Introduction
Glioma is the most common neoplasm of the central
nervous system (CNS). Glioblastoma multiforme (GBM) is one subtype
of glioma, presenting with high-grade malignancy, high rates of
disease recurrence and a poor patient prognosis. Despite
improvements in radiation therapy delivery, systemic cytotoxic
therapy options and surgical techniques, the median survival time
for patients with newly-diagnosed GBM subsequent to trimodality
therapy is 15 months (1). Thus,
patients with GBM require comprehensive treatment to achieve a
positive therapeutic outcome (2,3).
A number of active and passive immunotherapy
approaches that are being developed have demonstrated potential to
improve the survival time of patients with GBM, whilst exerting
minimal severe side effects (4).
Research regarding brain tumor immunology has resulted in the
development of novel immunotherapy strategies, including cytotoxic
T-lymphocyte therapies and dendritic cell (DC) vaccines (5,6). In
pediatric patients with glioma, DC-based immunotherapies may exert
certain clinical benefits, particularly in individuals with minimal
residual disease; however, additional investigation of such
treatment is necessary (7–9).
Unconventional treatment options, including gene
therapy combined with immunocyte-based immunotherapy, also offer
potential therapeutic approaches to reduce glioma-associated
mortality (10). Gene therapies have
been implicated in the treatment of brain tumors, with animal
models demonstrating preclinical efficacy, in addition to
encouraging safety profiles observed in phase I clinical trials;
however, in phase III clinical trials, such therapies have thus far
failed to demonstrate significant therapeutic efficacy (11,12).
The melanoma-associated antigen (MAGE) family
consists of 10 subfamilies, with 1–15 genes in each one (13,14).
MAGE-D4a is expressed in a large percentage of human CNS tumors,
indicating that it may serve as a potential target for
immunotherapy treatment (15,16). In the present study, ecto-mesenchymal
stem cells (EMSCs) were isolated and cultured, and subsequently
infected with an adenoviral plasmid containing MAGE-D4a
(pAd/MAGE-D4a). The MAGE-EMSCs were then induced to differentiate
into DCs, and their cytotoxicity on the human glioma U251 cell line
was analyzed.
Materials and methods
Isolation and culture of human
EMSCs
A human embryo (50 days of pregnancy) recovered from
a spontaneous abortion, initiated by trauma, was donated for tissue
isolation. The maxillary and mandibular processes of the embryo
were dissected and divided into <1 mm sections. The sections
were then incubated in a 0.025% trypsin solution (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) at 37°C for 10 min. The
solution was blown slightly to scatter the pieces into a single
cell suspension. Enzyme digestion was ceased by neutralization with
the equal volume of fresh Dulbecco's modified Eagle's medium (DMEM;
Gibco; Thermo Fisher Scientific, Inc.) containing 10% fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc.). The cell
suspension was centrifuged at 800 rpm (160 xg) for 6 min. The cell
pellet was resuspended by DMEM-F12 containing leukemia inhibitory
factor (LIF), filtered through a 75-µm steel mesh screen to remove
cell aggregates and undigested tissues, and was plated in DMEM into
tissue culture flasks (Corning Incorporated, Corning, NY, USA). The
cells were maintained at 37°C in 5% CO2 for 40 min. The
majority of the cells were observed to be adherent under an
inverted microscope (CK40; Olympus Corportion, Tokyo, Japan). The
culture medium was then switched to DMEM-F12 containing LIF, and
2–3 days later the cells were analyzed under the inverted
microscope and were subsequently resuspended for magnetic-activated
cell sorting (MACS).
Primary human EMSC (hEMSC)
purification by MACS
The present study utilized the indirect immune MACS
method to purify the hEMSCs. The magnetic beads were washed,
thoroughly, oscillated to achieve sufficient mixing and transferred
to a test tube. Following 2 min of standing, the supernatant was
discarded. Phosphate-buffered saline (PBS) containing 0.1% calf
serum (Qing Lake Calves, JinHua, China) was added to repeat the
aforementioned washing procedure. Following three washes, the
magnetic beads were resuspended and the hEMSCs were resuspended in
PBS containing 0.1% serum. Low-affinity nerve growth factor
receptor (LNGFR) rabbit anti-rat polyclonal antibody (dilution,
1:200; catalog no., sc-8317; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA), using a kit according to the manufacturer's
protocols (Dynal Biotech, Carlsbad, CA, USA) was then added into
the resuspended solution and incubated at 4°C for 30 min. PBS
containing 0.1% serum was used to wash and wipe off the residual
antibody. The cell suspensions were incubated with magnetic beads
at 4°C for 6 min at a concentration of 1×1011 beads/ml
cell suspension. The tube was held stirring on a magnetic rack for
2 min, with the supernatant subsequently discarded. PBS containing
0.1% serum was applied to resuspend and wash the cells twice.
