:
Angioleiomyoma: A clinicopathologic and immunohistochemical
reappraisal with special reference to the correlation with
myopericytoma. Hum Pathol. 38:645–651. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
LaBruna A, Reagan B and Papageorge A:
Leiomyoma of the maxillary sinus: A diagnostic dilemma. Otolaryngol
Head Neck Surg. 112:595–598. 1995. View Article : Google Scholar : PubMed/NCBI
|