Introduction
Desmoplastic small round cell tumors (DSRCTs) are
rare and aggressive malignant tumors with a poor prognosis, which
were initially described by Gerald and Rosai in 1989 (1). Currently, ~200 cases have been reported
in the literature (2). DSRCT
primarily affects young males between the ages of 15–25 years
(3,4).
The most commonly affected region is the pelvis, with other sites
including the omentum, the retroperitoneal space and the mesentery
(3–6).
The manifestations of DSRCT are non-specific, and patients
typically present with vague abdominal or pelvic discomfort,
including abdominal pain and/or distension, ascites, constipation
and urinary disorders (2,6,7).
Histologically, DSRCT is characterized by well-defined nests or
clusters of small, round tumor cells embedded in an abundant,
desmoplastic stroma (8,9). DSRCT is associated with the reciprocal
chromosomal translocation t(11:22)(p13;q12), which involves the
EWSR1 and WT1 genes (9). The
prognosis of patients with DSRCT is poor, with a mean survival time
of <30 months (10). Postoperative
radiotherapy and chemotherapy are reported to have no survival
advantage, and successful surgical excisions are extremely rare
(10,11). Previous studies of DSRCT have focused
on its pathological features (1,8,9), however, few studies have assessed the
computed tomography (CT) and fluorodeoxyglucose positron emission
tomography (FDG-PET)/CT results of this disease (3,4,6,10–14). The present study aimed to characterize
the CT and FDG-PET/CT imaging results of 4 DSRCT patients, and to
associate these observations with the pathological findings.
Materials and methods
Patients
The present study retrospectively reviewed 4
patients with DSRCT, clinically diagnosed by histopathology, who
were treated at the Affiliated Hospital of Qingdao University
Medical School (Shandong, China) between January 1, 2009, and March
31, 2011. All patients were male, with a mean age of 22.25 years
(range, 14–31 years). Clinical manifestations included an abdominal
mass (n=2), abdominal pain and distension (n=1), and jaundice
(n=1).
CT scanning and image analysis
Plain and triple-phase dynamic CT scans were
performed using a 64-slice CT scanner (SOMATOM Sensation Cardiac
64; Siemens, Munich Germany) in 3 patients or using a 16-slice CT
scanner (Brightspeed; GE Healthcare Bio-Sciences, Pittsburgh, PA,
USA) in 1 patient. The CT scan included a section thickness of 5
mm, a pitch of 1.375:1 and a field of view of 248×330 mm. Patients
were administered 1L of 3% meglumine diatrizoate (Shanghai Xudong
Haipu Pharmaceutical Co. Ltd., Shanghai, China), an oral contrast
agent, a total of 30 min prior to examination. Patients were
additionally administered 100 ml iopromide (Ultravist 300; Bayer
HealthCare Pharmaceuticals, Berlin, Germany), a non-ionic iodinated
contrast material. All patients initially underwent plain CT
scanning, followed by triple-phase CT examination that included
arterial, portal and delayed phases. Contrast material was
administered at a rate of 3.0 ml/sec with an automatic power
Ultravist® 300 injector (Bayer AG, Berlin, Germany).
Enhanced CT was performed in the arterial, portal and delayed
phases, with a delay time of 25, 60 and 120 sec, respectively,
following initiation of injection of the contrast materials. All CT
images were reviewed retrospectively by 2 professional radiologists
each with >20 years experience in abdominal CT studies. The
imaging results were evaluated for tumor location, shape, size,
number, margin, density and intensity of contrast enhancement. When
compared with adjacent tissue, the tumor density on plain CT was
defined as low density, isodensity or high density. The intensity
of enhancement, when performing contrast-enhanced CT, was classed
as no, mild, moderate or distinct enhancement.
FDG-PET/CT scanning and image
analysis
FDG-PET/CT was performed in 1 patient using a
dedicated PET/CT system (Discovery ST 16; GE Healthcare
Bio-Sciences). The patient was instructed to fast for at least 6 h
prior to injection of 18F-FDG. The patient's blood
glucose level was <11.1 mmol/l (normal range, 4.2–6.9 nmol/l).