Subsequent to the supernatant being discarded, the magnetic bead
separation fluid (0.2 mol/l sodium citrate; pH 2) was added and
lightly shaken to blend for 2 min. Following 2 min of stewing, the
supernatant was collected. The procedures were repeated several
times to discard the residual magnetic beads, and the collected
supernatant was incubated to expand the cell population. The growth
and morphology of the cells were observed under an inverted
microscope every 2–3 days. The third passage of hEMSCs were
obtained, centrifuged, washed and fixed by glutaraldehyde. The
prepared ultrathin sections were observed under a transmission
electron microscope (JEM-2000EX; JEOL, Ltd., Tokyo, Japan).
Construction of the recombinant
Ad-MAGE-D4a
The MAGE-D4a sequence was obtained by reverse RNA
transcription of the human glioma U251 cell line (Type Culture
Collection of the Chinese Academy of Sciences, Shanghai, China),
and was inserted into the adenoviral shuttle vector-pTracer-green
fluorescent protein (GFP; Agilent Technologies, Inc., Santa Clara,
CA, USA). MAGE-D4a DNA was linearized with PmeI and
co-transformed with the pAdEasy-1 Vector (cat no. 240005; Agilent
Technologies, Inc.) into the competent bacterial strain BJ5183,
which facilitates efficient recombination. Following screening,
recombinants for the adenoviruses, Ad/MAGE-D4a and Ad-GFP
(containing no insert sequence as a control), were generated. The
recombinant DNA was identified by the restriction endonuclease
PacI and transfected into human embryonic kidney (HEK)293
cells subsequent to linearization. The cells were collected once
cytopathogenic effects had appeared, and the generated recombinant
adenoviruses were isolated. Expression of the MAGE-D4a gene was
detected by polymerase chain reaction (PCR) using a
PicoTiterPlate™. Briefly, the DNA amplification procedure was
performed on an extremely small platform in picolitre quantities.
Subsequently the products were available to be transferred to solid
supports for transcription, translation or sequencing (17), which were performed by AuGCT (Beijing,
China). The virus titer was measured on day 14 post-infection. The
primers used were as follows: MAGE-D4a forward,
5′-ACGCGGTACCCATGGCTGAGGGAAGCTTCAGCGTGC-3′ and reverse,
5′-GGCTCGAGACGGTGCTGGATCCAGGAGAAGAAG-3′ (Augct DNA-Syn
Biotechnology Co., Ltd., Beijing, China).
pAd/MAGE-D4a adenoviral infection of
the hEMSCs
The sixth passage hEMSCs following MACS were seeded
in a 6-well plate at a concentration of 2×106 cells per
well. The cells were transduced with pAd/MAGE-D4a once they had
achieved ~80% confluency. Infection was performed with a
multiplicity of infection (MOI) of 0.1, 1, 10 and 100
plaque-forming units/cell in 1 ml infection buffer at room
temperature for 60 min. Subsequent to infection, the hEMSCs were
incubated for the indicated time periods at 37°C with 5%
CO2 in a humidified atmosphere. Following a total of 48
h, pAd/MAGE-D4a expression was analyzed by identifying the
GFP+ cells under a fluorescence microscope (IX71;
Olympus Corporation). In addition, total RNA was extracted from
pAd/MAGE-D4a adenoviral infected hEMSCs using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol, in order to check the expression of
MAGE-D4a. RNA (1 µg) was used to perform reverse transcription (RT)
to provide complementary DNA using the PrimeScript RT Master Mix
(Perfect Real Time; Takara Biotechnology Co., Ltd., Dalian, China).
For the quantitative polymerase chain reaction (qPCR), 10 µl SYBR
Green Premix Ex Taq™ (Takara Biotechnology Co., Ltd.), 1 µl primers
and 2 µl cDNA were used. The primer sequences were as follows and
were obtained from Beijing AuGCT Biotechnology Co., Ltd. (Beijing,
China): MAGE-D4a, forward 5′-CCAGCTTCTTCTCCTGGATC-3′ and reverse
5′-GTAACACTGATACCCAAAACATG-3′. Glyceraldehyde 3-phosphate
dehydrogenase was employed as a control, with the primer sequence
as follows: forward, 5′-TCACCAGGGCTGCTTTTAAC-3′; and reverse,
5′-GACAAGCTTCCCGTTCTCAG-3′. PCR was performed on a C1000 Touch™
Thermal Cycler (Bio-Rad Laboratories, Inc., Hercules, CA, USA), and
the cycling conditions were as follows: Initial denaturation at
95°C for 10 min, followed by 40 cycles of 15 sec at 95°C and 1 min
at 60°C. Each cDNA sample was run three times. MAGE-D4a mRNA
expression was normalized to glyceraldehyde 3-phosphate
dehydrogenase and fold differences were determined using the
2−ΔΔCq method (18).
Induction of differentiation of the
pAd/MAGE-D4a-hEMSCs to DCs
Subsequent to screening, the GFP+ hEMSCs
were counted at a concentration of 1×104 cells/ml. To
distinguish whether the infection was successful, GFP+
cells were determined by making comparisons between green
fluorescence and white light. Recombinant human
granulocyte-macrophage colony stimulating factor (GM-CSF; 100
ng/ml; Sigma-Aldrich, St. Louis, MO, USA) and recombinant human
interleukin-3 (IL-3; 200 U/ml; Sigma-Aldrich) were added to the
culture medium for 17 days. During this time, the medium was
changed for half dose with the combination of the above factors
every 3 days. On day 19, recombinant human tumor necrosis factor
(200 U/ml; Sigma-Aldrich) was added to continue the induction. The
cell morphology was subsequently analyzed under a fluorescence
microscope.
