Introduction
Colorectal cancer is the second most prevalent
malignancy and the leading cause of cancer-associated mortality
worldwide (1). The incidence rates of
colorectal cancer have been rapidly increasing in Asia due to
smoking, the increased tendency for being overweight and the
excessive consumption of meat and alcohol (2,3). Multiple
phytochemicals have been identified through epidemiological studies
as potential cancer-fighting agents that may be obtained from
commonly consumed fruits and vegetables (4,5).
Although the toxicity of chemotherapy is a major
obstacle for the successful treatment of cancer, chemo and
radiation therapies are the major tools for cancer treatment, at
present. For these reasons, the identification of safe components
with a high selectivity for killing cancer cells from natural
sources is required (6,7). Certain secondary metabolites from
natural plants exhibit biological activities, which are considered
to be critical for maintaining human health. The development of
high quality varieties that contain increased levels of bioactive
components may improve the medicinal value of plants (8).
Nelumbo nucifera, the lotus, is an aquatic
perennial plant that is commonly cultivated in water gardens in
tropical Asia and Australia (9). The
flowers, seeds, young leaves, rhizomes and roots of N.
nucifera are used for edible and medicinal sources in Asian
regions. The roots of N. nucifera have been identified as
being rich in dietary fiber, vitamins B1, B2, B6 and C, potassium,
phosphorus, copper and manganese (10). N. nucifera contains
biologically effective flavonoids, including kaempferol, quercetin,
rutin and hyperoside, exhibits anti-inflammatory, -oxidant,
-fungal, -microbial and tyrosinase inhibitory activities, and has
protective properties in carbon tetrachloride-induced liver damage
(11–18).
In a previous report, the anti-proliferative effect
of a 70% ethanol extract of the root of N. nucifera on HT-29
human colon cancer cells was investigated, which was based on cell
viability, Hoechst 33342 nuclear staining, apoptosis analysis,
western blotting and reverse transcription-polymerase chain
reaction (RT-PCR) analyses. N. nucifera was indicated to
inhibit the growth of colon cancer cells by the activation of the
mitochondria-dependent apoptotic pathway via the modulation of
Bcl-2-associated X protein (Bax) and B-cell lymphoma 2 (Bcl-2)
expression (19).
Flavonoids are polyphenol compounds that are
commonly identified in plants and have gained considerable interest
and attention in recent years, due to the bioactive functions they
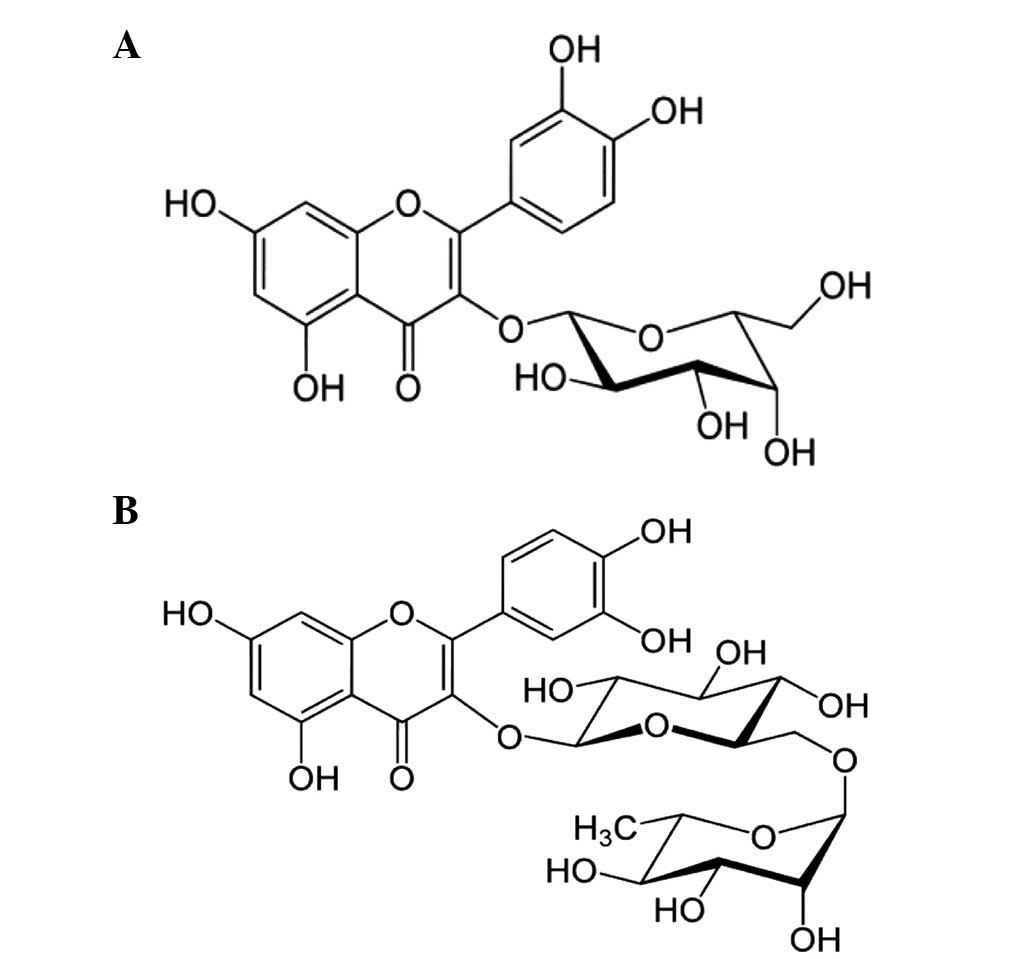
possess. Among these flavanoids, hyperoside and rutin are composed
of flavan-3-ol glycosidic linkages, which are molecules of joined
together glucose and glucose and rhamnose, respectively (Fig. 1). Despite evidence for the numerous
biological effects of flavan-3-ols, the anti-cancer effects of
hyperoside and rutin-induced apoptosis have not been
investigated.
Numerous previous studies aimed to identify
anticancer components from natural sources (20–23).
Therefore, the effects of hyperoside and rutin on the proliferation
and apoptosis in HT-29 human colon cancer cells, through
activity-guided fractionation and isolation methods, are
investigated in the present study.