Following intravenous injection of 18F-FDG (0.14
mCi/kg), the patient lay comfortably in a quiet and dark room for 1
h. PET, CT and fused PET/CT images of the whole body were reviewed
by 2 nuclear medicine physicians. The imaging results were
evaluated for tumor location, shape, size, number, margin, density
and maximum standard uptake value (SUVmax) of all lesions.
Histopathology
The tumors in 3 patients were resected totally (2
cases) or partially (1 case), and CT-guided needle biopsy of the
tumor was performed in the fourth patient. All cases demonstrated
no complications following tumor resection. Gross examination,
including analysis of tumor shape, size, number, margin and capsule
wall, was conducted in all specimens prior to routine histological
evaluation. The samples were fixed in formalin (Liaocheng Jianhua
Chemical Products Co., Ltd., Shandong, China), embedded in paraffin
(Taicang City Haotian Technology Co., Ltd., Jiangsu, China), and
stained with hematoxylin and eosin (Labest Biotechnology Co., Ltd,
Beijing, China). A BX43 microscope (Olympus Corporation, Tokyo,
Japan) was used to observe specimens. Immuno-histopathological
examinations were additionally performed, which included assays for
mouse anti-human anti-cluster of differentiation (CD)99 monoclonal
antibody (catalog no., ZM-0296), mouse anti-human anti-neuron
specific encolase (NSE) monoclonal antibody (catalog no., ZM-0203),
