Introduction
Neurofibromatosis type 2 (NF2) is a rare autosomal
dominant syndrome that is characterized by the presence of
bilateral vestibular schwannomas (VS; acoustic neuroma). In certain
patients, the disease may be complicated by meningioma, ependymoma,
spinal schwannomas or vitreous opacities. The growth of VS may lead
to brain stem compression, tinnitus, progressive hearing loss,
deafness, ataxia and eventual mortality. The incidence of NF2 in
the population is 1 in 40,000–33,000, and 50% of the cases
represent sporadic mutation, while the other half inherit the
disease from their parents (1).
Microsurgery for tumor resection and stereotactic radiotherapy are
the standard treatment strategies; however, these may sacrifice
hearing to achieve tumor control.
A vast amount of data has indicated that
angiogenesis is pivotal for tumor growth, and vascular endothelial
growth factor (VEGF) is a vital factor affecting angiogenesis and
vascular permeability. Therefore, molecular targeted treatment may
be promising for NF2-related tumors. Bevacizumab is the first Food
and Drug Administration-approved monoclonal antibody that is able
to neutralize the activity of VEGF in order to inhibit
angiogenesis, tumor metastasis and growth, specifically (2). Bevacizumab has been used to treat
malignant tumors, including metastatic rectal carcinoma and
advanced non-small cell lung cancer. Plotkin et al (3) previously achieved a favorable outcome by
treating NF2 with bevacizumab [5 mg/kg/2 weeks; intravenous
injection (IV)], including radiographic tumor regression and
hearing improvement. To the best of our knowledge, the optimal
regimen remains to be defined. A dosage of 5 mg/kg/2 weeks is
widely used; however, this may induce adverse effects, including
hypertension, thrombosis or delayed wound healing. Thus,
establishing a well-tolerated therapy would be promising for
patients, given the adverse effects of bevacizumab.
In the present case involving a patient with
bilateral VS in NF2, a lowered dose of bevacizumab and protracted
infusion interval was trialled so as to explore the efficacy of
low-dose bevacizumab regimen in inhibiting tumor growth and
minimizing the adverse effects.
Case report
A 55-year-old man who was suffering with left
progressive hearing loss and facial weakness was misdiagnosed with
nerve deafness in 2007 at Binzhou Medical University Hospital
(Binzhou, China). The patient presented right hearing loss in April