Materials and methods
Chemicals and reagents
Propidium iodide (PI) and
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
were purchased from Merck Millipore (Darmstadt, Germany) and
Sigma-Aldrich (St. Louis, MO, USA), respectively. Primary
antibodies included anti-mouse β-actin (sc-47778), and rabbit
monoclonal anti-Bcl-2 (sc-492) and anti-Bax (sc-493) (dilution,
1:1,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), and were
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Rabbit monoclonal antibodies against cysteinyl aspartate-specific
cleaved caspase-3 (9661), −8 (8592) and −9 (7237) and
poly-(ADP-ribose) polymerase (PARP; 5625) antibodies were purchased
from Cell Signaling Technologies, Inc. (Danvers, MA, USA).
Secondary antibodies included goat anti-rabbit IgG-horseradish
peroxidase (HRP; sc-2004) and goat anti-mouse IgG-HRP (sc-2005)
(dilution, 1:2,000), and were purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). All other chemicals and
reagents were of the highest analytical grade.
Plant materials
The roots of N. nucifera were purchased at
Nonghyup Hanaro mart (Yangjae, Seoul, Korea) in May 2012 (19). Roots were dried using an automatic
food dryer (LD-528WG; L'equip Co., Ltd., Seoul, Korea) and then
were ground to fine powders.
Instrumental analysis
The uncorrected melting points (MPs) were determined
using Mitamura-Riken Kogyo MEL-TEMP apparatus (Mitamura Riken Kogyo
Inc., Tokyo, Japan). Ultraviolet-visible (UV-Vis) spectra were
obtained using a JP/U3010 spectrometer (Hitachi, Ltd.,Tokyo, Japan)
and infrared (IR) spectra were obtained on a FT/IR-5300
spectrometer (Jasco International Co., Ltd., Tokyo, Japan). The
electron ionization (EI) and fast-atom bombardment-mass
spectrometry (FAB-MS) spectra were obtained on a JMS-700
spectrometer (JEOL, Ltd., Tokyo, Japan). The nuclear magnetic
resonance (NMR) spectra were measured on an Avance-500 spectrometer
(500 MHz; Bruker Corporation, Billerica, MA, USA), and the chemical
shifts were referenced against tetramethylsilane (TMS) and
deuterium dimethylsulfoxide (DMSO-d6) as NMR solvents. Column
chromatography (CC) was run on a silica gel 60 (70–230 or 230–400
mesh; Merck Millipore) and Sephadex LH-20 (25–100 mm; GE Healthcare
Life Sciences, Uppsala, Sweden). Thin layer chromatography (TLC)
was performed on silica gel 60F254 and RP-18254S plates. TLC plates
were visualized using UV light, stained with FeCl3,
aniline hydrogen phthalate and 20% H2SO4, and
then heated at 70–80°C for 5 sec.
Extraction and isolation of
compounds
The dried powdered roots of N. nucifera (800
g) were extracted 3 times using 70% ethyl alcohol (EtOH) under
70–80°C reflux conditions to produce 92 g extracts. The 70% EtOH
extract was suspended in water and successively partitioned with
n-hexane, dichloromethane (CH2C12), ethyl
acetate (EtOAc) and n-butanol (n-BuOH) to yield solvent-soluble
fractions. A 12-g portion of the EtOAc fraction was subjected to
silica gel CC and eluted with CH2C12-methanol
(MeOH) mixtures with increasing concentrations of MeOH (0, 20, 50,
70 and 100%) to produce 12 fractions (E-01-E12). Fraction E-08 was
submitted to silica gel CC using CH2C12-MeOH,
in the ratios of 1:9, 1:7, 1:5 and 1:2, to yield fifteen
sub-fractions. The sub-fractions E-08-8 (110 mg) and E-08-11 (180
mg) were successively purified using Sepadex LH-20 CC and vacuum
liquid chromatography (VLC) with MeOH to produce hyperoside (28 mg)
and rutin (34 mg).
Cell culture
The human colon cancer HT-29 cell line was obtained
from the Korean Cell Line Bank (Seoul, Korea). The normal colon
epithelium FHC cell line was obtained from the American Type
Culture Collection (Manassas, VA, USA). HT-29 cells were maintained
in Roswell Park Memorial Institute (RPMI)-1640 medium, supplemented
with 10% Invitrogen fetal bovine serum (FBS; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), 100 units/ml penicillin and
100 µg/ml streptomycin (Gibco; Thermo Fisher Scientific, Inc.). FHC
cells were maintained in Dulbecco's modified Eagle medium nutrient
mixture F-12 (DMEM)/F-12, which contained 10 ng/ml cholera toxin,
0.005 mg/ml insulin, 0.005 mg/ml transferrin, 100 ng/ml
hydrocortisone, supplemented with 10 % FBS, 100 units/ml penicillin
and 100 µg/ml streptomycin. All of the cell lines were cultured in
a humidified chamber with 5% CO2 at 37°C. Cell counts
were performed using a hemocytometer from Hausser Scientific
(Horsham, PA, USA).
Cell viability assay
The cytotoxic effects of hyperoside and rutin on the
HT-29 and FHC cell lines were estimated colorimetrically using the
MTT method, which is based on the reduction of tetrazolium salt by
mitochondrial dehydrogenase in viable cells (24). Briefly, the cells were seeded in a
96-well plate (density, 1×104 cells/ml) and were then
treated with hyperoside and rutin at final concentrations of 0, 100
and 200 µm. Subsequent to 24 h incubation, MTT solution was added
to each well at a final concentration of 0.4 mg/ml. Subsequent to 2
h of incubation, the supernatants were aspirated and replaced with
150 µl of dimethyl sulfoxide (DMSO) to dissolve the formazan
product. The absorbance at 570 nm was then read using a
spectrophotometric plate reader (model 550; Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The results were calculated as
percentages of the unexposed control.
Nuclear staining with Hoechst
33342
The nuclear morphology of the cells was observed
using Hoechst 33342 DNA-specific blue fluorescent dye. The viable
cells were stained homogeneously, whereas the apoptotic cells that
had undergone chromatin condensation or nuclear fragmentation were
not stained (25). The HT-29 cells
were treated with hyperoside and rutin at the various
concentrations. Cells were then fixed for 30 min at 37°C in 100%
MeOH, washed with PBS, and stained with 2 µg/ml Hoechst 33342
(Sigma-Aldrich). The cells were observed under a BX51 fluorescence
microscope and images were captured with a DP70 camera (Olympus
Corporation, Tokyo, Japan).