mouse anti-cytokeratin monoclonal antibody (catalog no., ZM-0069),
mouse anti-epithelial membrane antigen (EMA) monoclonal antibody
(catalog no., ZM-0095), mouse anti-desmin monoclonal antibody
(catalog no., ZM-0091) and mouse anti-vimentin monoclonal antibody
(catalog no., ZM-0260) (dilution, 1:200; Beijing Zhongshan Golden
Bridge Biotechnology Co., Ltd., Beijing, China).
Results
CT imaging findings
CT images revealed multiple intra-abdominal nodules
and/or masses with soft tissue density in all patients, and these
masses exhibited vague margins with adjoining organs. The tumor
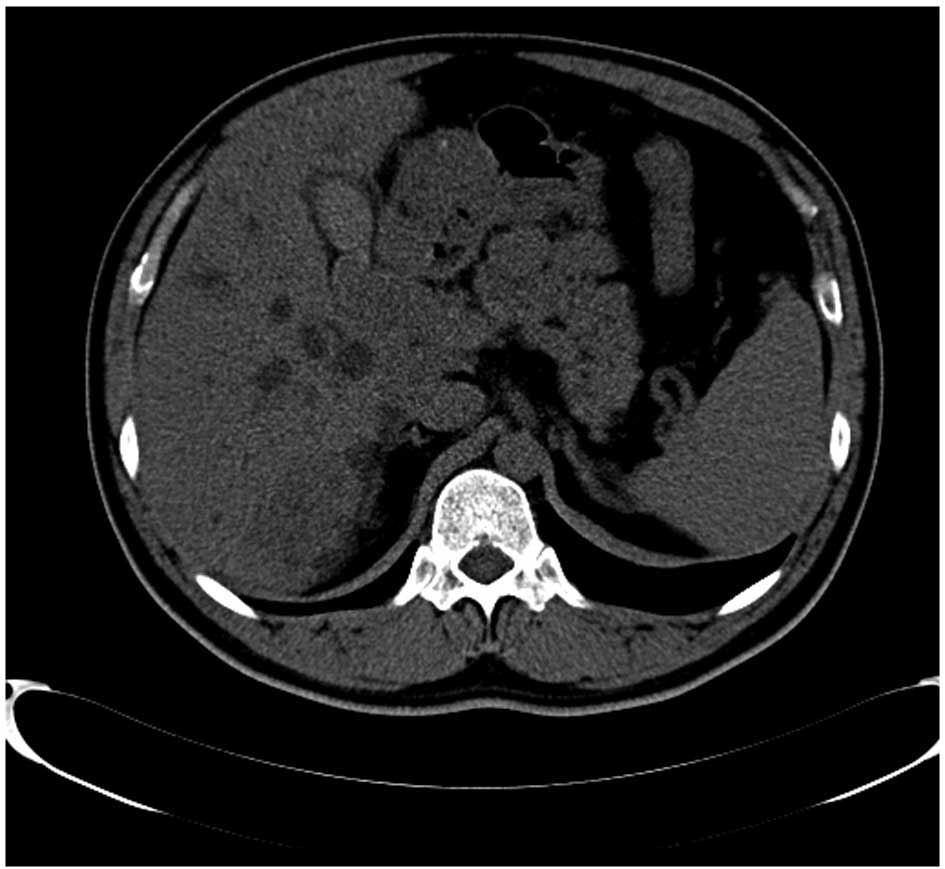
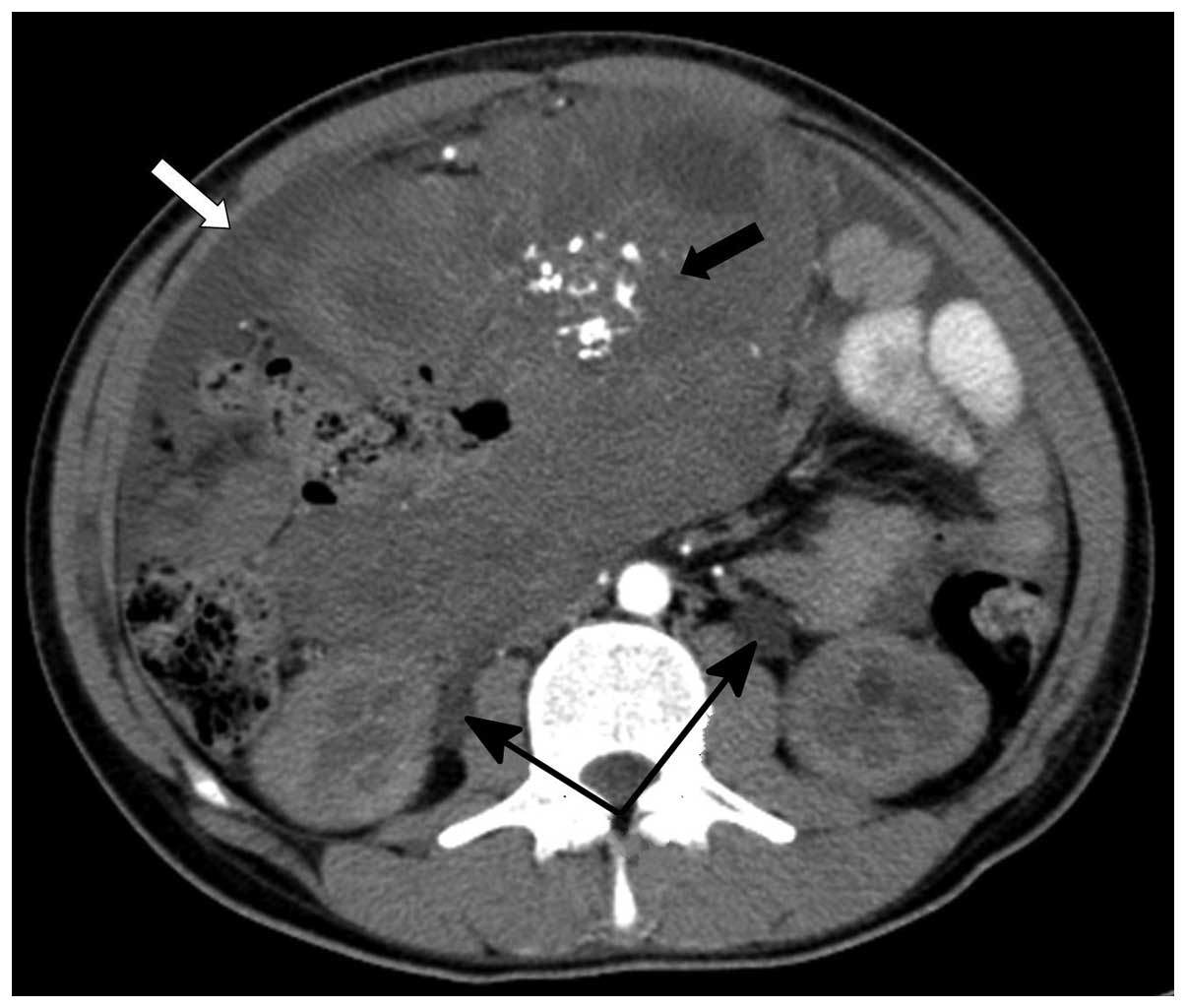
size was variable, with a maximum size of 170×170 mm (Fig. 1). Multi-node and patchy calcifications
were observed inside the foci in 2 cases (Fig. 2). Nodular thickening of the peritoneum
in 3 patients was noted as mild or moderate enhancement in the
contrast-enhanced CT. Multiple concurrent masses in the liver were
identified in 3 patients, with a maximum tumor size of 117×118 mm.
Although the majority of these masses were located close to the
superficial layer of the liver, 1 patient presented with a mass
occupying the hepatic hilum, which caused dilation of the
intrahepatic bile duct (Fig. 1). A
total of 3 patients exhibited multiple nodules with soft tissue
density surrounding the retroperitoneal abdominal aorta.
Contrast-enhanced CT of the tumor masses and nodules appeared as