Ex vivo cytotoxic T cell (CTL)
response
The human effector cell culture was formed using
peripheral blood mononuclear cells that had been separated using
the Ficoll-Hypaque density gradient centrifugation method and
resuspended in RPMI 1640 containing 10% calf bovine serum. The
cells were then seeded in a 6-well plate at a concentration of
5×106 cells per well and incubated at 37°C containing 5%
CO2 in a humidified atmosphere for 2 h. The non-adherent
cells were collected, counted at a concentration of
1×106 cells/ml and cultured at 37°C in a humidified
atmosphere containing 5% CO2. The cells were centrifuged
every 4 days, in addition to changing the culture medium. The cells
were mixed with the pAd/MAGE-D4a-hEMSC-induced DCs at a proportion
of 5:1.
Groups
The three groups of cells utilized were as follows:
Group 1, pAd/MAGE-D4a-hEMSC-induced DCs with effector lymphocytes,
termed MAGE-hEMSCs-DC + lymphocytes (MAGE-EMSCs-DC-L); Group 2,
hEMSCs transduced with pAd/MAGE-D4a with effector lymphocytes,
termed MAGE-hEMSCs + lymphocytes (MAGE-EMSCs-L); and Group 3,
lymphocytes (L).
CTL response assay
A methyl thiazolyl tetrazolium (MTT) assay was
performed to analyze the CTL response. The effector cells
(MAGE-EMSCs-DC-L, MAGE-EMSCs-L and L) and target cells (human
glioma U251 cell line) were mixed and seeded in a 96-well plate at
a ratio of 10:1 (effector cells, 105 cells per well;
target cells, 104 cells per well). The effector cells
and target cells were respectively seeded alone to serve as blank
controls. There were 8 samples within each group. Cells of each
groups were cultured for 24 h at 37°C in a humidified atmosphere
with 5% CO2. Following the MTT reaction, the absorbance
(A) of each wells was measured at 490 nm. The formula for the
cytotoxicity index (CTI) was as follows: CTI = [1 - (trial hole A -
effector cells A) / (target cells A)] x100.
Interferon-γ (IFN-γ) assay by
enzyme-linked immunosorbent assay (ELISA)
The MAGE-EMSC-DCs, or the normal control DC, was
incubated for 8 days in 10% FBS RPMI 1640 medium in a 5%
CO2 incubator. The MAGE-EMSC-DCs, GFP-EMSC-DCs or normal
control DCs were then co-incubated with the U251 cell line CTLs at
a ratio of 20:1 for 24 h. The cell culturing supernatant was
harvested, and ELISA was utilized to determine IFN-γ levels using
the human IFN-γ and biotinylated human IFN-γ detection antibodies,
according to the Human IFN-γ ELISA kit (catalog no., RAB0222;
Sigma-Aldrich).
Statistical analysis
The data were expressed as means and standard
deviations. The ex vivo CTL response analyses and IFN-γ
assay were performed according to the Student-Newman-Keuls test.
P<0.05 was considered to indicate a statistically significant
difference. All the data were analyzed using SPSS software, version
18.0 (SPSS, Inc., Chicago, IL, USA).
Results
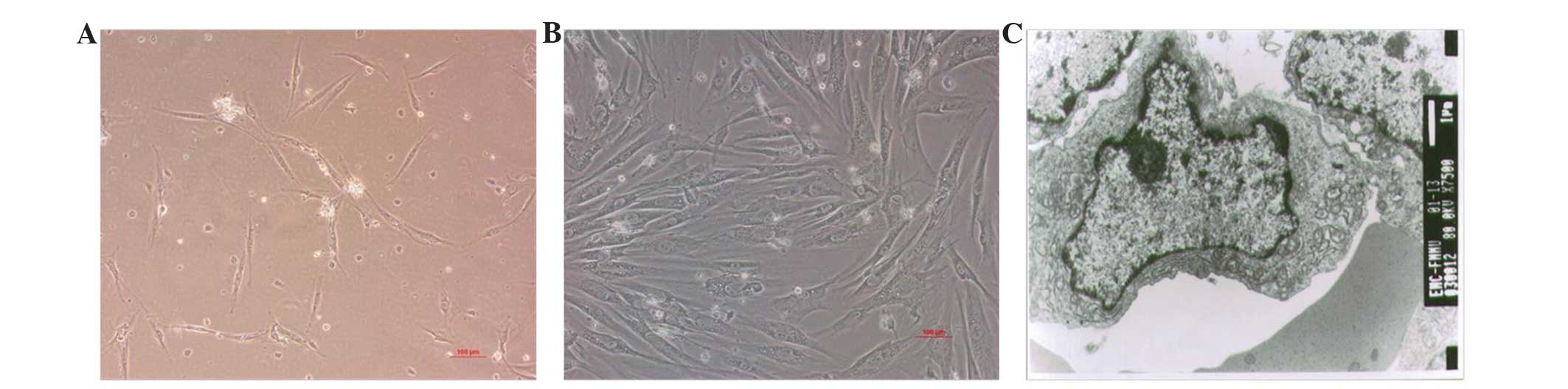
Characterization of the hEMSCs
Following MACS, the majority of LNGFR+
cells post-culture (37°C in a humidified atmosphere containing 5%
CO2) were attached, and demonstrated a fibroblast-like
morphology in the third passage (Fig. 1A
and B). The ultrastructure of the hEMSCs was characterized by
mesenchymal-like cells aggregated in an irregular shape. The
nucleolus was marked and close to the cell membrane, and contained
a large ratio of nucleuic to cytoplasmic material. There was a
greater amount of euchromatin than heterochromatin within the
cells, a large number of mitochondria and a small amount of rough
endoplasmic reticulum and ribosomes, which indicated the
undifferentiation and high proliferation activity of the hEMSCs
(Fig. 1C).