Apoptosis analysis
An Annexin V/PI double staining assay was carried
out in order to distinguish between the early and late apoptosis
stages. The stages were determined using an ApoScan™ Annexin V-FITC
apoptosis detection kit (BioBud, Seoul, Korea) in hyperoside and
rutin-treated HT-29 cells. The cells were trypsinized, harvested
and washed with PBS. The cells were resuspended in 1X binding
buffer (500 µl) and incubated with 1.25 µl of Annexin V-fluorescein
isothiocyanate (concentration, 200 µg/ml) at room temperature for
15 min. The supernatant was then removed following centrifugation
at 400 × g for 10 min. The cells were resuspended in 500 µl of 1X
binding buffer and the cell suspensions were then stained with 10
µl PI (concentration, 30 µg/ml) at 4°C in the dark. Fluorescence
was quantified using FACSCalibur flow cytometry (BD Biosciences,
Franklin Lakes, NJ, USA). The levels of early and late apoptosis
were determined as the percentage of Annexin
V+/PI− or Annexin
V+/PI+ cells, respectively.
Western blot analysis
Western blot analyses were performed, as previously
described (20). The cells were
cultured, harvested and lysed on ice for 30 min in lysis buffer
(120 mM NaCl; 40 mM Tris, pH 8.0; 0.1% nonyl
phenoxypolyethoxylethanol) and were then centrifuged at 13,000 × g
for 15 min. Lysates from each sample were mixed with 5X sample
buffer [0.375 M Tris-HCl; 5% sodium dodecyl sulfate (SDS); 5%
β-mercaptoethanol; 50% glycerol; 0.05% bromophenol blue, pH 6.8]
and were then heated to 95°C for 5 min. Equal amounts of protein
were separated by 12% SDS-polyacrylamide gel electrophoresis
(SDS-PAGE) and were transferred onto a nitrocellulose membrane. The
membranes were then washed with Tris-buffered saline (10 mM Tris,
150 mM NaCl) containing 0.05% Tween-20 (TBST), and were then
blocked in TBST containing 5% nonfat dried milk. The membranes were
incubated with respective specific primary antibodies overnight at
4°C. Subsequent to 3 washes in TBST, the membranes were incubated
with the appropriate secondary antibodies coupled to HRP for 1 h at
room temperature. The membranes were then washed 3 times in TBST
with 15 min between each step, and protein detection was performed
using an enhanced chemiluminescence western blotting detection kit.
The data of specific protein levels are presented as multiples
relative to the control.
RT-PCR
Cells were treated with dihydroxyflavone at 10 µg/ml
for various lengths of time, and were treated with hyperoside and
rutin at final concentrations of 0, 100 and 200 µm. Total RNA was
isolated from the cells using Gibco TRIzol® reagent (Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol, and
quantitated using spectrophotometry. Total RNA (5 µg) was reverse
transcribed into cDNA by incubating with Invitrogen SuperScript®™
RNase H reverse transcriptase (Thermo Fisher Scientific, Inc.). PCR
was conducted in a reaction composed of 40 µg of 1.25X reacting
mix, 1 µl enzyme mix, 1 µl forward primer (200 nM), 1 µl reverse
primer (200 nM) and RNA. cDNA was performed with following
conditions: cDNA synthesis at 45°C for 30 min, followed by
denaturation at 94°C for 2 min. A total of 40 cycles of PCR
amplification were then performed with following conditions: 94°C
for 15 sec, 60°C for 30 sec, and 68°C for 1 min. The last cycle was
followed by final extension step at 72°C for 5 min.
The primer pairs (Bionics, Seoul, Korea), forward
and reverse, respectively, were as follows: β-actin,
5-CCTCTATGCCAACACAGTGC-3 and 5′-ATACTCCTGCTTGCTGATCC-3; Bcl-2,
5-AGCTGCACCTGACGCCCTTCA-3′ and 5-AGCCAGGAGAAATCACAGAGG-3;
Bax,5-ATGGACGGGTCCGGGGAGCAG-3 and 5-CAGTTGAAGTTGCCGTCAGA-3. PCR was
performed for 40 cycles. Temperature cycling was initiated with
each cycle, using the Takara PCR Thermal Cycler Dice (Takara Bio,
Inc., Otsu, Japan), as follows: β-actin, 98°C for 10 sec
(denaturation), 55°C for 30 sec (annealing), 72°C for 1 min
(extension); Bcl-2, 98°C for 10 sec, 60°C for 30 sec, 72°C for 1
min; Bax, 98°C for 10 sec, 60.5°C for 30 sec and 72°C for 1 min.
The amplified products were resolved on 1% agarose gels, stained
with ethidium bromide (Sigma-Aldrich) and photographed under
ultraviolet light using a Mini BIS image analysis system (DNR
Bio-Imaging Systems Ltd., Jerusalem, Israel).
Statistical analyses
SPSS software version 22.0 (IBM SPSS, Armonk, NY,
USA) was used to analyse the data. The results were subjected to
analysis of variance, followed by the Tukey range test in order to
analyze the differences between conditions. In each case, the
P<0.05 was considered to indicate a statistically significant
difference. All measurements were made in triplicate, and all
values are given as the mean ± standard deviation.
Results
Isolation of pure compounds
The dried and ground compounds of the root of N.
nucifera were extracted with 70% EtOH and successively
fractionated using n-hexane, CH2C12, EtOAc
and n-BuOH to identify the cytotoxic solvent-soluble fractions of
the HT-29 human colon cancer cell line, through the activity-guided
fractionation and isolation method (26) Of the fractions, the EtOAc-soluble
fraction of N. nucifera demonstrated a strong cytotoxicity
with an half maximal inhibitory concentration value of 26.7±0.8
µg/ml (data not shown), based on the cell viability assay. CC was
applied to the EtOAc-soluble fraction for the isolation of pure
compounds. The chemical structures of the pure compounds were
characterized as hyperoside and rutin based on physicochemical
methods, co-TLC, MP, FAB-MS, UV-Vis, FT-IR, and proton (1H)- and
13C-NMR spectral data (Fig.
1).
