Introduction
Post-laparoscopic occurrence of port-site metastasis
refers to tumor foci either localized at single or multiple
locations under the skin or in the scar tissue of the abdominal
wall adjacent to the port (1).
Port-site metastasis is a rare complication that may occur
following laparoscopic surgery for malignant tumors of the urinary
system, with an incidence of 0.09–0.73% of all patients who undergo
laparoscopic surgery for urological malignancies (2,3). Previous
studies have reported ~50 cases of abdominal wall implantation
metastasis following surgical resection of malignant tumors of the
urinary system (4), of which, 9 cases
occurred following surgical resection of renal carcinoma (5–18,15). Thus, this indicates that the
occurrence of port-site metastasis subsequent to laparoscopic
radical resection of renal carcinoma and nephron-sparing surgery is
relatively rare. Although previous studies have reported the
pathogenesis, risk factors and prevention of port-site metastasis
following laparoscopic radical resection of renal carcinoma and
nephron-sparing surgery (14,15), few studies have reported the mechanism
and prognosis of port-site metastasis occurring in the case of
renal pelvis carcinoma (19,20). The present study reports the clinical
data of two patients with renal pelvis carcinoma and one patient
with renal carcinoma who developed port-site metastasis following
retroperitoneal laparoscopic surgery with the aim of identifying
the cause and prognosis of port-site metastasis occurring in such
circumstances. In addition, a review of the literature is conducted
in the present study.
Case report
Case 1
A 71-year-old male was admitted to The Second
Affiliated Hospital of Dalian Medical University (Dalian, China)
August 28, 2009, presenting with hematuria. Computed tomography
(CT; SOMATOM Definition AS 64; Siemens AG, Munich, Germany)
urography was performed following patient admission, which revealed
fluid collection in the left renal pelvis and left ureter. A
cystoscopy was subsequently performed, which identified blood