Transgene expression in vitro
To identify whether there was an increase in
MAGE-D4a expression, the total and MAGE-D4a RNA were subjected to
agarose gel electrophoresis. It was identified that the intensity
of the 28S band was much more potent than that of the 18S band,
whilst no band was detected at 5S; therefore demonstrating that the
integrity of the total RNA was marked. RNA A260/280 was 2.00,
indicating that it was suitable for RT-PCR.
Construction of T-easy/MAGE-D4a
T vector-linked MAGE was screened through DH-5a, and
the smaller clones were picked out. The plasmids were then
extracted following bacteria expansion, identified by
KpnL/XhoI double-enzyme cuts. The gel bands obtained
through electrophoresis were sent to Shanghai Shenggong Co. Ltd.
(Shanghai, China) for sequencing; the results were consistent with
those obtained from GenBank (www.ncbi.nlm.nih.gov/genbank), and no mutation was
detected.
Construction of pTrack-cmv-GFP
Through enzyme cutting, it was identified that
MAGE-D4a was successfully introduced into the pTrack-cmv-GFP
plasmid. Lanes 1 to 6 were multiple plasmids extracted from
positive clones, and were subjected to KpnL/XhoI double-enzyme
cutting.
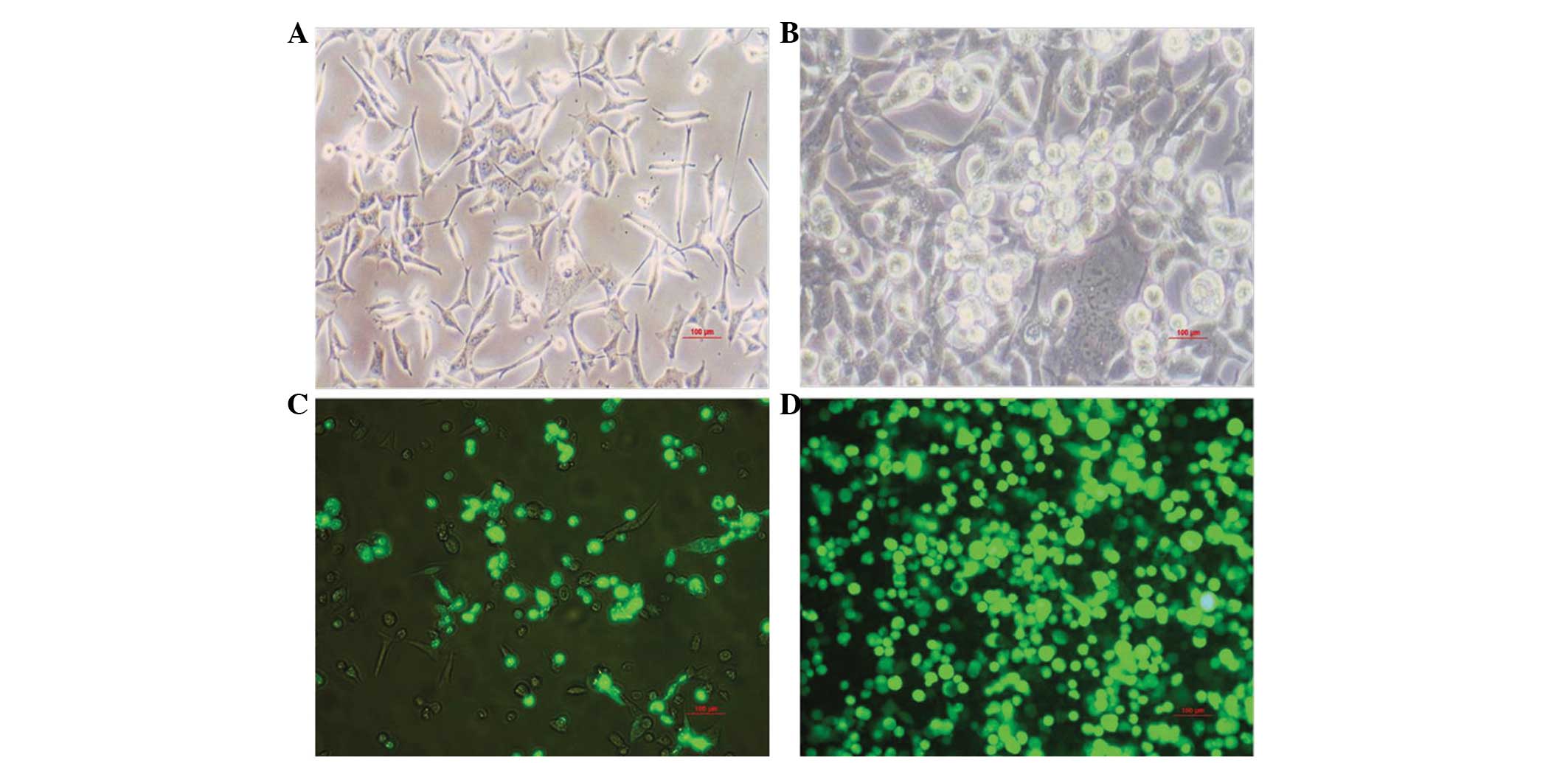
Infection of the HEK293 cells
Monolayer HEK293 cells were infected by
pAd/MAGE-D4a. The infected cells demonstrated characteristic
cytopathogenic effects, initially being round in shape, and once
refractivity had increased, they detached from the bottom of the
culture bottle and formed grape-like aggregates (Fig. 2A and B). Protein amplification was
analyzed by detecting GFP expression. The GFP expression level had
significantly increased by the fourth round of amplification
(Fig. 2C and D).