Spectral data of hyperoside
The spectral data were as follows: Pale yellowish
powder (MeOH); FeCl3 and aniline hydrogen phthalate
color reaction, positive; MP, 197–202°C; UV (MeOH) λmax,
280 (4.54), 364 (4.36) nm; IR vmax (KBr),
cm−1: 3,380 (OH), 1,660 (α,β-unsaturated C=O), 1,612,
1,490 (aromatic C=C), 1,240 (aromatic C-O), 1,062 and 1,012
(glycosidic C-O); FAB-MS, 465 [M+H]+ m/z;
1H-NMR (500 MHz, DMSO-d6): δ 8.27 (1H, s, 5-OH), 7.66
(1H, dd, J=2.1, 8.4, H-6′), 7.56 (1H, d, J=2.1,
H-2′), 6.82 (1H, d, J=8.4), 6.43 (1H, d, J=1.9, H-8),
6.22 (1H, d, J=1.9, H-6) and 5.36 (1H, d, J=7.6,
anomeric proton); 13C-NMR (125 MHz, DMSO-d6):
δ 181.4 (C-4), 164.4 (C-2), 162.4 (C-7), 161.0 (C-5), 160.1 (C-7),
156.6 (C-9), 150.2 (C-4′), 145.2 (C-3′), 121.2 (C-1′), 119.1
(C-6′), 116.1 (C-5′), 113.2 (C-2′), 105.1 (C-10), 103.1 (C-3), 99.1
(C-8), 97.7 (Glc-1″), 77.0 (Glc-5″), 76.7 (Glc-2″), 76.1 (Glc-3″),
70.1 (Glc-4″) and 60.4 (Glc-6″).
Spectral data of rutin
The spectral data were as follows: Pale yellowish
powder (MeOH); FeCl3 and aniline hydrogen phthalate
color reaction, positive; MP, 202–204°C; UV λmax (MeOH),
268 (4.50), 375 (4.26) nm; IR vmax (KBr),
cm−1: 3,360 (OH), 1,670 (α,β-unsaturated C=O), 1,600,
1,512 (aromatic C=C), 1,240 (aromatic C-O), 1,060 and 1,015
(glycosidic C-O); FAB-MS, 611 [M+H]+m/z;
1H-NMR (500 MHz, DMSO-d6): δ 7.68 (1H, d, J=2.1,
H-2′), 7.54 (1H, dd, J=2.1, 8.4, H-6′), 6.85 (1H, d,
J=8.4, H-5′), 6.40 (1H, d, J=1.8, H-8), 6.18 (1H, d,
J=1.8, H-6), 5.31 (1H, d, J=7.6, anomeric proton) and
0.92 (3H, d, J=5.5, Rha-6); 13C-NMR (125 MHz,
DMSO-d6), δ 177.24 (C-4), 163.97 (C-7), 161.09 (C-9),
156.50 (C-5), 156.30 (C-2), 148.29 (C-4′), 144.63 (C-3′), 133.16
(C-3), 121.48 (C-6′), 121.05 (C-1′), 116.15 (C-5′), 115.11 (C-2′),
103.83 (C-10), 101.04 (C-Glc″), 100.64 (C-Rha″), 98.58 (C-6), 93.49
(C-8), 76.30 (Rha-5″), 75.77 (Glc-5″), 73.95 (Glc-2″), 71.70
(Rha-4″), 70.43 (Rha-3″), 70.25 (Rha-2″), 69.86 (Glc-4″), 68.14
(Rha-5″), 66.89 (Glc-6″) and 17.63 (Rha-6″).
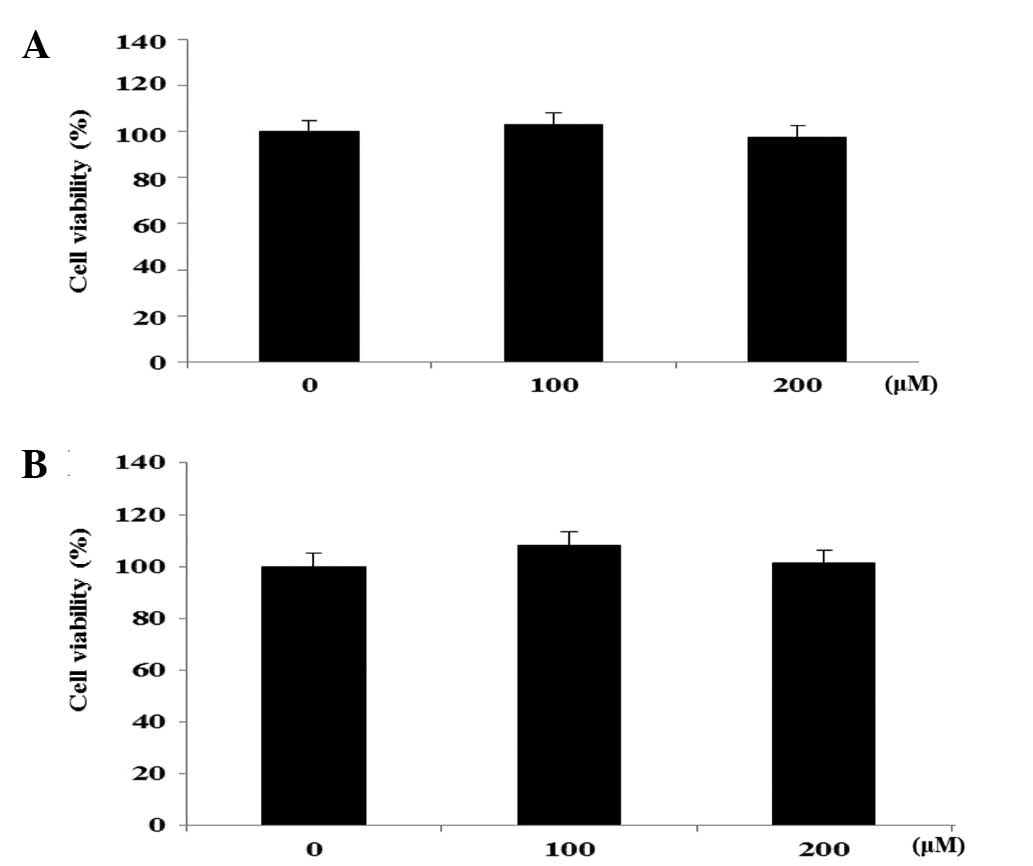
Cell viability in FHC and HT-29 cell
lines
In the preliminary investigation, the EtOAc-soluble
fractions of the roots of N. nucifera were observed to
exhibit significant cytotoxicity on the HT-29 human colon cancer
cells (IC50, 26.7±0.8 µg/ml). A bioactivity-guided
isolation of the cytotoxic EtOAc-soluble fraction on HT-29 human
colon cancer cells was performed, leading to the isolation of 2
flavonol glycosides, hyperoside and rutin. The effects of
hyperoside and rutin on the growth of FHC human colon normal cells
and HT-29 human colon cancer cells was examined using an MTT assay.