mild to moderate edge enhancement of the lesion, however, a lower
density and no appearance of enhancement was observed within the
centre of the larger lesions (Figs. 2
and 3). Furthermore, 1 patient with a
large tumor in the pelvic cavity demonstrated dilation of the
proximal ureters, renal pelvicalyses and hydronephrosis due to
pressure on the bilateral ureters (Fig.
2). Furthermore, 2 patients exhibited a low level of abdominal
ascites.
FDG-PET/CT imaging findings
PET/CT imaging revealed multiple nodular foci of FDG
uptake in the abdominopelvic cavity, liver and peritoneum (Fig. 4). The SUVmax of all masses ranged
between 4.0 and 12.9.
Intraoperative results
Large intra-abdominal masses were observed in all 3
patients who underwent tumor resection, and these masses adhered to
the omentum and adjacent organs. Numerous firm nodules of variable
size were distributed on the surface of the abdominal viscera and
omentum. In addition, multiple firm masses were identified inside
the liver in 2 patients, 1 of whom was treated with a complete
resection of the intrahepatic tumor.
Histological results and
immunohistochemistry findings
In terms of gross appearance, the surgically removed
tumor masses from 3 patients were poorly circumscribed, grey-white
in color and had a crisp texture. The majority of tumor masses
exhibited areas of tissue necrosis in the centre of the lesion, and
a number of tumor nodules had a greyish-yellow appearance, similar
to rotting flesh.