ejection at the orifice of the left ureter. Three consecutive urine
exfoliative cytology tests suggested the presence of urothelial
cell carcinoma. Retroperitoneal laparoscopic radical
nephroureterectomy and bladder cuffing resection, including the
ureteral orifice, was performed. The specimen was completely
excised, enveloped in an impermeable specimen bag and pulled out
through external rectus incision. Formalin-fixed paraffin-embedded
tissues (FFPET) were cut into 4-µm sections and stained with
hematoxylin-eosin (HE) to evaluate the cell pattern. The sections
were scanned under a light microscope and images were captured at a
magnification of ×200. Post-operative pathology revealed tumor
cells in papillary and solid nests with a disordered arrangement
and loss of polarity, which confirmed the diagnosis of high-grade
infiltrative urothelial cell carcinoma of the left renal pelvis and
ureter. Approximately 7 months after the initial surgery, a
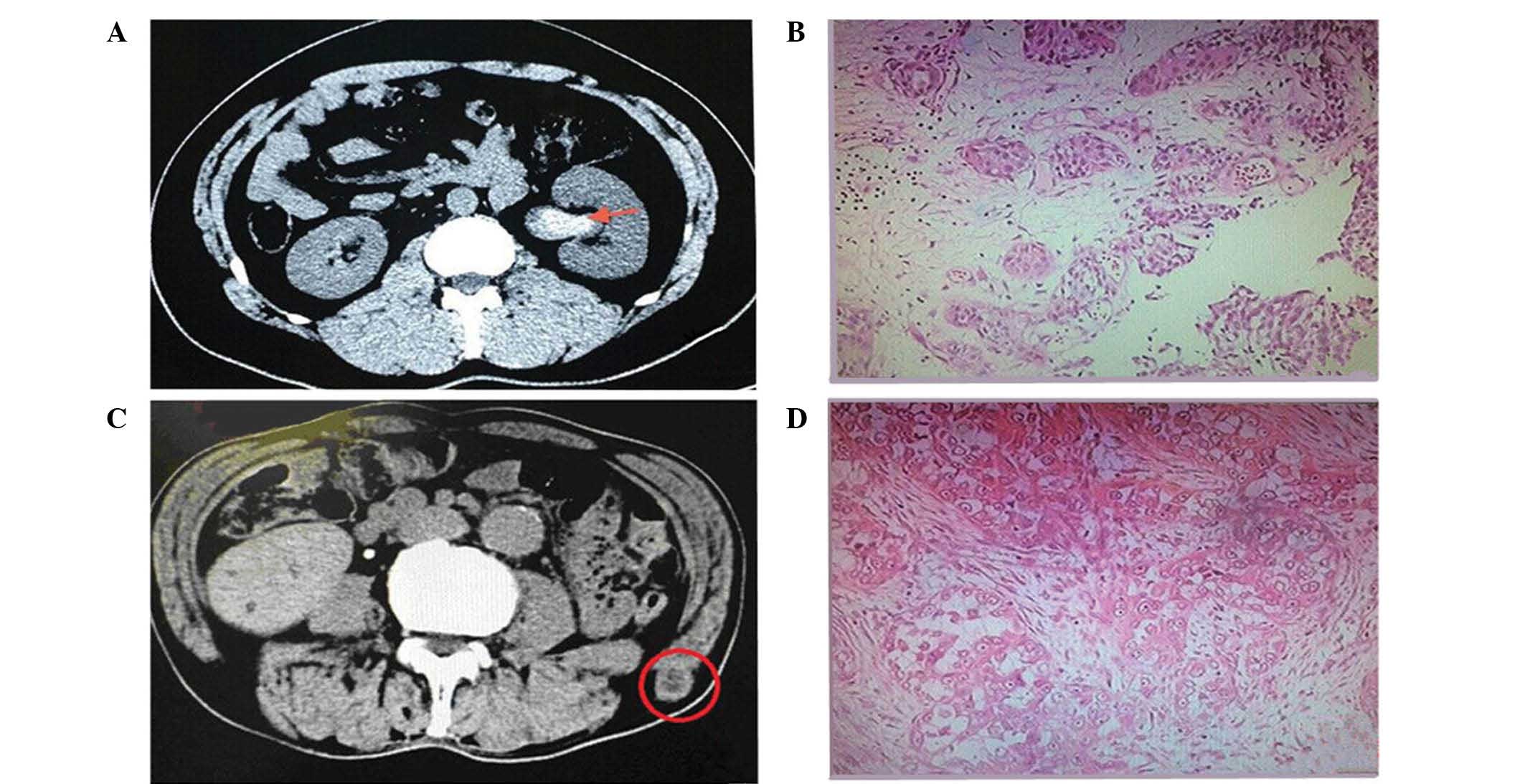
painless hard mass was detected under the lumbar skin. A CT scan
performed at the hospital revealed a subcutaneous metastatic tumor
in the left ilium measuring ~2.0 cm in size. Left abdominal wall
tumor resection was subsequently performed. The aforementioned
tissue preparation method for histopathological examination was
used. The post-operative pathology revealed tumor cells with large
nucleoli and the formation of cancerous cell nests in the fibrous
cords, which confirmed the diagnosis of a metastatic malignant
tumor (Fig. 1). At 2 weeks
post-resection, a rapidly progressive tumor was detected in the
left lumbar region, which was confirmed by emission CT as multiple
bone metastases. Abdominopelvic CT suggested the presence of liver
metastasis. The patient eventually succumbed to the disease despite
undergoing gemcitabine plus cisplatin (GC) chemotherapy [3 cycles
of gemcitabine (1,000 mg/m2) on days 1, 8 and 15 plus cisplatin (70
mg/m2) on day 2 every 28 days, each administered via intravenous
drip].