The cells were exposed to various concentrations (0–200 µm) of the
flavanoids for 24 h, and the cytotoxicity was determined as a
percentage of the viable treated cells in comparison with the
number of viable untreated control cells. As shown in Fig. 2, hyperoside and rutin did not affect
the proliferation on FHC colon normal cells in a dose-dependent
manner. However, hyperoside and rutin significantly inhibited the
proliferation of HT-29 human colon cancer cells in a dose-dependent
manner. Subsequent to 24 h of exposure, hyperoside and rutin
induced 25.6 and 37.1% growth inhibition at the concentration of
200 µm, respectively (Fig. 3).
Therefore, hyperoside and rutin were selected for the subsequent
experiments from the flavonoids isolated from N.
nucifera.
Induction of apoptosis in HT-29
cells
The apoptogenic properties of the compounds were
investigated through morphological changes in HT-29 cells. Nuclear
Hoechst 33342 staining was performed in order to determine whether
the anti-proliferative effect of hyperoside and rutin was due to
apoptosis. As shown in Fig. 4, HT-29
cells that were treated with hyperoside and rutin exhibited a
number of morphological changes, including cell shrinkage,
condensed chromatin, rounding, blebbing and an increased density of
apoptotic bodies compared with the untreated control cells. The
number of apoptotic cells increased in a dose-dependent
fashion.
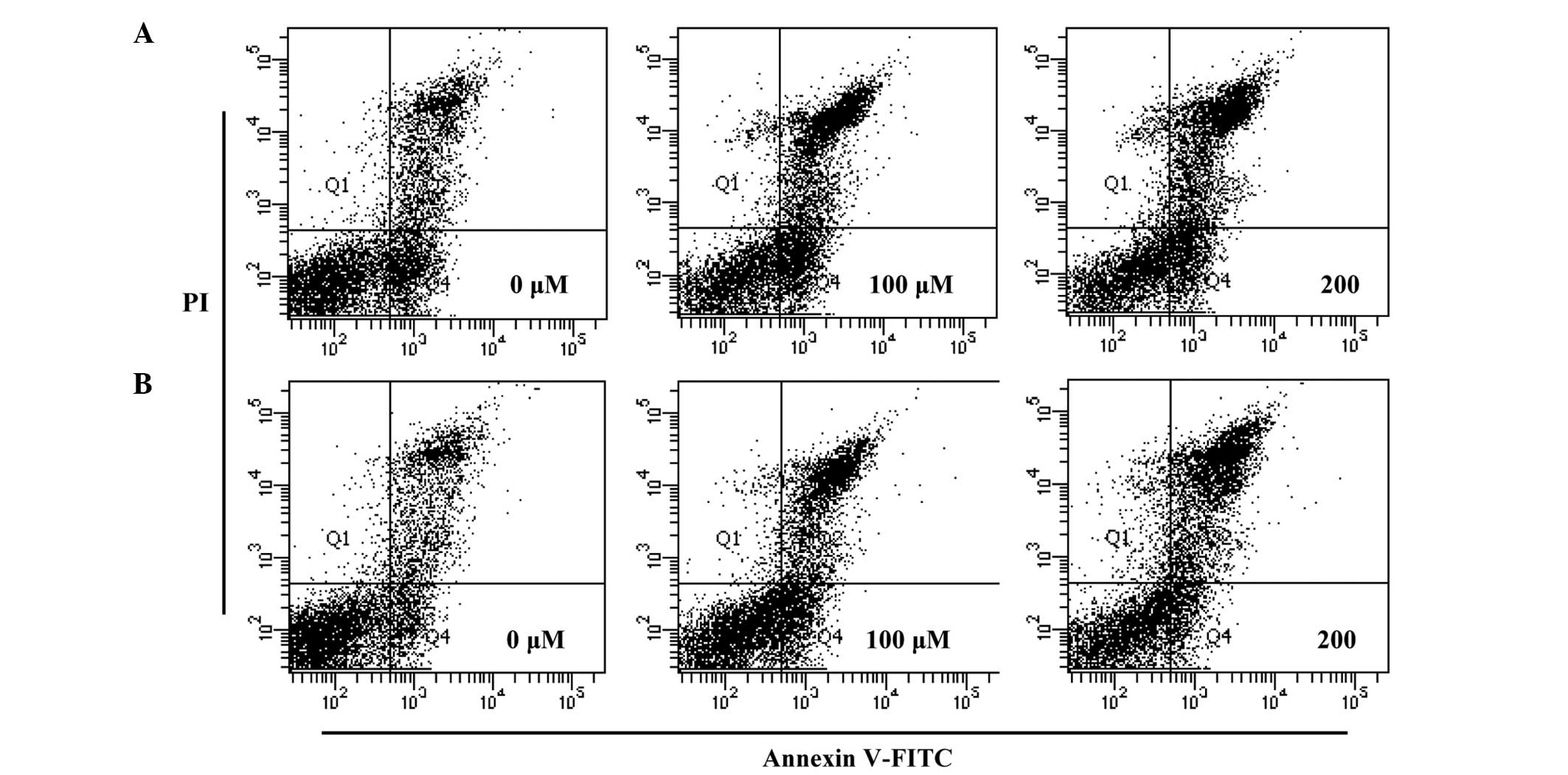
Effects on apoptosis in HT-29
cells
In order to quantify the percentage of apoptotic
cells, flow cytometry analysis was performed using double staining
with Annexin V and PI. The Annexin V−/PI−
population was considered to account for unaffected cells, the
Annexin V+/PI− population for early
apoptosis, Annexin V+/PI+ for late apoptosis
and Annexin V−/PI+ for necrosis. The results
showed that the treatment of the cells with hyperoside and rutin
significantly increased the percentage of apoptotic cells compared
with untreated control cells (Fig.
5). The total apoptotic cell populations of hyperoside and
rutin were increased by 52.4 and 56.5% at 200 µm, respectively,
compared with the control. These results indicate that hyperosde
and rutin may induce apoptosis in HT-29 human colon cancer
cells.