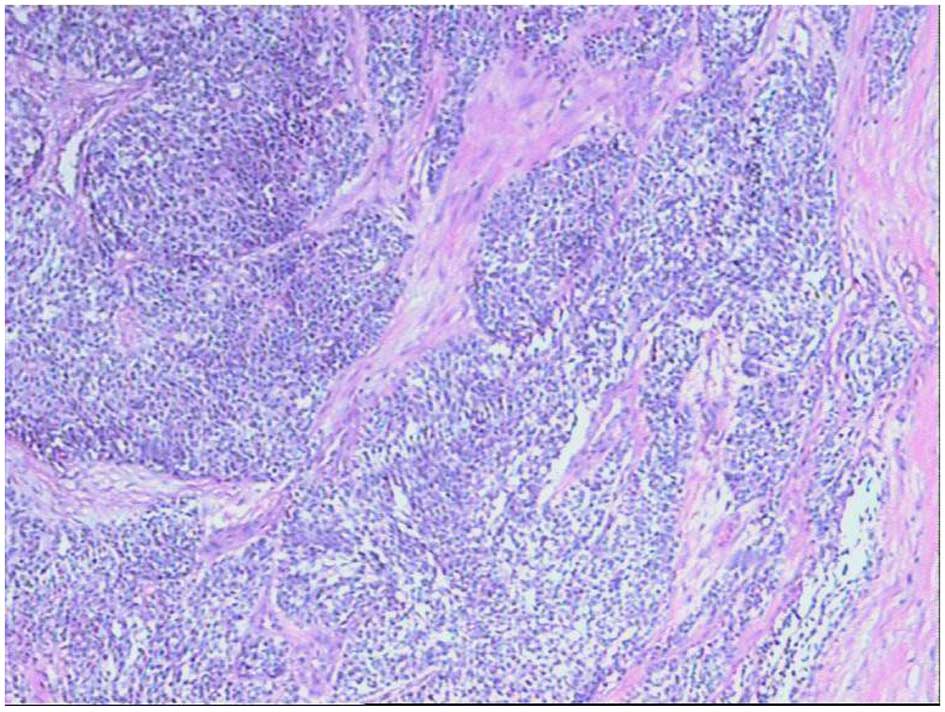
Microscopically, the DSRCT cells varied in size and
spherical or ovoid in appearance; however, there were additionally
a small number of spindle-shaped cells. The tumor cells had reduced
cytoplasm, and acidophilic and hyperchromatic nuclei. Clusters of
these undifferentiated tumor cells infiltrated the surrounding
dense connective tissue, and appeared as solid nests that were
well-defined and had differing shapes and sizes (Fig. 5).
Immunohistochemistry revealed positive results for
NSE, CK, and EMA expression in the DSRCT cells from all 4 patients,
whereas only 2 cases demonstrated positive staining for either CD99
or desmin. In addition, vimentin was strongly expressed in only 1
patient.
Discussion
DSRCT is a rare and highly malignant neoplasm, which
commonly arises in the peritoneal cavity and has a poor prognosis.
It is now widely recognised to be a member of the family of small
round blue cell tumors (12).
Previous studies have indicated that DSRCT primarily
occurs in male adolescents and young adults (male:female, 4–5:1)
(12,15,16),
however, a case has been reported in a 65-year-old woman (17). The clinical findings are non-specific,
and the disease most commonly presents as abdominal pain and
distension, ascites and hydronephrosis (3–15). In the
present study, the patients (male adolescents and young adults with
an age range of 14–31 years) complained of abdominal pain and
distension, and the presence of an abdominal mass, with the
exception of 1 patient, who presented with jaundice due to
oppression of the hepatic bile duct by tumor masses.
DSRCT shows a higher rate of occurrence on serosal
surfaces, where it appears as single or multiple nodular masses on
the surface of intra-abdominal organs (12). The tumor may additionally occur in the
omentum, with involvement of the surrounding viscera. Other common
areas of involvement are the scrotum, lungs, chest wall, skull,
pleura, mediastinum, thighs, soft tissues, bones, sinonasal
regions, ovaries, kidneys and parotid glands, as well as the
retroperitoneal space (12).
DSRCT frequently disseminates along the peritoneum
or invades neighbouring organs inside the peritoneal cavity. Local
metastasis of the tumor most commonly occurs in the liver, but may
be observed in abdominal and pelvic lymph nodes, with varying
degrees of ascites (5,12). Although the hematogenous metastasis of
tumor cells is rare, there have been numerous reports of metastasis
to remote organs, including the lungs, pleura, ilium and scrotum
(9,12,14).