2009. On February 25, 2013, contrast-enhanced magnetic resonance
imaging (MRI; Signa HDx 3.0T and 1.5T MR; GE Healthcare Life
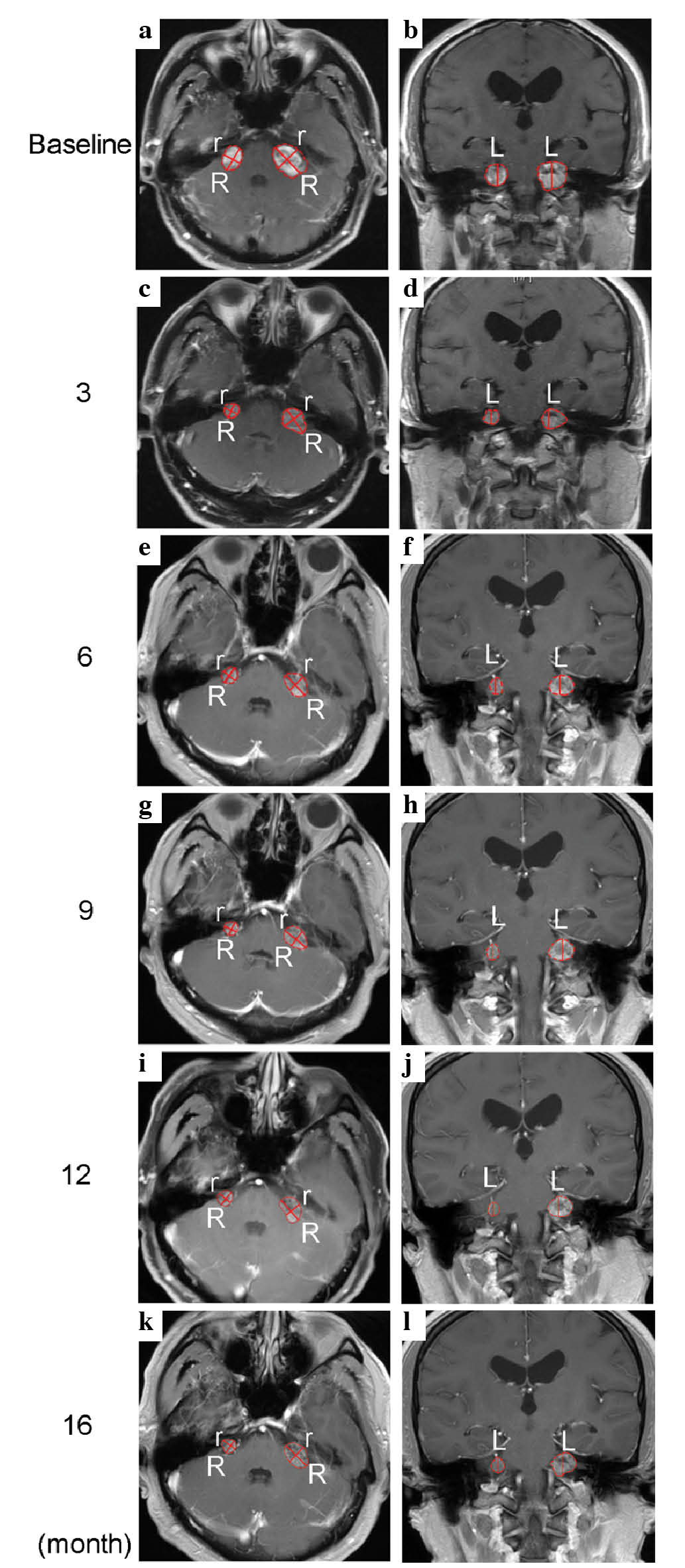
Sciences) (Fig. 1) revealed bilateral
VS measuring 5.25 and 2.54 cm3 on the left and right,
respectively (Fig. 1a–b). No mass
effects were detected on MRI of the spinal cord. Café au
lait spots (3×2 cm) were noted in the left anterior tibial
skin. The patient was conscious without nystagmus, and his left
nasolabial groove was shallower than the right. These findings
confirmed a diagnosis of NF2. The pure tone average (PTA) threshold
of 0.5, 1 and 2 kHz tones presented by bone-conduction indicated an
impaired capacity for bilateral language recognition (Fig. 2). However, the patient refused
microsurgical tumor resection and gamma knife therapy due to fears
of surgery complications. On February 28, 2013, bevacizumab (Roche
Diagnostics, Indianapolis, IN, USA) was administered by IV to
inhibit tumor growth and avoid further deterioration of hearing
initially. The drug dose was calculated according to patient's body
weight (91 kg). Initially, 300 mg (91 kgx3.3 mg/kg) was
administered every 2 weeks for 3 months. Subsequently, the dose of
bevacizumab was gradually lowered to 2.2 mg/kg every 4 weeks in
order to avoid adverse effects. Given that the patient had history
of hypertension, felodipine (5 mg/day) was administered orally to
maintain the patient's blood pressure within a normal range.
Following bevacizumab treatment (3.3 mg/kg, every 2
weeks) for a period of 3 months, the patient's hearing level
remained stable without deterioration and the bilateral VS
regressed by 56% and 76% on the left and right, respectively
(Figs. 1c–d and 2). The central part of tumor was not well
enhanced due to the rapid inhibition of angiogenesis by
bevacizumab. On June 17, 2013, the patient was recommenced on
bevacizumab (3.3 mg/kg, every 3 weeks), administered by IV, for the
next 3 months. His hearing remained stable as before and the left
VS further regressed by 13%, while the right VS remained stable
(Figs. 1e–f and 2). Given the adverse effects induced by
bevacizumab, including anemia, neutropenia and lymphocytopenia
(4), on September 14, 2013, the dose
was further lowered to 2.2 mg/kg and the infusion interval
protracted to 4 weeks so as to reduce the risk of hypertension
aggravated by bevacizumab whilst preventing tumor recurrence
following drug discontinuation.