Case 2
A 47-year-old female was admitted to The Second
Affiliated Hospital of Dalian Medical University on July 4, 2013,
due to the previous detection of a tumor in the urinary bladder
during a physical examination. The tumor was removed via
transurethral resection, and the tissue resected was observed to be
deep to the muscular layer. Post-operative pathology confirmed the
diagnosis of low-grade urothelial cell carcinoma. The patient
received regular pirarubicin perfusion chemotherapy (20 mg THP
dissolved in 40 ml 5% dextrose was administered intravesically once
a week for 6 weeks, then once a month) post-operatively. Blood
ejection was detected at the right ureter 10 months after the
initial surgery during a cystoscopic examination at the clinic of
the hospital. A lower abdominal contrast-enhanced magnetic
resonance imaging (MAGNETOM Verio 3T; Siemens AG) scan revealed a
tumor in the upper pole of the right kidney measuring ~4.0×3.6 cm
in size with uneven enhancement. Three consecutive urine
exfoliative cytology tests suggested the presence of urothelial
cell carcinoma. Retroperitoneal laparoscopic radical
nephroureterectomy and bladder cuffing resection, including the
ureteral orifice, was consequently performed. The specimen was
completely excised, enveloped in an impermeable specimen bag and
pulled out through external rectus incision. FFPET with HE staining
were observed in 4-µm sections by light microscope under
magnification of ×200. Post-operative pathology confirmed the
diagnosis of high-grade infiltrative urothelial cell carcinoma of
the right renal pelvis. Microscopically, the tumor was composed of
high-grade malignant urothelial cells. Numerous pleomorphic, giant
and multinucleated cells with one or more prominent nucleoli were
also observed. The tumor invaded the renal parenchyma with tumor
emboli detected in the venous lumen; however, no tumor tissue was
observed in the vascular stump of the renal hilum and the right
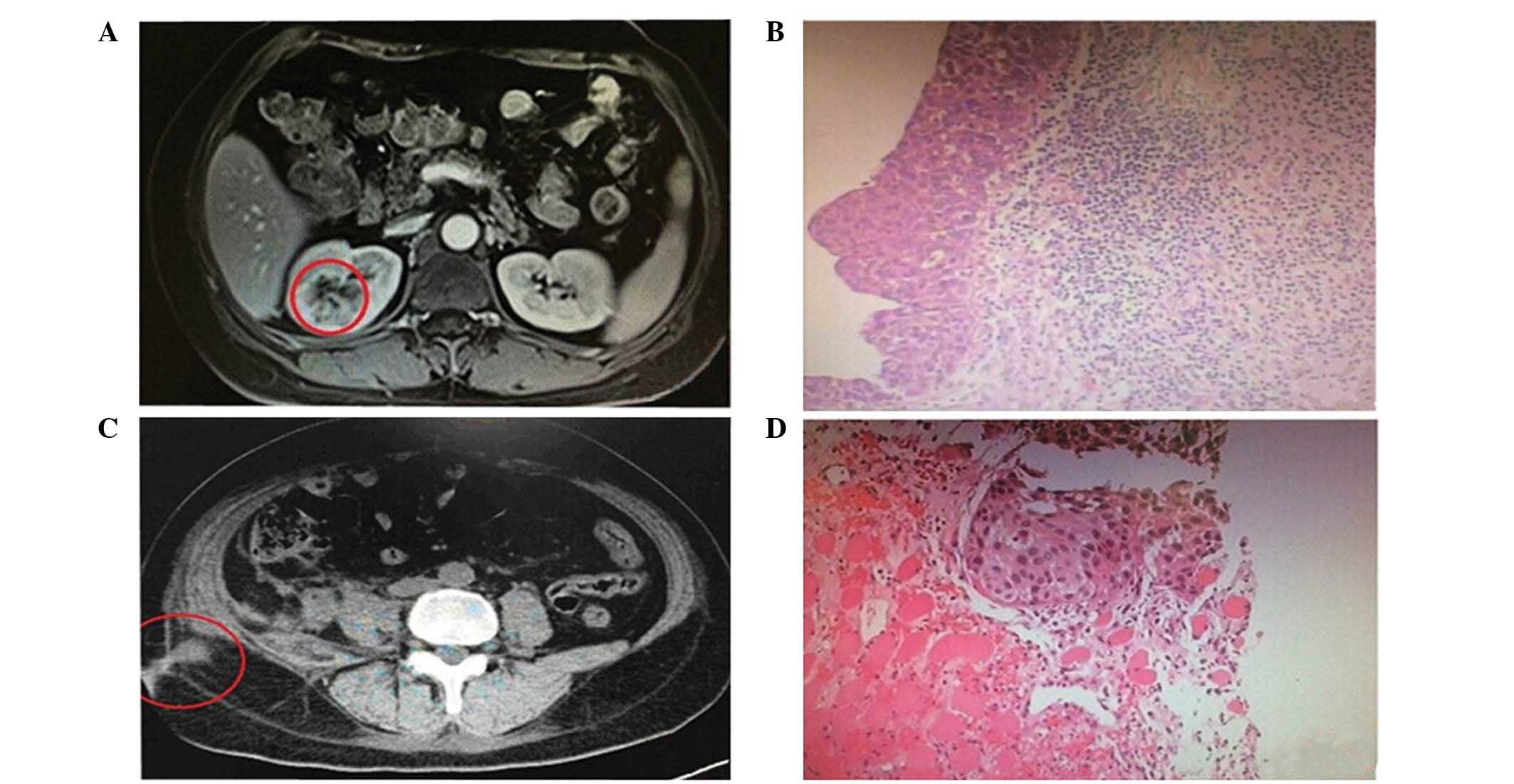
ureteral stump. Multiple hard, painless masses were detected at the
right abdominal port-site and the right lumbodorsal port-site
within 1 month of the initial surgery. An abdominal CT scan
performed at the hospital revealed metastatic tumors in the right
abdominal wall. Pathological biopsy of these two sites demonstrated
cancer cells distributed in small masses in the striated muscle,
which were consistent with an urothelial origin. The aforementioned
