Introduction
The incidence of primary carcinoma of the ureteral
stump following a simple nephrectomy is quite rare. Kim et
al (1) identified that, out of
318 patients who underwent a simple nephrectomy for benign renal
disease or renal organ donation, 8 presented with ureteral stump
carcinoma. Among the 8 patients, 6 patients exhibited transitional
cell carcinoma and 2 patients exhibited squamous cell carcinoma. No
concomitant bladder tumors were identified at stump tumor
diagnosis. Hematuria was the main presenting symptom in 3 of the 8
patients and 4 patients were diagnosed by follow-up imaging studies
(1). Ureteral stump carcinomas were
typically diagnosed by X-ray and ureteroscopic examination.
Excision of the ureteral stump and bladder cuff was performed in 7
patients while radical cystectomy, ureterectomy of the left
residual ureter and ileal conduit was performed in 1 patient.
Segawa et al (2) reported the
21st case in the Japanese literature of a patient with ureteral
stump carcinoma following a simple nephrectomy in 2006. By
contrast, primary carcinoma of ureteral stump following a radical
nephrectomy for renal cell carcinoma is rare. Previously, 7
patients with the disease have been reported worldwide; and all 7
patients were men with transitional cell carcinoma (3–9). The
current study presents a case report of an eighth patient, who is
the first female case; and reviews the literature concerning
patients with ureteral stump carcinoma following radical
nephrectomy. Written informed consent was obtained from the
patient.
Case report
A 61-year-old woman was admitted to Peking
University Shougang Hospital (Beijing, China) on 8 March 2012 with
asymptomatic intermittent gross hematuria, which had been ongoing
for 1 year. The patient had undergone a left radical nephrectomy
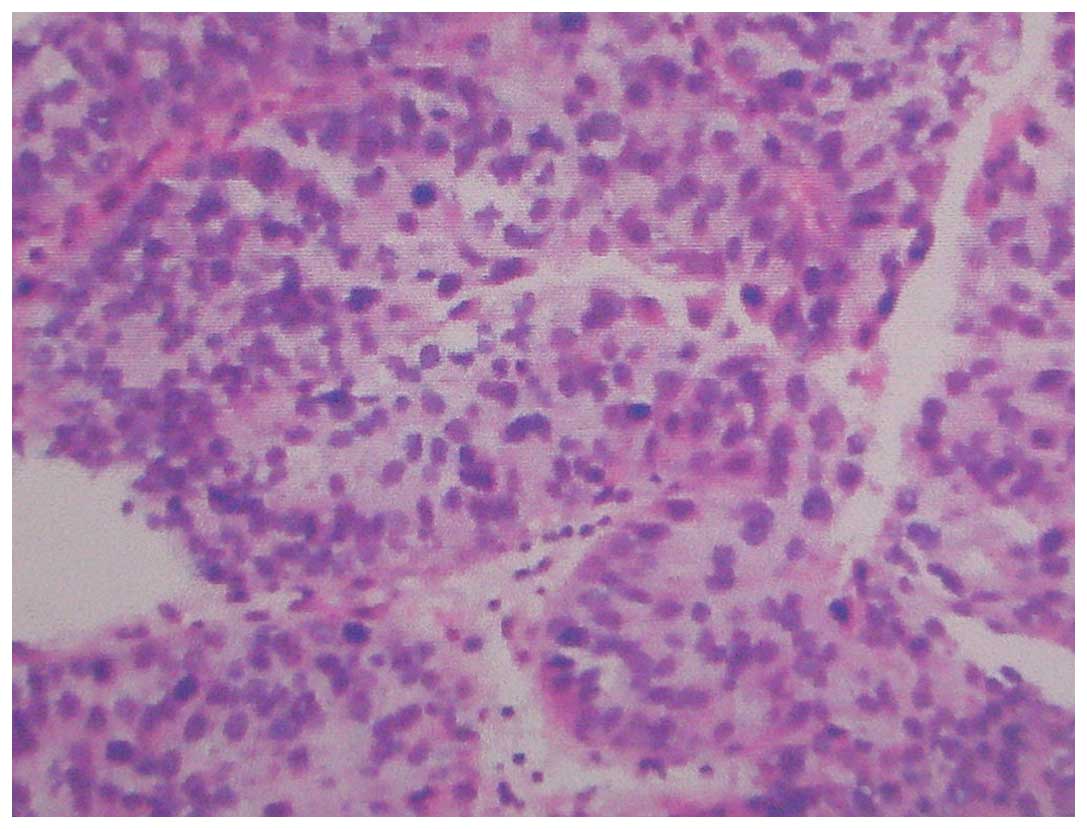
for local renal carcinoma 2 years prior. The pathological diagnosis
2 years prior was renal clear cell carcinoma, Furhman grade II
(10)(Fig.