After a constant treatment (2.2 mg/kg) for 1 year,
the patient's hearing was successfully preserved by low-dose
bevacizumab, without further progression (Fig. 3). Although no hearing improvement was
detected by pure tone audiometry (using the Madsen Midimate 622
audiometer; GN Otometrics, Taastrup, Denmark), the patient
subjectively experienced a significant hearing improvement as his
ability to communicate with people and distinguish voices was
restored. Compared with baseline measurements prior to treatment,
the bilateral VS regressed by 3.59 cm3 (68%) and 2.08
cm3 (82%) on the left and right, respectively (Fig. 1g–l; Table
I). At the time of writing, the patient was continuing to
receive bevacizumab treatment (2.2 mg/kg, every 4 weeks) by IV
without any significant adverse effects observed, and with no signs
of tumor progression or hearing deterioration.
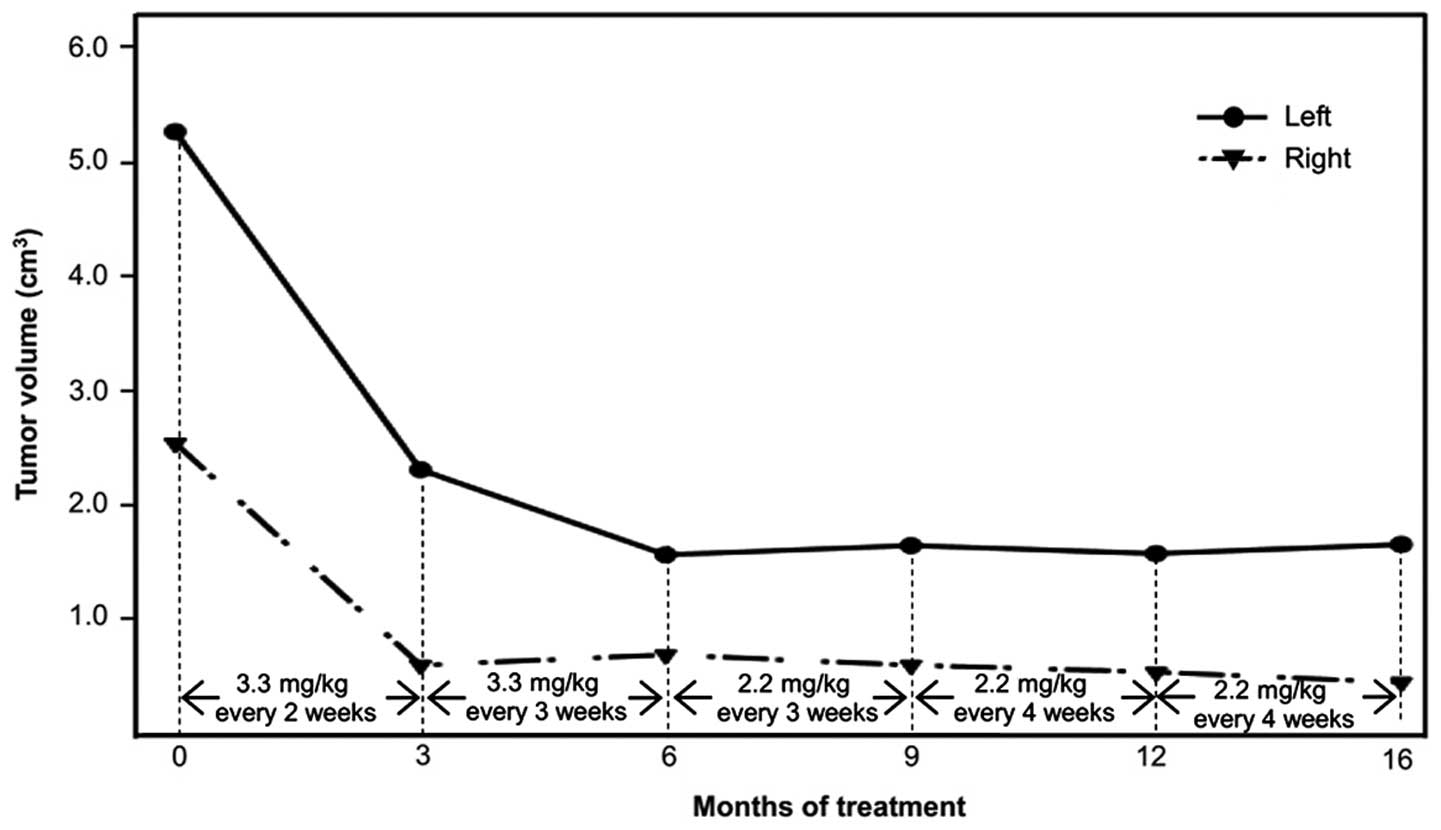
 | Table I.Tumor size analysis. |
Table I.