RT-PCR
An electrophoresis band at ~2,200 bp was amplified,
indicating that the virus contained the target gene. Under the same
conditions, there was no band detected in either the control or the
empty virus groups.
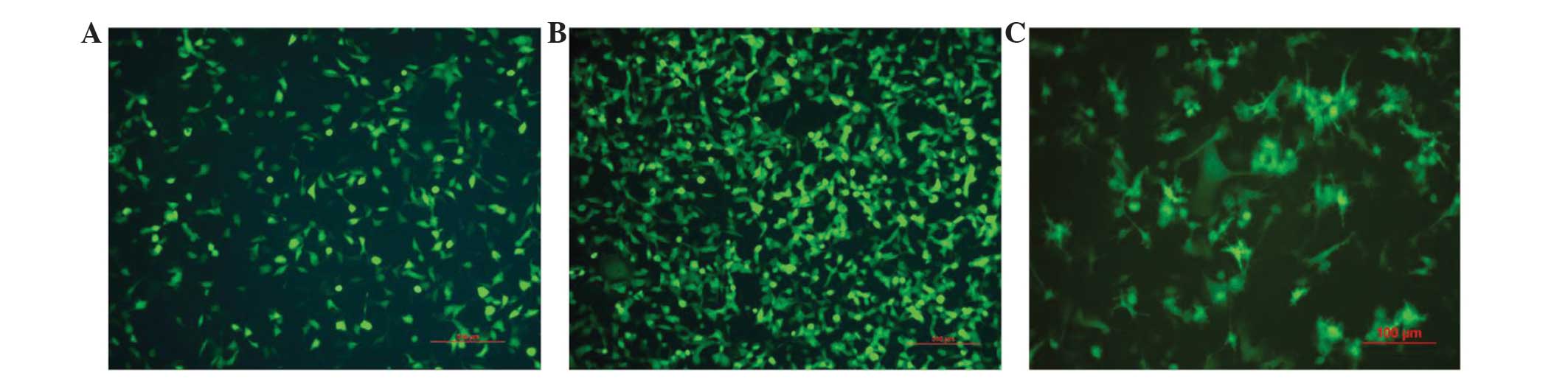
pAd/MAGE-D4a Titer measurement and
adenoviral infection of the hEMSCs
On day 14 post-infection, 10–100 wells of cells were
counted, and the virus titer was measured at 2×108
pfu/ml. Once the hEMSCs had been infected by the pAd/MAGE-D4a with
a MOI of 10, ~50% of the hEMSCs were GFP+ (Fig. 3A). When the hEMSCs were infected by
the pAd/MAGE-D4a with a MOI of 100, ~80% of hEMSCs were
GFP+, thus demonstrating that high biological activity
functions as a gene transfer vector (Fig.
3B).
Morphological features of the
pAd/MAGE-D4a-hEMSC derived DCs
The induced cells from the hEMSCs exhibited a veiled
dendritic morphology typical of DCs. The pricking and dendritic
eminences on the cell surfaces were observed under the microscope
(Fig. 3C).
Ex vivo CTL response
The MTT assay demonstrated that the cytotoxity index
in the MAGE-EMSC-DC-L, MAGE-EMSC-L and L groups were 65.1±3.4,
28.8±2.55 and 27.4±2.89%, respectively. The cytotoxic index was
significantly higher in the MAGE-EMSCs-DC-L group compared with the
other two groups (P<0.05; Fig.
4A).