In the present study, multiple intra-abdominal
nodules and masses were noted by the appearance of soft tissue
density on CT scanning and variable tumor sizes in all 4 patients,
and 3 cases showed involvement of the pelvic cavity. These 3
patients had exhibited nodular thickening of the peritoneum and
adhesion between the tumor and omentum when the tumor occurred in
the peritoneal cavity. Disseminated tumor masses on the visceral
surfaces were frequently multifocal and of varying sizes. Multiple
concurrent masses in the liver were observed in 3 patients,
featuring a maximum tumor size of 117×118 mm. Although the majority
of masses were located close to the superficial layer of the liver,
1 patient presented with a mass that occupied the hepatic hilum,
which caused dilation of the intrahepatic bile duct. The remaining
3 patients presented with slightly enlarged retroperitoneal lymph
nodes. The patient with a large mass in the pelvic cavity showed
dilation of the proximal ureters, renal pelvicalyses and
hydronephrosis due to pressure on the bilateral ureters. In
addition, 2 patients exhibited low levels of abdominal ascites.
CT scanning is the most frequently used method for
the diagnosis of abdominal DSRCT (7).
In plain CT, DSRCT appears as multiple and lobulated soft tissue
masses in the abdomen, pelvic cavity or retroperitoneal space, with
no clear site of origin (4). The
tumors are of varying sizes (up to 40 cm in diameter) and appear
with non-homogeneous density on CT images. The central areas of the
foci result in a diverse range of low-density images, which
correspond to hemorrhagic necrosis in gross specimens (14). Under contrast-enhanced CT, DSRCT
appears with mild-to-moderate or heterogeneous image enhancement,
although larger nodules or masses show only edge enhancement
(6,7,14).
Pickhardt et al (6) reported
that 7/9 patients showed a lower density change in the centre of
the tumor. The results of the present study are consistent with
this previous study. Contrast-enhanced CT scanning of tumor masses
and nodules revealed mild or moderate edge enhancement of the
lesion, and lower density or no appearance of enhancement within
the central area of larger lesions in the abdominal cavity and
liver, which represented the area of necrosis in the gross
specimens. Multinodal and patchy calcifications were observed
inside the foci in 2 cases, as reported previously (3,9).
Numerous CT findings of diagnostic importance exist
for DSRCT, including an adolescent age of onset, calcification of
the tumor tissue, extensive involvement of the peritoneum and the
absence of a clear site of origin. Zhang et al (3) and Bellah et al (12) suggested that the involvement of the
retrovesical region may be valuable for the diagnosis of DSRCT. In
the present study, 3 patients were diagnosed with DSRCT with
involvement of the peritoneal and retrovesical regions.
FDG-PET/CT has a significant role in tumor staging
and the identification of occult lesions that cannot be detected by
CT or magnetic resonance imaging (MRI) (3,18). In the
present study, masses with high FDG uptake and an SUVmax of
4.0–12.9 were indicative of malignancy. Compared with CT,
FDG-PET/CT is able to reveal smaller metastases and can distinguish
tumors from fibroses by whole-body metabolic imaging (3). PET/CT may be used for staging of DSRCT,
and is an improved tool compared with CT or MRI (3,10).
Additional studies utilizing FDG-PET/CT imaging are required.
Previous studies utilizing CT and FDG-PET/CT imaging
in abdominal DSRCT are rare, therefore, little diagnostic
experience is available for clinicians (10,12). The
radiological differential diagnosis for DSRCT is complicated by
other abdominal and retroperitoneal tumors, including
rhabdomyosarcoma, peritoneal leiomyosarcoma, mesothelioma,
intra-abdominal desmoid tumor, primitive neuroectodermal tumors
(PNETs), lymphoma and neuroblastoma (6,7,12). Rhabdomyosarcoma is primarily observed
in infants (70% of cases occur in children aged <10 years)
(19). Although these tumors may
involve the peritoneum (~10%), typical nodules and masses are
generally smaller compared with DSRCT, and calcification of tumor
tissue is rarely observed (7,12). Peritoneal leiomyosarcoma typically
affects women aged >24 years, however, DSRCT tends to occur in
male adolescents (12).