Tumor size analysis.
|
| Baseline | 3 months | 6 months | 9 months | 12 months | 16 months |
|---|
|
|
|
|
|
|
|
|
|---|
| Dimension | Left | Right | Left | Right | Left | Right | Left | Right | Left | Right | Left | Right |
|---|
| R, mm | 27.5 | 18.3 | 20.0 | 11.0 | 17.7 | 11.1 | 17.8 | 11.6 | 17.2 | 10.9 | 18.2 | 9.8 |
| r, mm | 18.7 | 15.6 | 15.1 | 10.5 | 12.1 | 10.6 | 13.4 | 10.6 | 12.6 | 10.3 | 11.3 | 9.4 |
| L, mm | 19.5 | 17.0 | 14.6 | 10.1 | 14.0 | 11.3 | 13.2 | 9.5 | 13.9 | 9.3 | 15.4 | 9.5 |
| V,
cm3 | 5.25 | 2.54 | 2.31 | 0.61 | 1.57 | 0.70 | 1.65 | 0.61 | 1.58 | 0.55 | 1.66 | 0.46 |
Discussion
A complete literature review was conducted using
computer search engines in PubMed (http://www.ncbi.nlm.nih.gov/pubmed) to identify all
cases of VS in NF2 treated with bevacizumab. The search was
performed using single or combined search terms, including
‘bevacizumab’, ‘bilateral vestibular schwannomas’,
‘neurofibromatosis 2’ and ‘adverse effects’. In total, 6 relevant
reports comprising 39 cases of VS in NF2, published between 2010
and 2014, were included (3,5–9). The
clinical characteristics are summarized in Table II, including drug dose, number of
patients, treatment duration, mean age and adverse effects.
Radiological response and hearing status are detailed in Table III. All patients received
bevacizumab 5 mg/kg/2 weeks by IV. The mean age was 26±2.2 years
(range, 12–73 years). The median duration of treatment was 12
months, and 85% of the patients (33/39) experienced tumor
regression. The median tumor volume reduction was 27.5% (range,
3–91%). The solid component regressed less than the cystic
component of the tumor (8). However,
the findings also indicate that tumor regrowth may occur after drug
discontinuation (9). The rate of
hearing improvement was 45.2% (14/31), whilst the rate of hearing
stability was 48.4% (15/31). Hearing loss was observed during
treatment intermission and disappeared after treatment resumed
(7). Plotkin et al (7) reported bevacizumab treatment for
progressive VS in 31 patients with a 3-year follow-up. The rates of
tumor stability or regression were 88% at 1 year, 67% at 2 years
and 54% at 3 years. The rates of hearing stability or improvement
were 90% at 1 year, 81% at 2 years, and 61% at 3 years. However,
168 adverse events were identified during 572 patient-months of
treatment. The frequency of adverse events was high, demonstrating
how adverse effects have become an obstacle for the clinical use of
bevacizumab.
 | Table II.Summary of patient characteristics and
adverse effects in previous studies. |
Table II.
Summary of patient characteristics and
adverse effects in previous studies.
| Author, year | Dose | Patients, n | Median duration
(range), months | Mean age (range),
years | Adverse effects | Ref. |
|---|
| Subbiah et al,
2012 | 5 mg/kg IV/2 wk | 2a | 9.5 (9–10) | 28.5 (16–41) | No significant
adverse effects | (5) |
| Eminowicz et
al, 2012 | 5 mg/kg IV/2 wk | 2 | 3.5 (3–4) | 34 (31–37) | No significant
adverse effects | (6) |
| Plotkin et al,
2012 | 5 mg/kg IV/2 wk | 31 | 14 (6–41) | 26 (12–73) | Hypertension,
proteinuria, menorrhagia, epistaxis, pneumonia | (7) |
| Plotkin et al,
2009 | 5 mg/kg IV/2 wk | 10 | 12 (3–19) | 25 (16–53) | Hypertension,
proteinuria, menorrhagia, epistaxis, pneumonia | (3) |
| Mautner et al,
2010 | 5 mg/kg IV/2 wk | 2 | 4.5 (3–6) | 32 (24–40) | No significant
adverse effects | (8) |
| Mautner et al,
2010 | 5 mg/kg IV/2–4
wk | 2 | 15 (12–18) | 30 (22–38) | Hypertension | (9) |
 | Table III.Summary of radiological response and
hearing status after the treatment. |
Table III.
