Introduction
Non-Hodgkin's lymphomas (NHLs) are a heterogeneous
group of lymphoproliferative disorders originating in B, T or
natural killer (NK) lymphocytes. In the United States, B-cell
lymphomas represent 80–85% of all NHL cases; 15–20% of cases are T-
and NK-lymphomas (1). The National
Comprehensive Cancer Network guidelines considers the following
common subtypes of B-cell lymphoma: Diffuse large B-cell lymphoma,
31% of cases; follicular lymphoma, 22%; chronic lymphocytic
leukemia/small lymphocytic lymphoma, 6%; mantle-cell lymphoma, 6%;
mucosa-associated lymphoid tissue lymphoma, 5% (2). Overall NHL mortality rates are ~10.9 per
100,000 individuals in one year. However, there is little
information concerning the mortality rates for the specific
subtypes (3).
Recent studies have demonstrated that the diagnostic
accuracy of fine-needle aspiration cytology (FNAC) may improve
significantly when FNAC is used in combination with flow cytometry
(FCM) or immunohistochemistry (4).
FNAC offers several advantages: The procedure is quick, inexpensive
and the aspiration procedure exhibits very few complications
(5). At present, treatment options
for B-cell lymphoma differ between patients. CHOP
(cyclophosphamide, doxorubicin, vincristine and prednisone)
chemotherapy has been the standard treatment for patients, and
subsequently rituximab was added to CHOP to improve the outcomes
for patients (2), High dose therapy
with autologous stem cell rescue is another alternative for
relapsed or refractory patients (3).
Non-Hodgkin's lymphoma as a secondary tumor has
recently gained attention following decades of neglect during the
diagnosis and treatment of primary tumors (6). Krikorian et al (7) were the first to report the increase risk
of secondary NHL (sNHL) in patients successfully treated for a
primary tumor. Krishnan and Morgan (8) reported that the lowest occurrence rates
of sNHL was 0.07%, which was obtained from multinational
population-based registries data in 1987, and the highest
occurrence rate was 3%, which was obtained from the Norwegian
Cancer Registry Database in 2002. Usually, sNHL develops after the
first 5 years of initial therapy of primary cancer. The majority of
sNHL cases develop after ~5 years of initial therapy for primary
cancers. sNHLs lead to increased morbidity and mortality in these
patients 8). Primary penile carcinoma has rarely been reported to
lead to the development of B-cell lymphoma (9). To the best of our knowledge, the present
study is the first to report a case of secondary B-cell lymphoma,
diagnosed by FNAC and flow cytometry (FCM), following the treatment
of penile carcinoma. Informed consent for the publication of this
data was obtained from the patient.
Case report
The patient, a 62-year-old man, had suffered from
frequent urination, repeated chest tightness, and weakness for 5
years. The situation worsened 1 month prior to his admission to the
People's Hospital of Zhejiang Province (Hangzhou, China) in May
2014. The patient was diagnosed with hyperplasia of the prostate
gland and cardiomyopathy.