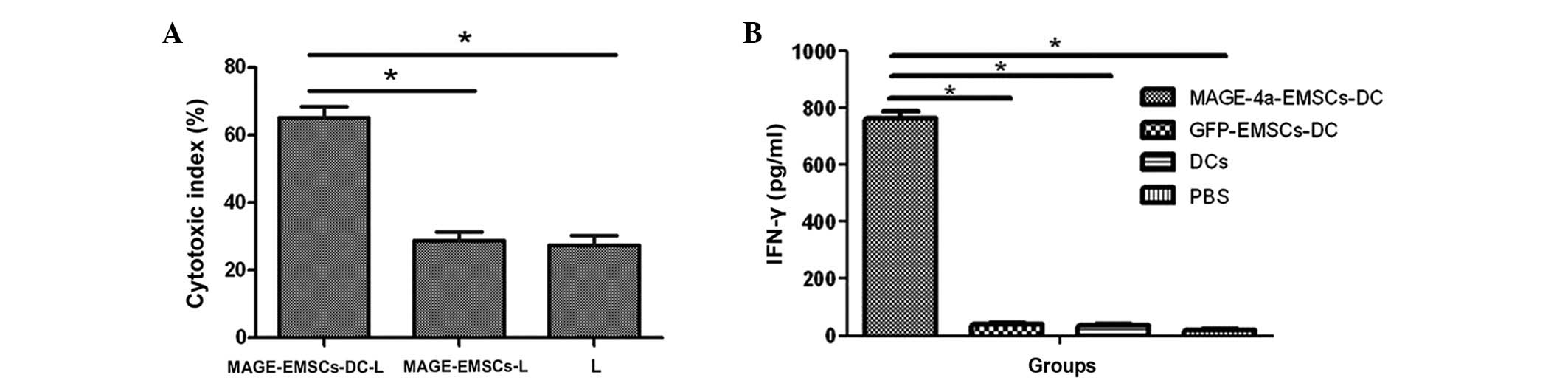 | Figure 4.(A) Cytotoxicity indexes in the
MAGE-EMSC-DC-L, MAGE-EMSC-L and L groups were 65.1±3.4, 28.8±2.55
and 27.4±2.89%, respectively. The cytotoxic index was significantly
greater in the MAGE-EMSC-DC-L group compared with the other two
groups. Error bars represent the mean ± SD from the three groups.
(B) IFN-γ release in the culture supernatants of the MAGE-4a
specific CTLs that were exposed to the MAGE-EMSC-DCs, GFP-EMSC-DCs
or the normal control DCs at a ratio of 20:1 (MOI, 100). The CTLs
induced by the MAGE-EMSC-DCs, co-cultured with the U251 cells for
24 h, produced 765.0 pg/ml IFN-γ, which was much higher compared
with the control wells. Error bars represent the mean ± SD from the
three groups. *P<0.05. MAGE, melanoma antigen family; EMSCs;
ecto-mesenchymal stem cells; DC, dendritic cell; SD, standard
deviation; IFN, interferon; CTLs, cytotoxic T-cell; MOI,
multiplicity of infection; GFP, green fluorescence protein; PBS,
phosphate buffered-saline. |
IFN-γ assay with ELISA
IFN-γ release in the culture supernatants of
specific CTLs was significantly higher in the wells of the
co-culture with MAGE-4a-EMSC-DCs (765.0 pg/ml) compared with the
wells containing control DCs (P<0.05; Fig. 4B); indicating that the MAGE gene may
be recognized by CTLs.
Discussion
Of all malignant primary brain tumors, GBM is the
most prevalent and the most lethal. Over the years, researchers in
basic and clinical studies have aimed to improve the survival rate
for patients with GBM; however, the prognosis remains to be poor
(19). Preclinical data and active
immunotherapy studies have concentrated on synthetic antigen
vaccination and tumor lysate strategies (20). Marsh et al (1) reviewed the existing clinical data
regarding immunological therapy in the treatment of GBM and
identified that the existing phase I and II data suggest that such
therapies may produce valid, and sometimes durable, responses in
patients with GBM with mild toxicity compared with other existing
therapies. The results of the present study demonstrate that DCs
derived from hEMSCs expressing MAGE-D4 can induce specific CTL
responses against U251 cells in vitro.
In the current study, hEMSCs were isolated and
cultured and then purified and expanded ex vivo using the
indirect immune MACS method. Cranial neural crest-derived hEMSCs
differentiate into a number of different cell types, including
peripheral nervous system glial cells, neurons, melanocytes and
certain paraendocrine and endocrine cells (21). Furthermore, the pluripotency of hEMSCs
presents the opportunity to obtain DCs and/or microglia in
vitro and in vivo (22).
The current study subsequently constructed recombinant Ad-MAGE-D4a,
which was used as a target (or tool) in the MAGE-D4a gene, and has
also been utilized in immunotherapy for malignant glioma and other
tumors (23,24). The purified hEMSCs were infected by
Ad-MAGE-D4a and induced into DCs. Under the GM-CSF and IL-3 culture
condition, the MAGE-EMSC-DCs exhibited typical DC morphology with
elongated dendritic processes, which upregulated the expression of
major histocompatibility complex II, cluster of differentiation
(CD)11c, CD86, CD80 and CD40, and downregulated the expression of
LNGFR (22). DCs are antigen
presenting cells, and currently DC-based vaccinations, which
provoke host immune responses against tumor antigens, are being
considered as promising immunotherapeutic agents (25,26).