Summary of radiological response and
hearing status after the treatment.
|
|
|
| Hearing status, %
(n)a |
|
|---|
|
|
|
|
|
|
|---|
| Author, year | Tumor reduction,
median (range) | No. of patients
experiencing tumor regression | Improved | Stable | Declined | Ref. |
|---|
| Subbiah et
al, 2012 | Stable | 0 of 2 | – | 100 (2 of 2) | – | (5) |
| Eminowicz et
al, 2012 | 30.5% (10–52%) | 2 of 2 | – | 100 (2 of 2) | – | (6) |
| Plotkin et
al, 2012b | 26%
(3–91%) | 27 of 31 | 57 (13 of 23) | 35 (8 of 23) | 8 (2 of 23) | (7) |
| Plotkin et
al, 2009 | 26%
(5–44%) | 9 of
10 | 57 (4 of 7) | 29 (2 of 7) | 14 (1 of 7) | (3) |
| Mautner et
al, 2010 | 42%
(41–43%) | 2 of 2 | – | 100 (2 of 2) | – | (8) |
| Mautner et
al, 2010 | 47.5% (43–52%) | 2 of
2c | 50 (1 of
2)d | 50 (1 of 2) | – | (9) |
The NF2 tumor suppressor gene has been shown to
reside on chromosome 22q12.2, with the highest mutation rate among
human genetic diseases (10). The
aberration of the NF2 gene may lead to neoplasia of ectodermal and
mesodermal tissues during embryonic development phases by affecting
a variety of signal transduction pathways associated with tumor
genesis and progression, including the phosphoinositide
3-kinase/protein kinase B/mammalian target of rapamycin pathway and
the Raf/Ras/mitogen-activated protein kinase kinase pathway
(5,11). Loss of a functional NF2 gene product,
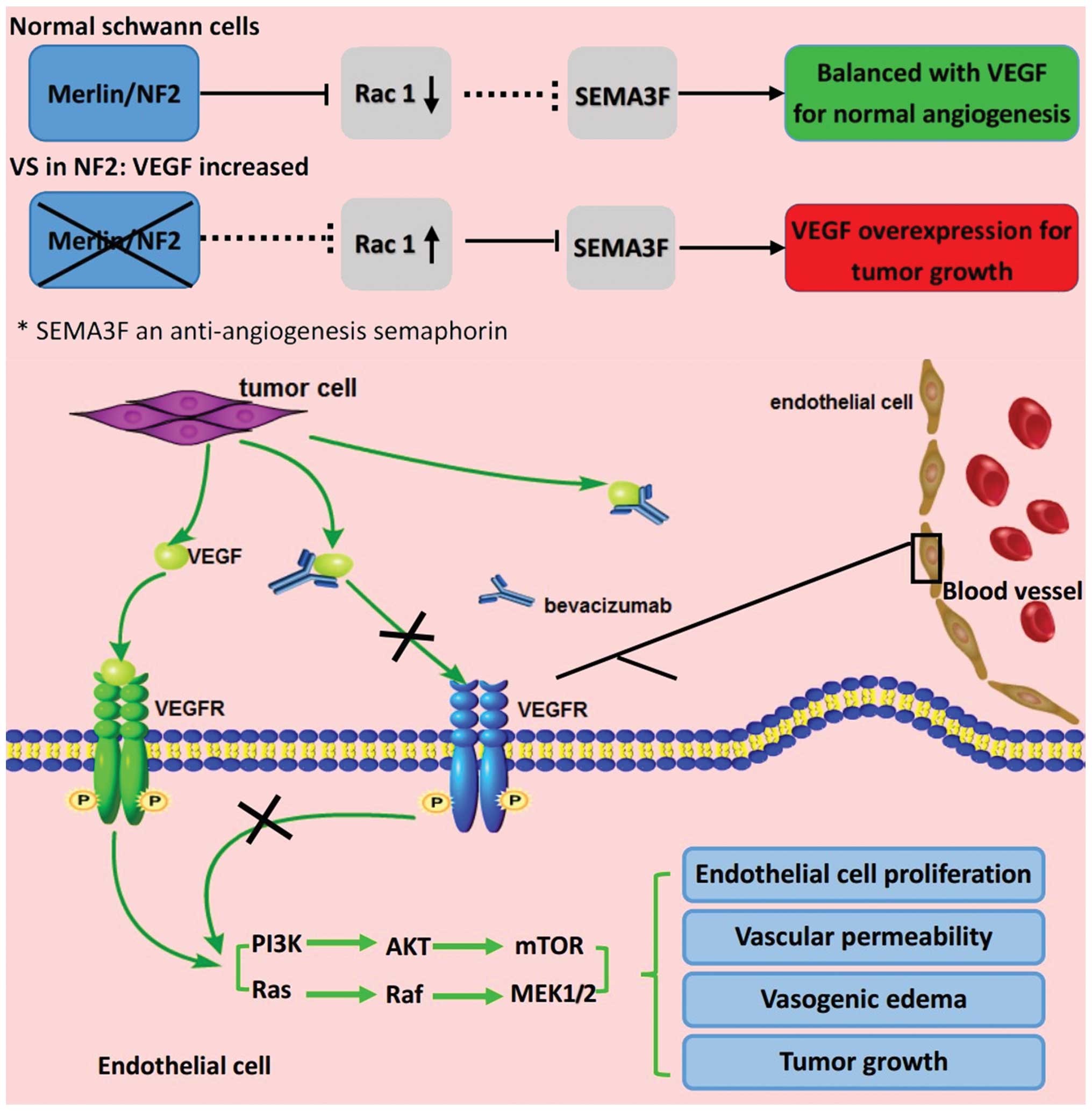
Merlin (a tumor suppressor protein), may inhibit the expression of
anti-angiogenesis semaphorin-3F by up regulating the Rac1 pathway
in VS, resulting in a relative surplus of VEGF (12). Furthermore, tumor necrosis occurs when
a tumor's blood supply cannot satisfy its growth, which may
activate the compensatory overexpression of VEGF to improve the
hypoxic microenvironment (12).
Hence, VEGF blockade using bevacizumab may normalize the
vascularity and decrease edema (Fig.
4) (12).
Microsurgery for tumor resection and stereotactic
radiotherapy are usually recommended for VS in NF2 (13). However, patients must abandon hearing