A physical examination of the patient revealed a
long foreskin and a thick and swollen cauliflower-like mass
(diameter, 3 cm) on the head of the penis. The right inguinal lymph
nodes were swollen but painless. A preoperative biopsy of the lymph
nodes indicated squamous cell carcinoma. The patient underwent a
penis and scrotal skin excision, and the postoperative pathological
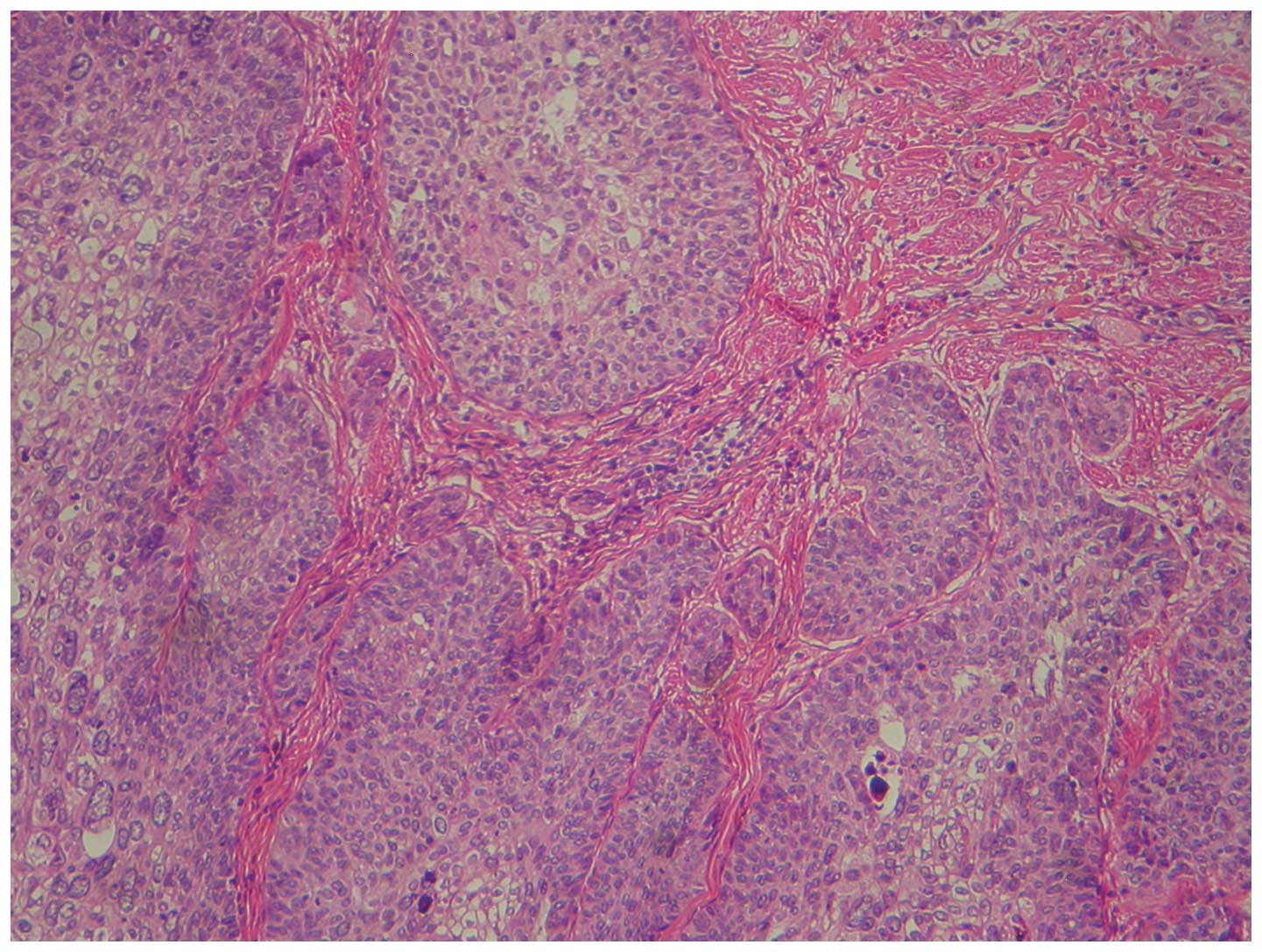
diagnosis revealed a moderately differentiated squamous cell
carcinoma (Fig. 1).
In July 2014, the patient returned to the People's
Hospital of Zhejiang Province with a swollen and painful cheek.