Leiomyosarcoma frequently appears as multiple well-defined nodules
or lumps inside the peritoneal cavity or along the mesenterium
(12). These tumors are prone to
metastasis, primarily by hematogenous or lymphatic routes, although
it is difficult to observe implantation metastases in the abdominal
wall. Malignant mesothelioma rarely occurs in patients aged <20
years (20). This tumor accounts for
~15% of tumors involving the peritoneum, and is typically
complicated by high levels of ascites. An intra-abdominal desmoid
tumor is a rare, benign proliferation of fibrous tissue, which is
typically solitary or associated with Gardner's syndrome (12). Intra-abdominal desmoid tumors that
occur inside the pelvic cavity, retroperitoneal space or on the
abdominal wall frequently manifest as single or multiple masses,
with identical or reduced density compared with normal muscle
tissue. These masses rarely show necrotic or cystic alterations,
even inside large tumor masses (12).
Furthermore, the absence of metastases is of differential
diagnostic value in DSRCT patients. PNETs primarily affects
adolescents and young adults, and is a highly aggressive tumor
(12). Although the CT
characteristics of PNETs are similar to those of DSRCT, the
occurrence of tumor calcification is rare in PNETs (12). Lymphoma, unlike DSRCT, frequently
occurs in middle-aged men, and manifests as enlarged lymph nodes in
the abdominal cavity and retroperitoneal space during the early
stages of the disease (12,21). By contrast, in its early stages, DSRCT
frequently appears as a solid mass with no sign of lymph node
enlargement (7,12). In addition, a distinguishing feature
of lymphoma is the occurrence of spleen enlargement prior to tumor
infiltration. Tumor calcification is also rarely observed in
lymphoma. Neuroblastoma commonly affects infants, with 79% of
patients aged <4 years (average age, 22 months), and typically
appears as a single, paravertebral mass (12).
In conclusion, DSRCT is a rare and highly malignant
neoplasm, which typically affects male adolescents and young
adults. CT results of abdominal DSRCT are relatively characteristic
and may assist with diagnosis. The CT imaging characteristics
include, but are not limited to, multiple soft-tissue masses in the
abdominal or pelvic cavity or retroperitoneal space, no clear site
of origin, mild or moderate edge enhancement under
contrast-enhanced CT, multinodal and patchy calcification of the
tumor, adjoining organ involvement, and implantation metastases of
the abdominal wall and/or hepatic metastasis. When a tumor with
these characteristics is identified, particularly in adolescents
and young adults, a diagnosis of DSRCT should be suspected.
FDG-PET/CT may have a significant role in tumor staging.
References
|
1
|
Gerald WL and Rosai J: Case 2.
Desmoplastic small cell tumor with divergent differentiation.
Pediatr Pathol. 9:177–183. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Briseño-Hernández AA, Quezada-López DR,
Corona-Cobián LE, Castañeda-Chávez A, Duarte-Ojeda AT and
Macías-Amezcua MD: Intra-abdominal desmoplastic small round cell
tumour. Cir Cir. 83:243–248. 2015.(In Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang WD, Li CX, Liu QY, Hu YY, Cao Y and
Huang JH: CT, MRI, and FDG-PET/CT imaging findings of
abdominopelvic desmoplastic small round cell tumors: Correlation
with histopathologic findings. Eur J Radiol. 80:269–273. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kis B, O'Regan KN, Agoston A, Javery O,
Jagannathan J and Ramaiya NH: Imaging of desmoplastic small round
cell tumor in adults. Br J Radiol. 85:187–192. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mainenti PP, Romano L, Contegiacomo A,
Romano M, Casella V, Cuccuru A, Insabato L and Salvatore M: Rare
diffuse peritoneal malignant neoplasms: CT findings in two cases.