in order to achieve tumor control, and are also subject to possible
postoperative complications, such as cerebrospinal fluid leak and
facial weakness. Unfortunately, postoperative tumor recurrence may
induce the need for reoperation in some patients, resulting in a
decline in quality of life (14). In
the current study, anti-angiogenesis therapy with bevacizumab
objectively induced tumor regression and hearing preservation in a
case of VS in NF2. A low-dose regimen was beneficial for avoiding
significant adverse effects, providing an alternative treatment
strategy instead of surgical intervention.
Compared with baseline measurements prior to
treatment, the bilateral VS regressed by 3.59 cm3 (68%)
and 2.08 cm3 (82%) on the left and right, respectively
(Fig. 1; Table I). The absolute change in tumor volume
was not as significant as the relative change; as the tumors in the
current patient were not very large, a small absolute volume change
may result in a large relative percentage reduction in tumor volume
compared with the baseline. To avoid the potential bias in tumor
volume analysis, relative percentage and absolute volume changes
from baseline were used for comparison. The tumor volume remained
stable in last 10 months without further regression. This suggests
that tumor stability may be a compromise between tumor regression
induced by low-dose regimen and tumor growth.
Although no objective hearing improvement was
detected in the patient, subjective hearing improvement did occur.
Furthermore, the patient's hearing declined during treatment
intermission and recovered after treatment was resumed, while the
tumor volume remained stable. Thus, we hypothesized that hearing
stability was drug-dependent. It is possible that higher-dose
regimens may be helpful for hearing improvement, whilst low-dose
regimens are not. In addition, the biological mechanisms underlying
the effects of bevacizumab for hearing stability may be different
from those for tumor regression, which have been reported
previously (15).