Upon physical examination, the superficial lymph nodes on the left
neck, and right axillary and inguinal regions all demonstrated
severe swelling, high activity and slight tenderness. Additional
auxiliary examinations were performed. B-mode ultrasonography
revealed that the lymph nodes of the bilateral axilla, groin and
neck were enlarged. A chest computed tomography (CT) scan revealed
multiple masses at the mediastinum, hilum of the right lung and the
right axilla. In addition, bilateral lung lesions, a tubercle at
the anterior side of the right upper lobe, which does not rule out
lymph node infiltration, a calcified lesion at the apex of the
right lung, small levels of bilateral pleural effusion, and
pericardial effusion were identified. The laboratory examination
data were as follows: White blood cell count, 4.64×109
cells/l (normal range, 4–10×109 cells/l), including
77.1% neutrophils (normal range, 50–75%), 14.8% lymphocytes (normal
range, 20–40%), 6.1% monocytes (normal range, 2–12%), 1.8%
eosinophils (normal range, 0.5–5%) and 0.2% basophils (normal
range, 0–2%); hemoglobin, 106 g/l (normal range, 120–160 g/l);
platelet count, 233×109/l (normal range,
100–300×109/l); Na+, 3.81 mmol/l (normal
range, 135–145 mmol/l); K+, 137 mmol/l (normal range,
3.5–5.5 mmol/l); Cl−, 96.7 mmol/l (normal range, 96–108
mmol/l); total protein, 49.5 g/l (normal range 65–85 g/l); albumin,
27.1 g/l (normal range, 40–55 g/l); globulin, 22.4 g/l (normal
range, 20–40 g/l); aspartate transaminase, 20 units/l (normal
range, 0–50 units/l); alanine transaminase, 28 units/l (normal
range, 10–52 units/l); alkaline phosphatase, 93 units/l (45–125
units/l); blood urea nitrogen, 7.75 mmol/l (normal range, 2.85–7.14
mmol/l); creatinine, 93.3 µmol/l (normal range, 44–133 µmol/l); and
uric acid, 774 µmol/l (normal range, 210–440 µmol/l). A routine
urine test revealed no abnormality; however, levels of tumor
markers in the serum increased, including the levels of
carbohydrate antigen 125 at 52.4 units/ml (normal range, 0–35
units/ml) and cytokeratin 19 at 5.9 ng/ml (normal range, 0–3.8