Abdom Imaging. 28:827–830. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pickhardt PJ, Fisher AJ, Balfe DM, Dehner
LP and Huettner PC: Desmoplastic small round cell tumor of the
abdomen: Radiologic-histopathologic correlation. Radiology.
210:633–638. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chouli M, Viala J, Dromain C, Fizazi K,
Duvillard P and Vanel D: Intra-abdominal desmoplastic small round
cell tumors: CT findings and clinicopathological correlations in 13
cases. Eur J Radiol. 54:438–442. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim HJ, Sohn BS, Kwon JE, Kim JY and Park
K: ThinPrep cytological findings of desmoplastic small round cell
tumor with extensive glandular differentiation: A case study.
Korean J Pathol. 47:182–187. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dufresne A, Cassier P, Couraud L,
Marec-Bérard P, Meeus P, Alberti L and Blay JY: Desmoplastic small
round cell tumor: Current management and recent findings. Sarcoma.
2012:7149862012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ben-Sellem D, Liu KL, Cimarelli S,
Constantinesco A and Imperiale A: Desmoplastic small round cell
tumor: Impact of F-FDG PET induced treatment strategy in a patient
with long-term outcome. Rare Tumors. 1:e192009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nathan JD, Gingalewski C and Salem RR:
Intra-abdominal desmoplastic small round cell tumor. Yale J Biol
Med. 74:287–293. 1999.
|
|
12
|
Bellah R, Suzuki-Bordalo L, Brecher E,
Ginsberg JP, Maris J and Pawel BR: Desmoplastic small round cell
tumor in the abdomen and pelvis: Report of CT findings in 11
affected children and young adults. AJR Am J Roentgenol.
184:1910–1914. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim JH, Goo HW and Yoon CH:
Intra-abdominal desmoplastic small round-cell tumor: Multiphase CT
findings in two children. Pediatr Radiol. 33:418–421. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tateishi U, Hasegawa T, Kusumoto M, Oyama
T, Ishikawa H and Moriyama N: Desmoplastic small round cell tumor:
Imaging findings associated with clinicopathologic features. J
Comput Assist Tomogr. 26:579–583. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gerald WL, Miller HK, Battifora H,
Miettinen M, Silva EG and Rosai J: Intra-abdominal desmoplastic
small round-cell tumor. Report of 19 cases of a distinctive type of
high-grade polyphenotypic malignancy affecting young individuals.
Am J Surg Pathol. 15:499–513. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Roberts P, Burchill SA, Beddow RA,
Wheeldon J, Cullinane C and Lewis IJ: A combined cytogenetic and
molecular approach to diagnosis in a case of desmoplastic small
round cell tumor with a complex translocation (11;22;21). Cancer
Genet Cytogenet. 108:19–25. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gao DX, Liao SL, Shi XJ and Hui ZY:
Desmoplastic small round cell tumor: A report of 5 cases. Clin Exp
Pathol. 16:353–356. 2000.
|
|
18
|
Magnan H, Abramson SJ, Price AP, Grewal
RK, Merchant MS, LaQuaglia MP and Meyers PA: Positron emission
tomography for response assessment in desmoplastic small round cell
tumor. J Pediatr Hematol Oncol. 35:e190–e193. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chung CJ, Bui V, Fordham LA, Hill J and
Bulas D: Malignant intraperitoneal neoplasms of childhood. Pediatr
Radiol. 28:317–321. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Haliloglu M, Hoffer FA and Fletcher BD:
Malignant peritoneal mesothelioma in two pediatric patients: MR
imaging findings. Pediatr Radiol. 30:251–255. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Johnson KA, Tung K, Mead G and Sweetenham
J: The imaging of Burkitt's and Burkitt-like lymphoma. Clin Radiol.
53:835–841. 1998. View Article : Google Scholar : PubMed/NCBI
|