Whether bevacizumab may be used for long-term
treatment remains controversial due to the risk of adverse effects,
which include hypertension, proteinuria, thrombosis and hemorrhage
(7). Furthermore, considering tumor
recurrence following drug discontinuation (9), the optimal dose and duration of
treatment is undetermined. As reported by Zuniga et al
(16), disease recurrence and more
aggressive progression were also detected in the treatment of
malignant glioma following bevacizumab discontinuation. However, no
evidence has indicated that bevacizumab discontinuation leads to
accelerated disease progression. Therefore, the current patient is
undergoing close monitoring to observe the efficacy of low-dose
regimen in avoiding severe adverse effects and tumor recurrence
following drug discontinuation.
Given the adverse effects induced by systemic IV
administration of bevacizumab and the insufficient drug
concentration at the tumor site due to the blood-tumor barrier
(BTB), Riina et al (17)
reported a novel approach involving super-selective intra-arterial
cerebral infusion of bevacizumab following BTB disruption. Of 3
patients, 1 experienced tumor regression of 11% and 19% on the left
and right, respectively. All 3 patients presented hearing
improvement or stability. Appropriate cerebrovascular
interventional therapy may be better in certain circumstances. For
example, with regard to local chemotherapy, cerebrovascular
interventional therapy results in less neurotrauma than craniotomy.
Furthermore, cerebrovascular interventional therapy may benefit
patients exhibiting tumors which extend into the internal auditory
canal, as gross total resection is difficult, patients that cannot
tolerate the adverse effects induced by systemic chemotherapy, and
patients with a Karnofsky performance scale score of ≥60 (18).
As the pathogenesis of NF2 depends on multiple
signaling pathways, the combination of bevacizumab with other
molecular targeted drugs, such as erlotinib and lapatinib
(inhibitors of epidermal growth factor receptor), may generate
synergistic effects (5,19). However, the efficacy of this treatment
must be confirmed by clinical trials containing a larger cohort of
patients.
For the patients who exhibit no response to
bevacizumab, a surgical intervention is recommended following a
4-6-week drug metabolism phase to minimize the risk of
postoperative hemorrhage and delayed wound healing (6,8).
In summary, a low-dose regimen of bevacizumab (2.2
mg/kg/4 weeks, IV) would appear to be promising for patients with
VS in NF2, given the adverse effects triggered by bevacizumab
(4). However, the minimum dose
required to sustain a response to bevacizumab in NF2 patients is
still unknown, and may differ from patient to patient. Finding the
minimum effective dose for individual patients sufficient to
sustain hearing and/or volumetric response would aid in decreasing
toxicity and long-term tolerability. With the scientific
advancements, more comprehensive and safe regimens may be
determined.
Acknowledgements
The authors would like to thank Dr Shilei Ni from
the Department of Neurosurgery, Qilu Hospital (Jinan, China) for
the valuable suggestions on study design.
References
|
1
|
Di Maio S, Mrak G, Juric-Sekhar G, Born D,
Mantovani A and Sekhar LN: Clinicopathologic assay of 15 tumor
resections in a family with neurofibromatosis type 2. J Neurol Surg
B Skull Base. 73:90–103. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Claudio PP, Russo G, Kumar CA, Minimo C,
Farina A, Tutton S, Nuzzo G, Giuliante F, Angeloni G, Maria V, et
al: pRb2/p130, vascular endothelial growth factor, p27 (KIP1) and
proliferating cell nuclear antigen expression in hepatocellular
carcinoma: Their clinical significance. Clin Cancer Res.
10:3509–3517. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Plotkin SR, Stemmer-Rachamimov AO, Barker
FG II, Halpin C, Padera TP, Tyrrell A, Sorensen AG, Jain RK and di
Tomaso E: Hearing improvement after bevacizumab in patients with
neurofibromatosis type 2. N Engl J Med. 361:358–367. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yonezawa S, Miwa K, Shinoda J, Nomura Y,
Asano Y, Nakayama N, Ohe N, Yano H and Iwama T: Bevacizumab
treatment leads to observable morphological and metabolic changes
in brain radiation necrosis. J Neurooncol. 119:101–109. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Subbiah V, Slopis J, Hong DS, Ketonen LM,
Hamilton J, McCutcheon IE and Kurzrock R: Treatment of patients
with advanced neurofibromatosis type 2 with novel molecularly
targeted therapies: From bench to bedside. J Clin Oncol.