ng/ml). The other laboratory findings were also normal. A bone
marrow smear analysis revealed the presence of hyperplastic
myelocytes, with a large number of myelocyte and metamyelocyte
cells and a low number of segmented granulocytes with toxic
particles (data not shown). FNAC was performed on the lymph node of
the present patient. The standard surgical procedure is as follows:
Firstly, the skin above the mass to undergo biopsy is swabbed with
an antiseptic solution; the skin, underlying fat and muscle may be
numbed with a local anesthetic, although this is often not
necessary with superficial masses. Secondly, a needle of extremely
fine diameter is passed into the mass and withdrawn several times
for sampling of cells. Finally, these cells are used in a smear and
examined under a microscope or rendered into a suspension used for
flow cytometry (10). A large number
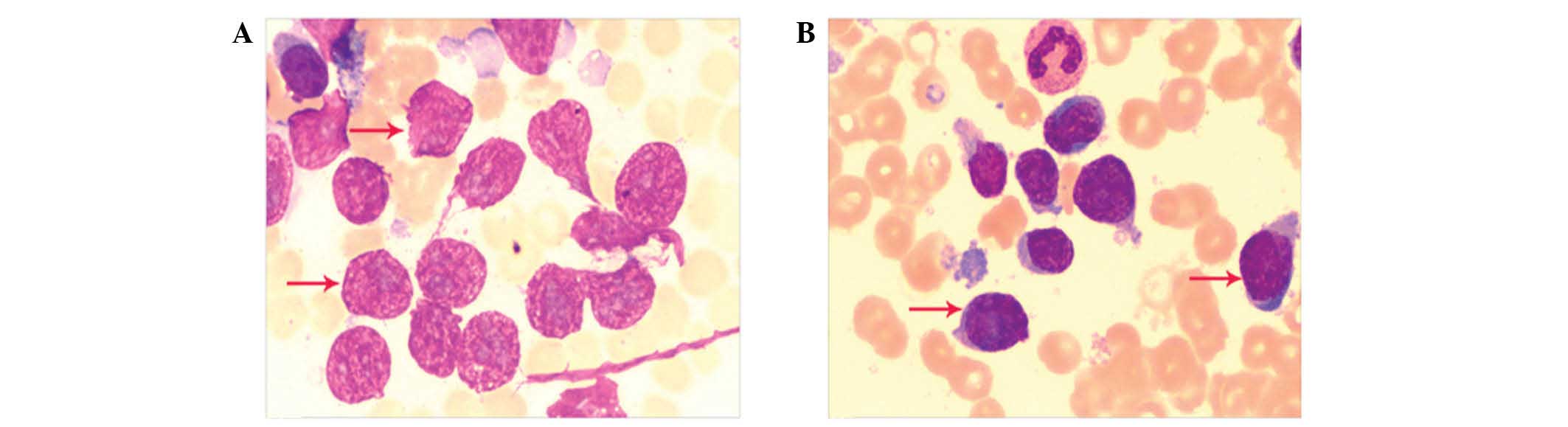
of abnormal and bare nuclei cells were distributed in the current
smears obtained using FNAC, which were indicative of lymphoma cells
(Fig. 2). In the present study,
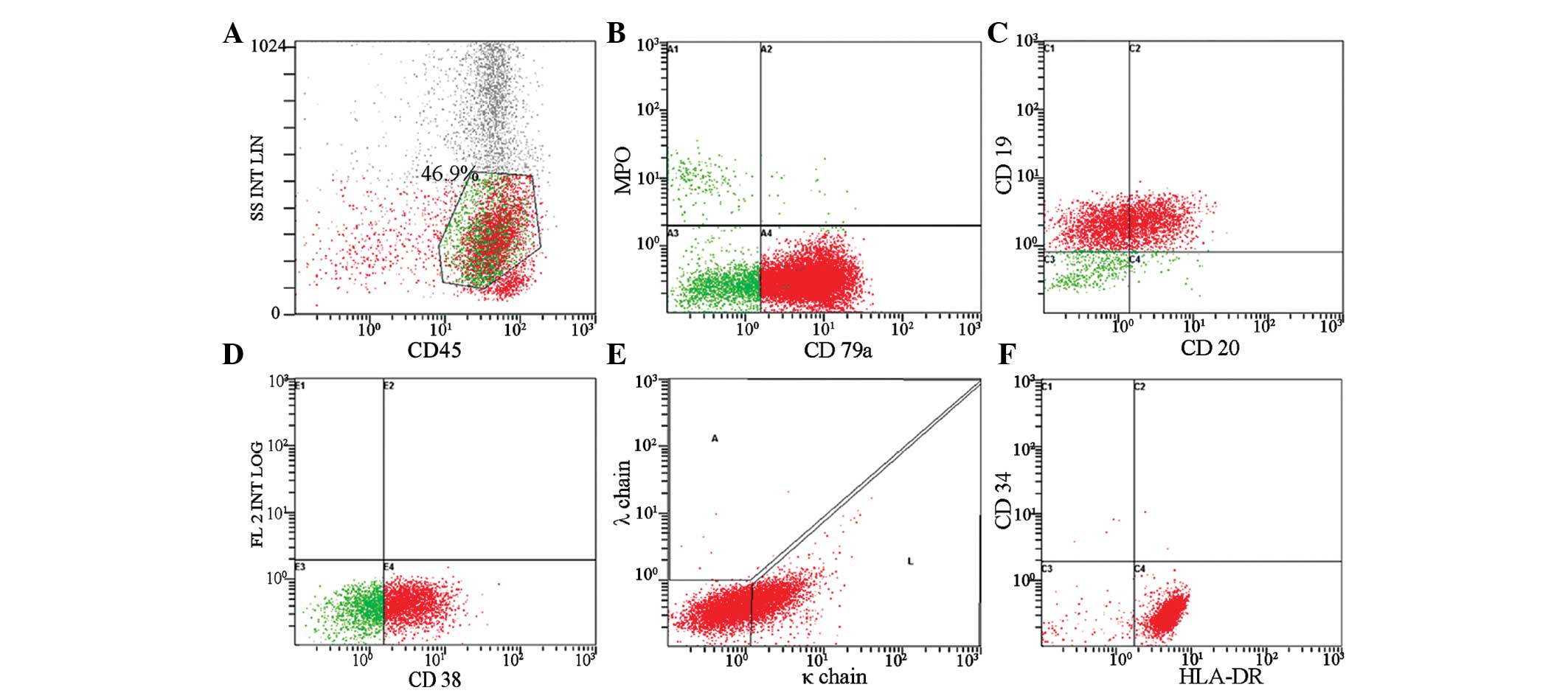
immunophenotyping of the aspirate obtained from FNAC revealed the
following data, which supported the concept of a B-cell origin:
Cluster of differentiation (CD)3+, 6.8%;
CD3+CD4+, 1.9%;
CD3+CD8+, 3.2%; natural killer, 2.7%;
CD19+, 88.1%; CD10+, 0.2%; CD20+,
42.7%; CD79a, 84.8%; CD38, 64.9%; CD23+, 6.5%; κ chain,
31.0%; and λ chain, 1.4% (Fig.
3).
Discussion
The number of studies reporting lymphoma as a
secondary cancer has gradually increased, and the condition has
been described following a number of types of primary tumor,
including neuroblastoma (11), breast
cancer (12), follicular thyroid
carcinoma (13) and parotid gland
cancer (14). There are a number of
potential mechanisms acting in the pathogenesis of NHL, which may
be associated with radiation, cytotoxic drugs and immunosuppression
(8). In the present report, a patient
with penile cancer developed enlarged lymph nodes two months
subsequent to penile cancer surgery. The recurrence of enlarged
lymph nodes at the left neck, and right axillary and inguinal