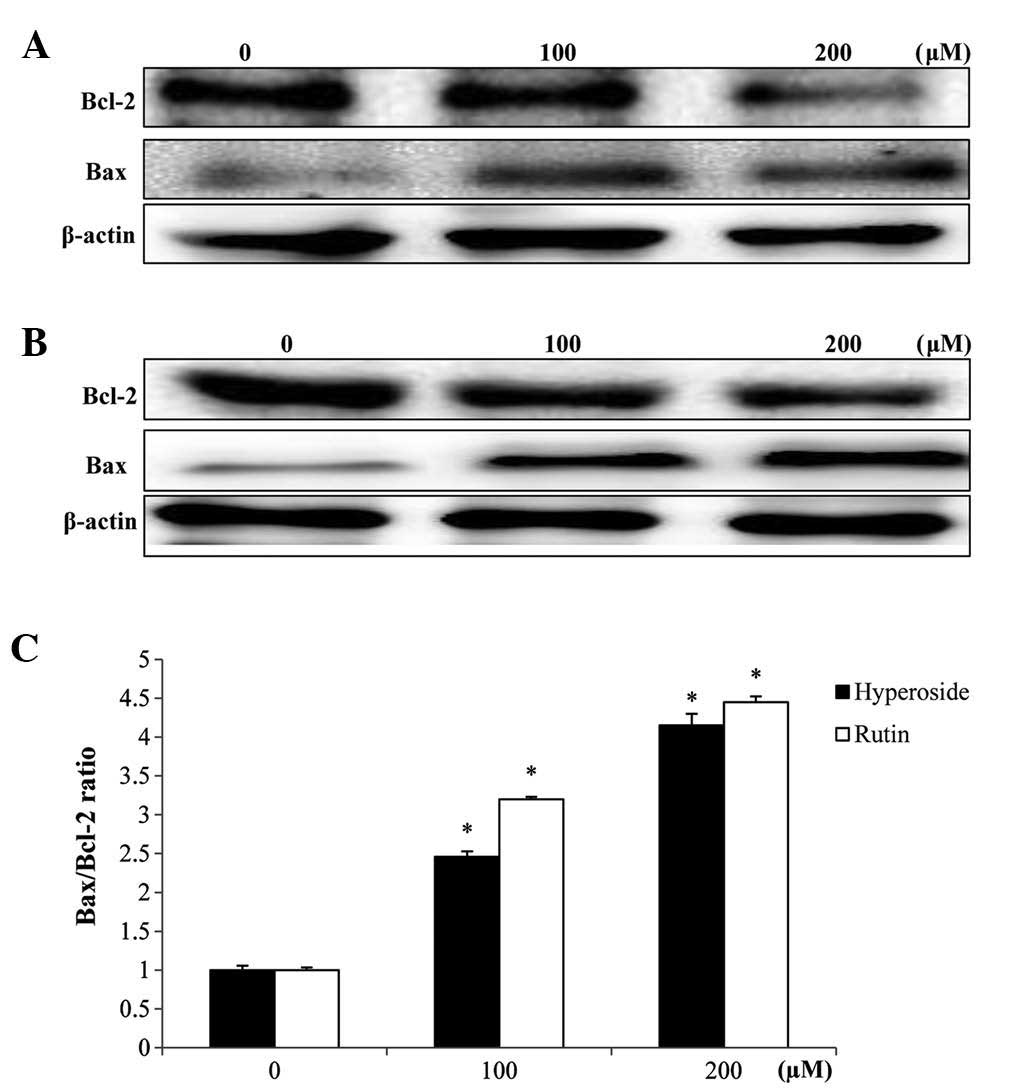
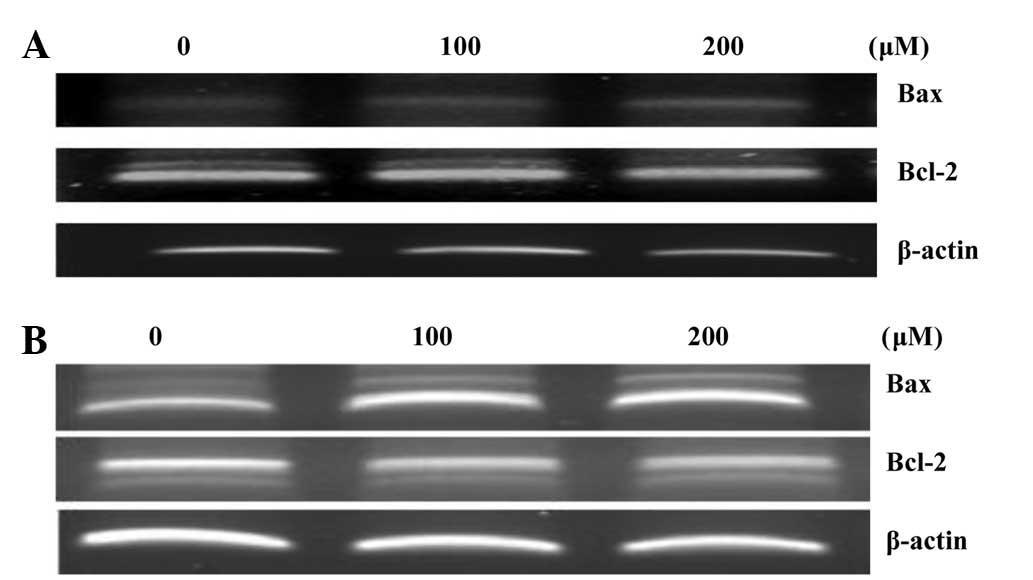
Effects on Bcl-2 and Bax
expression
In order to study the effects of hyperoside and
rutin on apoptosis in HT-29 cells, the expression levels of
apoptosis regulatory proteins, including Bcl-2 and Bax were
examined. The mitochondrial pathway is an important apoptosis
pathway as it regulates the apoptotic cascade via a convergence of
signaling at the mitochondria. Bcl-2 interacts with the
mitochondrial plasma membrane and prevents mitochondrial membrane
pores from opening during apoptosis, blocking the signals of
apoptotic factors (22). As shown in
Fig. 6, hyperoside and rutin
increased Bax expression but decreased the expression of Bcl-2,
each in a dose-dependent manner. Also, a densitometric analysis of
the bands showed that hyperoside and rutin caused a dose-dependent
increased the Bax/Bcl-2 ratio (Fig.
6C).