1), with no evidence of extracapsular extension or regional
lymph node metastasis. Following radical nephrectomy, the renal
function of the patient was abnormal and serum creatinine (Cre)
levels increased to 120–140 µmol/l (normal range, <98 µmol/l).
The medical history of the patient included hypertension for 3
years prior to the current study. The physical examination of the
patient following readmission in March 2012 was unremarkable.
Laboratory examinations, including routine blood, urine, liver
function and renal function tests, were unremarkable, with the
exception of the Cre level of the patient, which was 773 µmol/l.
Urine cytology was negative. A computed tomography (CT) scan
demonstrated the presence of a 5.0×1.0 cm solid mass in the left
ureteral stump at the site of the iliac artery, with no metastasis
or invasion (Fig 2). The mean CT
value of the mass was 37 HU. Hydronephrosis of the right kidney and
dilatation of the upper and middle region of right ureter was
observed using magnetic resonance hydrography, but the reason for
stenosis was unclear. A cystoscopy was performed, which revealed
that urine passing through the right ureteral orifice was cloudy
and blood was draining from the left ureteral orifice. A double J
stent was placed in the right ureter to drain the urine for 10
days. The Cre level decreased to 300 µmol/l and stabilized. A
flexible ureteroscopy was performed on the right-side of the urine
collecting system, and a 1-cm stenosis was observed at the lower
region of the right ureter. However, there was no sign of a
neoplasm of the bladder. Samples were taken from the site of
stenosis and the stenosis was dilated with a rigid ureteroscopy. A
double J stent was re-inserted into the right ureter. An excision
of the left ureteral stump and bladder cuff was performed. The
histological diagnosis following surgery was high-grade
transitional carcinoma with focal interstitial cancer cell
infiltrates, without invasion of the ureteral muscle layer
(Fig. 3). Chronic inflammation was
detected in the right ureter, but no carcinoma was observed. The
patient did not receive adjuvant therapy following surgery. The
patient was alive at the 35-month follow-up; however, the patient
possessed chronic renal failure and a double J stent remained in
the right ureter of the patient, which was replaced every 3 months.
There was no evidence of recurrence of either renal carcinoma or
transitional carcinoma of the urinary tract.
Discussion
The occurrence of primary carcinoma of the ureteral
stump following a simple nephrectomy is rare. Kim et al
(1) identified that, out of 318
patients who underwent a simple nephrectomy for benign renal
disease or renal organ donation, 8 presented with ureteral stump
carcinoma; the incidence rate of the disease was 2.51%. Among the 8
patients, there were 6 patients with transitional cell carcinoma
and 2 patients with squamous cell carcinoma (1).
The synchronous occurrence of ipsilateral renal cell
carcinoma and urothelial carcinoma in the same kidney is rare
(11). Approximately 50 cases of
synchronous renal carcinoma and transitional cell carcinoma have
been reported in the literature (12,13).
Notably, synchronous presentation of both tumors has not been found
to result in a worse prognosis (12,13). A
small number of cases of synchronous renal carcinoma and ureter
carcinoma have also been reported (14–16).
Chuang et al (17) reported
the case of a 67-year-old woman with renal cell carcinoma and
urothelial carcinoma of the bladder, pelvis and ureters.