regions was the major reason for the readmission of the patient to
the hospital. The tissues specimens that had been previously
diagnosed with penile cancer were immunohistochemically examined
and markers for B-cell lymphoma (CD19, CD20, CD22 and CD79a) were
negative (data not shown); therefore, the possibility of penile
cancer and B-cell lymphoma appearing at the same time was
eliminated, and the B-cell lymphoma was concluded to be a secondary
tumor that developed subsequent to penile cancer.
Immunophenotypic analysis may be performed using
flow cytometry or immunohistochemistry, the choice depends on the
antigens as well as the expertise and resources available to the
hematopathologist. Immunohistochemistry has certain limitations on
the diagnosis of certain tumors, including in cases where the lymph
node is not easily accessible, such as Hodgkin's lymphoma (15). FNAC is a simple, safe, minimally
invasive and fast technique that is well tolerated by patients. In
addition, the single cell suspension that is suitable for FCM
analysis is easy to prepare (16).
FCM is a rapid and sensitive multi-parameter analysis technique,
which is important in the diagnosis of patients with lymph node
enlargement (17). In the present
study, a fine-needle aspiration biopsy of the lymph node was
performed and FCM analyses were conducted. Immunophenotyping by FCM
revealed increased expression of CD79a, CD19, CD20 and κ chain, and
a diagnosis of B-cell lymphoma was confirmed.
The present study demonstrates that the combination
of FNAC and FCM with lymph node aspiration analysis may improve the
accuracy and sensitivity of lymphoma diagnoses. Therefore, the
combined technique used in the present study may be used as the
routine method in lymphoma diagnosis (18). The application of this technology in
clinical practice will contribute to the earlier diagnosis and
treatment for patients with secondary lymphoma.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81301406) and the
Provincial Natural Science Foundation of Zhejiang (grant nos.
LQ13H190005 and LQ12H16019).