In the present study, ex vivo CTL response
analysis demonstrated that the cytotoxic index of MAGE-EMSC-DC-L
was significantly higher than the indexes of the other two groups,
which indicated that a potent and specific antitumor immune
response may be induced by the MAGE-EMSC-DC vaccine. The CTLs
induced by the MAGE-EMSC-DCs, co-cultured with the U251 cells for
24 h, produced greater IFN-γ release compared with the control
groups. Interferon-γ is a cytokine that interacts with cell-surface
receptors, subsequently activating the transcription of genes. It
is considered that the genes activated offer therapeutic potential
by inhibiting tumor angiogenesis, disrupting proliferative
mechanisms and increasing tumor immunogenicity (27).
Despite the notable results from the present study,
there are certain issues that require clarification. Firstly, cell
apoptosis was not analyzed with flow cytometry following the
construction of pAd/MAGE-D4a; the present study instead depended on
GFP fluorescence post-infection, which detected that the
pAd/MAGE-D4a infection had no significant effect on the apoptosis
of the hEMSCs when compared with the mock-infected pAd/MAGE-D4a.
Secondly, no data from animal experiments was utilized to
demonstrate that the MAGE-D4-EMSCs could differentiate into
microglial cells in the CNS and serve as antiglioma effectors.
Therefore, further investigation is required to assess the
effectiveness and safety of the application of MAGE-EMSC-DCs to
induce antigen-specific CTL responses in vivo.
Recurrence of GBM primarily occurs due to the tumor
itself developing several mechanisms that allow it to escape the
immune system (28). Thus, gene and
immunotherapy are currently the most promising strategies to solve
this problem. The results of the present study have demonstrated
that EMSCs may be effectively infected by adenovirus vectors to
express MAGE-D4a. MAGE-D4-EMSCs differentiate into DCs, which
subsequently induce MAGE-D4a-specific CTL responses against tumor
cells in vitro. Based on the results from the current study,
MAGE-D4-EMSC-DCs may serve as a feasible and effective approach for
the treatment of glioma and other CNS tumors. Additional
immunotherapy strategies are currently in development for the
treatment of GBM, and future studies may consider the application
of combinatorial gene and immunotherapy strategies in preclinical
trials.
Acknowledgements
The present study was supported by the Youth Science
Fund Project of the National Natural Science Foundation of China
(grant no. 81101710).
Glossary
Abbreviations
Abbreviations:
|
EMSCs
|
ecto-mesenchymal stem cells
|
|
DC
|
dendritic cells
|
|
IFN-γ
|
interferon-γ
|
|
MOI
|
multiplicity of infection
|
|
CNS
|
central nervous system CNS
|
|
GBM
|
glioblastoma multiforme
|
|
MAGE
|
melanoma antigen
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
MACS
|
magnetic activated cell sorting
|
|
LNGFR
|
low-affinity nerve growth factor
receptor
|
|
IL-3
|
interleukin-3
|
|
GM-CSF
|
granulocyte-macrophage colony
stimulating factor
|
References
|
1
|
Marsh JC, Goldfarb J, Shafman TD and Diaz
AZ: Current status of immunotherapy and gene therapy for high-grade
gliomas. Cancer Control. 20:43–48. 2013.PubMed/NCBI
|
|
2
|
Chen J and Xu T: Recent therapeutic
advances and insights of recurrent glioblastoma multiforme. Front
Biosci (Landmark Ed). 18:676–684. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ajeawung NF, Wang HY and Kamnasaran D:
Progress from clinical trials and emerging non-conventional
therapies for the treatment of Medulloblastomas. Cancer Lett.
330:130–140. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu Q, Yuan X and Yu JS: Glioma stem cell
research for the development of immunotherapy. Adv Exp Med Biol.
746:216–225. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Daga A, Bottino C, Castriconi R, Gangemi R
and Ferrini S: New perspectives in glioma immunotherapy. Curr Pharm
Des. 17:2439–2467. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Petrosiute A, Auletta JJ and Lazarus HM:
Achieving graft-versus-tumor effect in brain tumor patients: From
autologous progenitor cell transplant to active immunotherapy.
Immunotherapy. 4:1139–1151. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ardon H, Van Gool SW, Verschuere T, Maes
W, Fieuws S, Sciot R, Wilms G, Demaerel P, Goffin J, Van Calenbergh
F, et al: Integration of autologous dendritic cell-based
immunotherapy in the standard of care treatment for patients with
newly diagnosed glioblastoma: Results of the HGG-2006 phase I/II
trial. Cancer Immunol Immunother. 61:2033–2044. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Prins RM, Wang X, Soto H, Young E, Lisiero
DN, Fong B, Everson R, Yong WH, Lai A, Li G, et al: Comparison of
glioma-associated antigen peptide-loaded versus autologous tumor
lysate-loaded dendritic cell vaccination in malignant glioma
patients. J Immunother. 36:152–157. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lasky JL III, Panosyan EH, Plant A,
Davidson T, Yong WH, Prins RM, Liau LM and Moore TB: Autologous
tumor lysate-pulsed dendritic cell immunotherapy for pediatric
patients with newly diagnosed or recurrent high-grade gliomas.