The occurrence of primary carcinoma of the ureteral
stump following a radical nephrectomy for renal cell carcinoma is
particularly rare. The present study performed a literature review
and, to the best of our knowledge, there were 7 previous cases
worldwide of ureteral stump carcinoma following radical nephrectomy
for renal cell carcinoma (3–9). All 7 patients presented with
transitional cell carcinoma (3–9). The
patient described in the current study is the eighth patient with
the disease, following transitional cell carcinoma. The previous 7
patients were men, and therefore the present patient is the first
female patient reported. The occurrence of ureteral stump carcinoma
following radical nephrectomy is markedly lower compared with the
occurrence following a simple nephrectomy; however, the reason for
this is unclear. From the present literature review, the mean age
of patients at diagnosis was 68.1 years (range, 49–88 years). The
mean interval between the nephrectomy and diagnosis of the ureteral
stump carcinoma was 7.3 years (range, 0.9–23.0 years). This is
longer than the 6.4-year average interval in patients that
underwent a nephrectomy for benign renal disease (1). Among the 8 patients with ureteral stump
carcinoma following radical nephrectomy for renal cell carcinoma, 4
patients were from Japan, 1 patient was from China and 3 patients
were from Europe and America.
The development of ureteral stump carcinoma may
occur due to a number of factors, including the following:
Hyperplasia and metaplasia resulting from chronic irritation due to
infection or urinary calculi; malignant metamorphosis in urinary
leukoplakia; and stimulation by an unrecognized carcinogenic agent
(4). However, there was no evidence
of significant inflammation or the presence of urinary calculi in
the 8 patients with ureteral stump carcinoma following radical
nephrectomy for renal cell carcinoma. Haritopoulos et al
(9) identified a patient that was
first diagnosed with transitional cell carcinoma of the bladder,
which was completely resected by transurethral resection. The
patient presented with 3 tumor recurrences in the bladder following
surgery. In total, 11 years subsequent to the first occurrence of
transitional cell carcinoma of the bladder, ureteral stump
carcinoma was diagnosed. Among the 8 patients described by the
literature review in the present study, bladder carcinoma was
concurrent in 2 patients (6,8). In 1 patient, ureteral stump carcinoma
was detected by histological examination following radical
cystectomy and ureterectomy of the left residual ureter due to the
presence of a bladder carcinoma (8).
Therefore, a history of prior bladder cancer or concurrent bladder
cancer should be considered as a notable risk factor for the
development of ureteral stump carcinoma due to the implantation of
cancer as a result of vesicoureteral reflux. Regardless of
vesicoureteral reflux, the possibility of ureteral stump carcinoma
should be considered due to the multifocal development of
transitional cell carcinoma of the urinary tract.
The first symptom in all 8 patients identified by
the present study was asymptomatic hematuria, including macroscopic
hematuria and gross hematuria. Therefore, the ureteral stump must
be carefully evaluated when hematuria is observed in a patient
following a nephrectomy for renal cell carcinoma. The possibility
of ureteral stump carcinoma should not be dismissed, including in
cases in which bladder carcinoma has already been diagnosed. Urine
cytology may aid diagnosis, but the sensitivity of this method is
poor (18). A retrograde ureterogram
may reveal a mid-ureteral filling defect. Ureteral stump carcinoma
may be identified using a CT scan, which may be enhanced during a
contrast enhancement scan. In addition, a ureteroscopy may be used
to observe the ureteral stump and to take samples for pathological
examination; however, it should be performed with care. A ureteral
stump and bladder cuff excision should be performed once ureteral
stump carcinoma is diagnosed.
In conclusion, the occurrence of primary carcinoma
of the ureteral stump following a radical nephrectomy for renal
cell carcinoma is rare. However, if a patient that has undergone a
radical nephrectomy possesses hematuria, the possibility of
ureteral stump carcinoma should be considered, particularly in East
Asian countries. In addition, a history of prior bladder carcinoma
or the current existence of bladder carcinoma should be considered
as a serious risk factor for the development of ureteral stump
carcinoma. A ureteral stump and bladder cuff excision should be
performed once ureteral stump carcinoma is diagnosed.
References
|
1
|
Kim YJ, Jeon SH, Huh JS and Chang SG:
Long-term follow-up of ureteral stump tumors after nephrectomy for
benign renal disease. Eur Urol. 46:748–752. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Segawa N, Kotake Y, Noumi H, Uchimoto S,
Azuma H, Katsuoka Y and Tsuji M: Ureteral tumor occurring from
remaining stump: A case report. Hinyokika Kiyo. 52:565–567.
2006.(In Japanese). PubMed/NCBI
|
|
3
|
Grey LF, Sorial RF and Levin HJ:
Transitional cell carcinoma in ureteral stump after radical
nephrectomy for renal cell carcinoma. Urology. 29:209–210. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Suzuki T, Tsuchiya N, Otomo R, Kakinuma H,
Satoh S, Sato K, Ogawa O and Kato T: Primary tumor of the ureteral
stump following a nephrectomy for renal cell carcinoma. Int J Urol.