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer Statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
NCCN: The National Comprehensive Cancer
Network Clinical Practice Guidelines in Oncology: Non-Hodgkin's
Lymphomas. Version 2. 2011.
|
|
3
|
Howlader N, Morton LM, Feuer EJ, Besson C
and Engels EA: Contributions of subtypes of non-Hodgkin lymphoma to
mortality trends. Cancer Epidemiol Biomarkers Prev. 25:174–179.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zeppa P, Marino G, Troncone G, Fulciniti
F, De Renzo A, Picardi M, Benincasa G, Rotoli B, Vetrani A and
Palombini L: Fine-needle cytology and flow cytometry
immunophenotyping and subclassification of non-Hodgkin lymphoma: A
critical review of 307 cases with technical suggestions. Cancer.
102:55–65. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Silas OA, Ige OO, Adoga AA, Nimkur LT and
Ajetunmobi OI: Role of fine needle aspiration cytology (FNAC) as a
diagnostic tool in paediatric head and neck lymphodenopathy. J Otol
Rhinol. 4:2015.PubMed/NCBI
|
|
6
|
El-Mallawany NK and Cairo MS: Advances in
the diagnosis and treatment of childhood and adolescent B-cell
non-Hodgkin lymphoma. Clin Adv Hematol Oncol. 13:113–123.
2015.PubMed/NCBI
|
|
7
|
Krikorian JG, Burke JS, Rosenberg SA and
Kaplan HS: Occurrence of non-Hodgkin's lymphoma after therapy for
Hodgkin's disease. N Engl J Med. 300:452–458. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Krishnan B and Morgan GJ: Non-Hodgkin
lymphoma secondary to cancer chemotherapy. Cancer Epidemiol
Biomarkers Prev. 16:377–380. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sonpavde G, Pagliaro LC, Buonerba C, Dorff
TB, Lee RJ and Di Lorenzo G: Penile cancer: Current therapy and
future directions. Ann Oncol. 24:1179–1189. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Berner A, Lund-Iversen M and Nesland JM:
Fine needle aspirations in oncology. Arkh Patol. 73:21–26.
2011.PubMed/NCBI
|
|
11
|
Imashuku S, Hibi S, Kosaka K, Tabata Y,
Naya M, Hohjo M and Todo S: Secondary lymphoid malignancy in two
children with neuroblastoma. Med Pediatr Oncol. 27:54–56. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fritzsche FR, Pahl S, Petersen I,
Burkhardt M, Dankof A, Dietel M and Kristiansen G: Anaplastic
large-cell non-Hodgkin's lymphoma of the breast in periprosthetic
localisation 32 years after treatment for primary breast cancer - A
case report. Virchows Arch. 449:561–564. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lu J, Frater JL, Kreisel FH, Marcus JN and
Hassan A: Secondary lymphoma involving metastatic follicular
thyroid carcinoma to the skull: A unique example of tumor-to-tumor
metastasis. Head Neck Pathol. 2:209–212. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Williams-Smith L, Gupta R and Osborne RF:
Secondary lymphoma of the parotid gland: Clinical experience. Ear
Nose Throat J. 92:632013.PubMed/NCBI
|
|
15
|
Dunphy CH: Applications of flow cytometry
and immunohistochemistry to diagnostic hematopathology. Arch Pathol
Lab Med. 128:1004–1022. 2004.PubMed/NCBI
|
|
16
|
Lieu D: Cytopathologist-performed
ultrasound-guided fine-needle aspiration and core-needle biopsy: A
prospective study of 500 consecutive cases. Diagn Cytopathol.
36:317–324. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mathiot C, Decaudin D, Klijanienko J,
Couturier J, Salomon A, Dumont J and Vielh P: Fine-needle
aspiration cytology combined with flow cytometry immunophenotyping
is a rapid and accurate approach for the evaluation of suspicious
superficial lymphoid lesions. Diagn Cytopathol. 34:472–478. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jorgensen JL: State of the Art Symposium:
Flow cytometry in the diagnosis of lymphoproliferative disorders by
fine-needle aspiration. Cancer. 105:443–451. 2005. View Article : Google Scholar : PubMed/NCBI
|