Anticancer Res. 33:2047–2056. 2013.PubMed/NCBI
|
|
10
|
Mineharu Y, Muhammad AK, Yagiz K, Candolfi
M, Kroeger KM, Xiong W, Puntel M, Liu C, Levy E, Lugo C, et al:
Gene therapy-mediated reprogramming tumor infiltrating T cells
using IL-2 and inhibiting NF-κB signaling improves the efficacy of
immunotherapy in a brain cancer model. Neurotherapeutics.
9:827–843. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tobias A, Ahmed A, Moon KS and Lesniak MS:
The art of gene therapy for glioma: A review of the challenging
road to the bedside. J Neurol Neurosurg Psychiatry. 84:213–222.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Assi H, Candolfi M, Baker G, Mineharu Y,
Lowenstein PR and Castro MG: Gene therapy for brain tumors: Basic
developments and clinical implementation. Neurosci Lett. 527:71–77.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
van der Bruggen P, Traversari C, Chomez P,
Lurquin C, De Plaen E, Van den Eynde B, Knuth A and Boon T: A gene
encoding an antigen recognized by cytolytic T lymphocytes on a
human melanoma. Science. 254:1643–1647. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chomez P, De Backer O, Bertrand M, De
Plaen E, Boon T and Lucas S: An overview of the MAGE gene family
with the identification of all human members of the family. Cancer
Res. 61:5544–5551. 2001.PubMed/NCBI
|
|
15
|
Ito S, Kawano Y, Katakura H, Takenaka K,
Adachi M, Sasaki M, Shimizu K, Ikenaka K, Wada H and Tanaka F:
Expression of MAGE-D4, a novel MAGE family antigen, is correlated
with tumor-cell proliferation of non-small cell lung cancer. Lung
Cancer. 51:79–88. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee MH, Son EI, Kim E, Kim IS, Yim MB and
Kim SP: Expression of cancer-testis genes in brain tumors. J Korean
Neurosurg Soc. 43:190–193. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Leamon JH, Lee WL, Tartaro KR, Lanza JR,
Sarkis GJ, deWinter AD, Berka J, Weiner M, Rothberg JM and Lohman
KL: A massively parallel PicoTiterPlate based platform for discrete
picoliter-scale polymerase chain reactions. Electrophoresis.
25:11762004. View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang X, Zhang W, Mao XG, Zhen HN, Cao WD
and Hu SJ: Targeting role of glioma stem cells for glioblastoma
multiforme. Curr Med Chem. 20:1974–1984. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Reardon DA, Wucherpfennig KW, Freeman G,
Wu CJ, Chiocca EA, Wen PY, Curry WT Jr, Mitchell DA, Fecci PE,
Sampson JH and Dranoff G: An update on vaccine therapy and other
immunotherapeutic approaches for glioblastoma. Expert Rev Vaccines.
12:597–615. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chai Y, Jiang X, Ito Y, Bringas P Jr, Han
J, Rowitch DH, Soriano P, McMahon AP and Sucov HM: Fate of the
mammalian cranial neural crest during tooth and mandibular
morphogenesis. Development. 127:1671–1679. 2000.PubMed/NCBI
|
|
22
|
Hu S, Shen X, Zhang R, Zhang Y, Zhang R,
Zhang W, Deng Z, Cao Y, Zhou Z, Chen J, et al: Development of rat
antigen-presenting cells from pluripotent ecto-mesenchymal stem
cells in vitro and in vivo. Mol Immunol.
45:3818–3826. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Syed ON, Mandigo CE, Killory BD, Canoll P
and Bruce JN: Cancer-testis and melanocyte-differentiation antigen
expression in malignant glioma and meningioma. J Clin Neurosci.
19:1016–1021. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Weeraratne SD, Amani V, Neiss A, Teider N,
Scott DK, Pomeroy SL and Cho YJ: miR-34a confers chemosensitivity
through modulation of MAGE-A and p53 in medulloblastoma.
Neuro-oncol. 13:165–175. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang JY, Cao DY, Liu WC, Zhang HM, Teng ZH
and Ren J: Dendritic cell generated from CD34+ hematopoietic
progenitors can be transfected with adenovirus containing gene of
HBsAg and induce antigen-specific cytotoxic T cell responses. Cell
Immunol. 240:14–21. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Candolfi M, King GD, Yagiz K, Curtin JF,
Mineharu Y, Muhammad AK, Foulad D, Kroeger KM, Barnett N, Josien R,
et al: Plasmacytoid dendritic cells in the tumor microenvironment:
Immune targets for glioma therapeutics. Neoplasia. 14:757–770.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kane A and Yang I: Interferon-gamma in
brain tumor immunotherapy. Neurosurg Clin N Am. 21:77–86. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Szabo AT and Carpentier AF: Immunotherapy
in human glioblastoma. Rev Neurol (Paris). 167:668–672. 2011.
View Article : Google Scholar : PubMed/NCBI
|