6:41–43. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nagatsuma K, Tachibana M, Miyakawa A,
Asanuma H and Murai M: Transitional cell carcinoma of ureteral
stump after radical nephrectomy for renal cell carcinoma. Int J
Urol. 6:627–629. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cher ML, Milchgrub S and Sagalowsky AI:
Transitional cell carcinoma of the ureteral stump 23 years after
radical nephrectomy for adenocarcinoma. J Urol. 149:106–108.
1993.PubMed/NCBI
|
|
7
|
Mitsui K, Yamada Y, Taki T, Akahori M,
Kato K, Honda N, Fukatsu H, Kawai Y and Yoshikawa K: A case of
asynchronous renal cell carcinoma, hepatocellular carcinoma and
residual ureteral cancer. Hinyokika Kiyo. 44:583–586. 1998.(In
Japanese). PubMed/NCBI
|
|
8
|
Gohji K, Ueno K, Higuchi A and Fujii A: A
case of asynchronous renal cell carcinoma and urothelial cancer of
the urinary bladder and left ureter. Hinyokika Kiyo. 39:927–930.
1993.(In Japanese). PubMed/NCBI
|
|
9
|
Haritopoulos K, Stravodimos K, Banias C,
Giaslakiotis V, Alamanis C and Giannopoulos A: Transitional cell
carcinoma of ureteral stump after radical nephrectomy in a patient
with a history of bladder carcinoma. Int Urol Nephrol. 36:337–338.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Konety BR and Williams RD: Renal
parenchymal neoplasms. Smith's General Urology. Tanagho EA and
McAninch JW: (17th). McGraw-Hill. (New York, NY). 331–332.
2008.
|
|
11
|
Leveridge M, Isotalo PA, Boag AH and
Kawakami J: Synchronous ipsilateral renal cell carcinoma and
urothelial carcinoma of the renal pelvis. Can Urol Assoc J.
3:64–66. 2009.PubMed/NCBI
|
|
12
|
Fernández Arjona M, Santos Arrontes D, De
Castro Barbosa F, Begara Morillas F, Cortes Aranguez I and González
L: Synchronous renal clear-cell carcinoma and ipsilateral
transitional-cell carcinoma: Case report and bibliographic review.
Arch Esp Urol. 58:460–463. 2005.(In Spanish). PubMed/NCBI
|
|
13
|
Mucciardi G, Galì A, D'Amico C, Muscarà G,
Barresi V and Magno C: Transitional cell carcinoma of the renal
pelvis with synchronous ipsilateral papillary renal cell carcinoma:
Case report and review. Urol Case Rep. 3:93–95. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tsujimura A, Takahara S and Koide T: A
case of synchronous ipsilateral renal cell carcinoma and
transitional cell carcinoma. Hinyokika Kiyo. 37:1303–1306. 1991.(In
Japanese). PubMed/NCBI
|
|
15
|
Merenciano Cortina FJ, Laforga J, De la
Morena E, Amat Cecilia M, Rafie Mazketli W and Romero Pérez P:
Transitional carcinoma of the ureter and ipsilateral synchronous
renal cell carcinoma in hydronephrotic atrophic kidney: Infrequent
association. Actas Urol Esp. 25:380–384. 2001.(In Spanish).
PubMed/NCBI
|
|
16
|
Ulamec M, Stimac G, Kraus O and Kruslin B:
Bilateral renal cell carcinoma and concomitant urothelial carcinoma
of the renal pelvis and ureter: Case report. Lijec Vjesn.
129:70–73. 2007.(In Croatian). PubMed/NCBI
|
|
17
|
Chuang HC, Chuang CK and Ng KF:
Simultaneous development of renal cell carcinoma and multifocal
urothelial carcinoma. Chang Gung Med J. 31:515–519. 2008.PubMed/NCBI
|
|
18
|
Flanigan RC: Urothelial tumors of the
upper urinary tract. Campbell-Walsh Urology. Wein AJ: 2:(9th).
Saunders Elsevier. (Philadelphia, PA). 1638–1652. 2007.
|