30:e64–e68. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eminowicz GK, Raman R, Conibear J and
Plowman PN: Bevacizumab treatment for vestibular schwannomas in
neurofibromatosis type two: Report of two cases, including
responses after prior gamma knife and vascular endothelial growth
factor inhibition therapy. J Laryngol Otol. 126:79–82. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Plotkin SR, Merker VL, Halpin C, Jennings
D, McKenna MJ, Harris GJ and Barker FG II: Bevacizumab for
progressive vestibular schwannoma in neurofibromatosis type 2: A
retrospective review of 31 patients. Otol Neurotol. 33:1046–1052.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mautner VF, Nguyen R, Kutta H, Fuensterer
C, Bokemeyer C, Hagel C, Friedrich RE and Panse J: Bevacizumab
induces regression of vestibular schwannomas in patients with
neurofibromatosis type 2. Neuro Oncol. 12:14–18. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mautner VF, Nguyen R, Knecht R and
Bokemeyer C: Radiographic regression of vestibular schwannomas
induced by bevacizumab treatment: Sustain under continuous drug
application and rebound after drug discontinuation. Ann Oncol.
21:2294–2295. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ferrer M, Schulze A, Gonzalez S, Ferreiro
V, Ciavarelli P, Otero J, Giliberto F, Basso A and Szijan I:
Neurofibromatosis type 2: Molecular and clinical analyses in
argentine sporadic and familial cases. Neurosci Lett. 480:49–54.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Akhmametyeva EM, Mihaylova MM, Luo H,
Kharzai S, Welling DB and Chang LS: Regulation of the
neurofibromatosis 2 gene promoter expression during embryonic
development. Dev Dyn. 235:2771–2785. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
London NR and Gurgel RK: The role of
vascular endothelial growth factor and vascular stability in
diseases of the ear. Laryngoscope. 124:E340–E346. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Carlson ML, Tveiten OV, Driscoll CL,
Goplen FK, Neff BA, Pollock BE, Tombers NM, Castner ML, Finnkirk
MK, Myrseth E, et al: Long-term quality of life in patients with
vestibular schwannoma: An international multicenter cross-sectional
study comparing microsurgery, stereotactic radiosurgery,
observation, and nontumor controls. J Neurosurg. 122:833–842. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Narita Y: Drug review: Safety and efficacy
of bevacizumab for glioblastoma and other brain tumors. Jpn J Clin
Oncol. 43:587–595. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fisher LM, Doherty JK, Lev MH and Slattery
WH: Concordance of bilateral vestibular schwannoma growth and
hearing changes in neurofibromatosis 2: Neurofibromatosis 2 natural
history consortium. Otol Neurotol. 30:835–841. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zuniga RM, Torcuator R, Jain R, Anderson
J, Doyle T, Ellika S, Schultz L and Mikkelsen T: Efficacy, safety
and patterns of response and recurrence in patients with recurrent
high-grade gliomas treated with bevacizumab plus irinotecan. J
Neurooncol. 91:329–336. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Riina HA, Burkhardt JK, Santillan A,
Bassani L, Patsalides A and Boockvar JA: Short-term
clinico-radiographic response to super-selective intra-arterial
cerebral infusion of bevacizumab for the treatment of vestibular
schwannomas in neurofibromatosis type 2. Interv Neuroradiol.
18:127–132. 2012.PubMed/NCBI
|
|
18
|
Burkhardt JK, Riina HA, Shin BJ, Moliterno
JA, Hofstetter CP and Boockvar JA: Intra-arterial chemotherapy for
malignant gliomas: A critical analysis. Interv Neuroradiol.
17:286–295. 2011.PubMed/NCBI
|
|
19
|
Lim SH, Ardern-Holmes S, McCowage G and de
Souza P: Systemic therapy in neurofibromatosis type 2. Cancer Treat
Rev. 40:857–861. 2014. View Article : Google Scholar : PubMed/NCBI
|