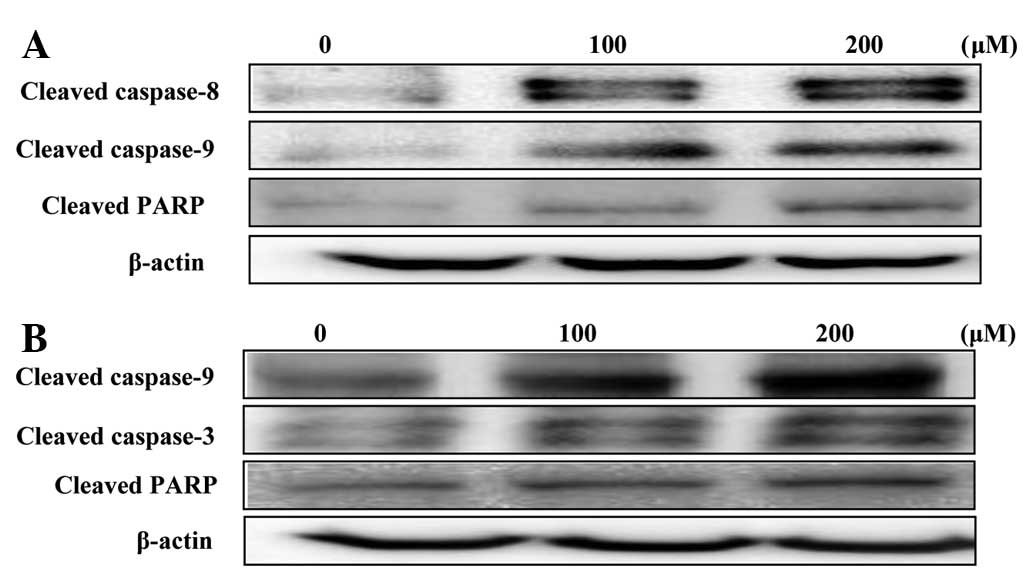
Effects on caspase expression
The disruption of the mitochondrial plasma membrane
by hyperoside and rutin was followed by the activation of the
cleaved caspases-3, 8 and 9 and target protein PARP, respectively
(Fig. 7). These results, together
with the Bax/Bcl-2 ratio suggested that hyperoside and rutin may
induce apoptosis through the regulation of apoptosis-associated
protein expression in HT-29 human colon cancer cells.
RT-PCR analysis
RT-PCR analysis was used to examine any alterations
in Bax and Bcl-2 expression. During apoptosis, Bcl-2, a negative
regulator of apoptosis, prevents mitochondrial membrane pores from
opening; however, the positive regulator, Bax, produces the
opposite effect. As a result, hyperoside and rutin increased Bax
expression, but decreased the expression of Bcl-2 in a
concentration-dependent manner (Fig.
8).
Discussion
Uncontrolled proliferation is a significant
biological feature of cancer cells, and the inhibition of cell
proliferation may achieve the arrest of tumor growth (27). Colorectal cancer is the second leading
cause of cancer-associated mortalities in the Western world
(28). Colorectal cancer is
hypothesized to arise as a result of the transformation of normal
colonic epithelial cells into a colorectal carcinoma, as an
adenomatous polyp. Increased understanding of cancer has led to the
development of numerous novel anticancer agents and associated
natural therapies, a variety of which clinically target the
inhibition of cancer cells, but not normal cells; however, numerous
anticancer agents are also toxic to normal cells. Therefore, novel
agents that are derived from natural sources, which specifically
target colorectal cancer but have a decreased toxicity for normal
colonic epithelial cells, hold enormous potential (20).
Apoptosis is an extremely important phenomenon due
to the maintenance of cellular homeostasis by the regulation of
cell division and cell death (29).
The mitochondrial-associated pathway is regulated by anti-apoptotic
members, including Bcl-2 and Bcl-2-like 1, and pro-apoptotic
members, including Bax, Bcl-2-antagonist/killer 1, BH3 interacting
domain death agonist, Bcl-2 associated agonist of cell death and
Bcl-2-like 11, of the Bcl-2 family. The anti-apoptotic proteins on
the outer membranes of the mitochondria maintain the integrity of
the mitochondria, through inhibiting apoptosis in the presence of
various apoptotic stimuli (30). In
response to an apoptotic stimulus, the pro-apoptotic proteins
reside in the cytosol and translocate to the mitochondria, which
leads to the formation of membrane pores at the mitochondrial
membranes (31). The data in the
present study showed that apoptosis with hyperoside and rutin is
associated with the upregulation of Bax protein and the
downregulation of Bcl-2 expression, each in a dose dependent
manner.
Caspase-3 is one of the key executioners of
apoptosis, and is either partially or totally responsible for the
proteolytic cleavage of numerous proteins, including PARP (32). PARP is an important factor that allows
cells to maintain viability, but the cleavage of PARP facilitates
cellular disassembly and acts as a major marker for
caspase-dependent apoptosis (33).
The cleaved form of PARP was detected in hyperoside and
rutin-treated HT-29 cells. These data indicate that hyperoside and
rutin induce apoptosis via the mitochondrial pathway. To the best
of our knowledge, the present study is the first to demonstrate the
cytotoxic effects of hyperoside and rutin on HT-29 human colon
cancer cells, and provides a possible mechanism for this activity.
The present study indicates that hyperoside and rutin may be the
promising candidates for chemotherapy of colon cancer.
References
|
1
|
Kwon YS, Lee DH, Lee KS and Nam KS:
Effects of deep-sea water on inhibition of metastatic regulators
expression in human colorectal adenocarcinomas by chitosan
oligosaccharide. J Chitin Chitosan. 4:229–234. 2012.
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hur SK, Kim SS, Heo YH, Ahn SM, Lee BG and
Lee SK: Effects of the grapevine shoot extract on free radical
scavenging activity and inhibition of pro-inflammatory mediator
production in RAW264.7 macrophages. Biomol Ther. 9:188–193.
2001.
|
|
4
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Siegel RL, Jemal A and Ward EM: Increase
in incidence of colorectal cancer among young men and women in the
United States. Cancer Epidemiol Biomarkers Prev. 18:1695–1698.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Møller H, Sandin F, Robinson D, Bray F,
Klint S, Linklater KM, Lambert PC, Påhlman L, Holmberg L and Morris
E: Colorectal cancer survival in socioeconomic groups in England:
Variation is mainly in the short term after diagnosis. Eur J
Cancer. 48:46–53. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kopetz S, Chang GJ, Overman MJ, Eng C,
Sargent DJ, Larson DW, Grothey A, Vauthey JN, Nagorney DM and
McWilliams RR: Improved survival in metastatic colorectal cancer is
associated with adoption of hepatic resection and improved
chemotherapy. J Clin Oncol. 27:3677–3683. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hyun JW and Chung HS: Medicinal plants
with cytotoxic and antioxidative activity. Recent Progress in
Medicinal Plants. Sharma AK, Singh VK, Govil JN and Goyal NK:
12:(1st). Studium Press LLC. (Houston, TX). 193–202. 2006.
|
|
9
|
Shen-Miller J, Schopf JW, Harbottle G, Cao
RJ, Ouyang S, Zhou KS, Southon JR and Liu G: Long-living lotus:
Germination and soil γ-irradiation of centuries-old fruits, and
cultivation, growth, and phenotypic abnormalities of offspring. Am
J Bot. 89:236–247. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park SH, Ham TS and Han JH: Nutritional
contents of beverage from lotus root and evaluation of its
physiological function in aorta relation. Kor J Ori Med Physiol
Pathol. 19:490–494. 2005.
|
|
11
|
Jung HA, Kim JE, Chung HY and Choi JS:
Antioxidant principles of Nelumbo nucifera stamens. Arch
Pharm Res. 26:279–285. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li S, Zhang Z, Cain A, Wang B, Long M and
Taylor J: Antifungal activity of camptothecin, trifolin, and
hyperoside isolated from Camptotheca acuminata. J Agric Food
Chem. 53:32–37. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kao ES, Wang CJ, Lin WL, Yin YF, Wang CP
and Tseng TH: Anti-inflammatory potential of flavonoid contents
from dried fruit of Crataegus pinnatifida in vitro and in
vivo. J Agric Food Chem. 53:430–436. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Choi JH, Kim DW, Yun N, Choi JS, Islam MN,
Kim YS and Lee SM: Protective effects of hyperoside against carbon
tetrachloride-induced liver damage in mice. J Nat Prod.
74:1055–1060. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xue YL, Miyakawa T, Hayashi Y, Okamoto K,
Hu F, Mitani N, Furihata K, Sawano Y and Tanokura M: Isolation and
tyrosinase inhibitory effects of polyphenols from the leaves of
persimmon, Diospyros kaki. J Agric Food Chem. 59:6011–6017.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Le Gall G, DuPont MS, Mellon FA, Davis AL,
Collins GJ, Verhoeyen ME and Colquhoun IJ: Characterization and
content of flavonoid glycosides in genetically modified tomato
(Lycopersicon esculentum) fruits. J Agric Food Chem.
51:2438–2446. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fattouch S, Caboni P, Coroneo V, Tuberoso
CI, Angioni A, Dessi S, Marzouki N and Cabras P: Antimicrobial
activity of Tunisian quince (Cydonia oblonga Miller) pulp
and peel polyphenolic extracts. J Agric Food Chem. 55:963–969.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shin SW, Lee Y, Moon SR, Koo IH, Hong H,
Shin E, Lee M, Park J and Chung HS: Identification of secondary
metabolites with antioxidant and antimicrobial activities from
Artemisia iwayomogi and Chrysanthemum zawadskii. J Kor Soc Appl
Biol Chem. 53:716–723. 2010. View Article : Google Scholar
|
|
19
|
Guon TE and Chung HS: Effects of
Nelumbo nucifera root extract on proliferation and apoptosis
in HT-29 human colon cancer cells. J East Asian Soc Diet Life.
24:20–27. 2014.
|
|
20
|
Ryu MJ and Chung HS: [10]-Gingerol induces
mitochondrial apoptosis through activation of MAPK pathway in
HCT116 human colon cancer cells. In Vitro Cell Dev Biol
Anim. 51:92–101. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shin HJ, Lee SY, Kim JS, Lee S, Choi RJ,
Chung HS, Kim YS and Kang SS: Sesquiterpenes and other constituents
from Dendranthema zawadskii var. latilobum. Chem Pharm Bull
(Tokyo). 60:306–314. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ryu MJ, Kim AD, Kang KA, Chung HS, Kim HS,
Suh IS, Chang WY and Hyun JW: The green algae Ulva fasciata
Delile extract induces apoptotic cell death in human colon
cancer cells. In Vitro Cell Dev Biol Anim. 49:74–81. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kang KA, Lee JH, Zhang R, Piao MJ, Chung
HS and Hyun JW: Oryzadine, a new alkaloid of Oryza sativa
cv. Heugjinjubyeo, attenuates oxidative stress-induced cell
damage via a radical scavenging effect. Food Chem. 119:1135–1142.
2010. View Article : Google Scholar
|
|
24
|
Carmichael J, DeGraff WG, Gazdar AF, Minna
JD and Mitchell JB: Evaluation of a tetrazolium-based semiautomated
colorimetric assay: Assessment of chemosensitivity testing. Cancer
Res. 47:936–942. 1987.PubMed/NCBI
|
|
25
|
Gschwind M and Huber G: Apoptotic cell
death induced by β-amyloid 1–42 peptide is cell type dependent. J
Neurochem. 65:292–300. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chung HS, Chang LC, Lee SK, Shamon LA, van
Breemen RB, Mehta RG, Farnsworth NR, Pezzuto JM and Kinghorn AD:
Flavonoid constituents of Chorizanthe diffusa with potential
cancer chemopreventive activity. J Agric Food Chem. 47:36–41. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu T, Song Y, Chen H, Pan S and Sun X:
Matrine inhibits proliferation and induces apoptosis of pancreatic
cancer cells in vitro and in vivo. Biol Pharm Bull.
33:1740–1745. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Strickland L, Letson GD and Muro-Cacho CA:
Gastrointestinal stromal tumors. Cancer Control. 8:252–261.
2001.PubMed/NCBI
|
|
29
|
Bold RJ, Termuhlen PM and McConkey DJ:
Apoptosis, cancer and cancer therapy. Surg Oncol. 6:133–142. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nagappan A, Park KI, Park HS, Kim JA, Hong
GE, Kang SR, Lee H, Kim EH, Lee WS, Won CK and Kim GS: Vitamin C
induces apoptosis in AGS cells by down-regulation of 14–3-3σ via a
mitochondrial dependent pathway. Food Chem. 135:1920–1928. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cory S and Adams JM: The Bcl2 family:
Regulators of the cellular life-or-death switch. Nat Rev Cancer.
2:647–656. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim KN, Ham YM, Moon JY, Kim MJ, Jung YH,
Jeon YJ, Lee NH, Kang N, Yang HM, Kim D and Hyun CG: Acanthoic acid
induces cell apoptosis through activation of the p38 MAPK pathway
in HL-60 human promyelocytic leukaemia. Food Chem. 135:2112–2117.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Oliver FJ, de la Rubia G, Rolli V,
Ruiz-Ruiz MC, de Murcia G and Murcia JM: Importance of
poly(ADP-ribose) polymerase and its cleavage in apoptosis. Lesson
from an uncleavable mutant. J Biol Chem. 273:33533–33539. 1998.
View Article : Google Scholar : PubMed/NCBI
|