tissue preparation method for histopathological examination was
used; microscopically, the carcinoma cells were arranged in the
solid nest-like distribution with a large deeply-dyed nucleolus and
evident pleomorphism (Fig. 2). The
patient stopped any further treatment and eventually succumbed to
the disease.
Case 3
A 68-year-old male was admitted to The Second
Affiliated Hospital of Dalian Medical University on September 18,
2013, due to the detection of a tumor in the right kidney during a
previous physical examination. The patient had a 13-year history of
hypertension. Following regular administration of amlodipine, the
blood pressure of the patient fluctuated between 130 and 90 mmHg.
The patient also had a history of renal syndrome for 5 years, for
which no regular treatment was administered. An abdominal CT scan
performed following admission detected a space-occupying lesion in
the middle portion of the right kidney, measuring ~3.0×4.0 cm in
size. A laparoscopic nephron-sparing nephrectomy was performed, and
the tumor was removed completely via a specimen bag. With the
method mentioned in Case 1, the tumor tissue was managed for
histopathological examination. Post-operative pathology confirmed
the diagnosis of Fuhrman grade 3 (21) renal clear cell carcinoma; tumor cells
exhibited clear cytoplasm and a sharply outlined cell membrane,
with alveolar nests and tightly packed tubules. The tumor involved
the renal capsule, but no cancer tissue was identified in the cut
edge. At 9 months after the initial surgery, the patient complained
of right lumbodorsal and abdominal pain. A CT plain scan was
performed, which identified multiple soft-tissue intensity nodular
shadows in the diaphragmatic muscle in the posterior of the right
kidney, which exhibited a circular enhancement and were suspected
to be metastatic tumors. CT-guided pathological biopsy of the
abdominal wall tumors resulted in the diagnosis of a metastatic
lesion, which was observed using a light microscope. The FFPET
showed a clear cell pattern that was identical, histologically, to
the previous renal cell carcinoma (Fig.
3). Sunitinib targeted therapy (50 mg daily, administered
orally) was administered, but was discontinued 2 weeks after
treatment initiation due to bone marrow suppression. The condition
of the patient is stable at present.
Discussion
Since the first case of successful laparoscopic
nephrectomy was reported in 1991 (16), minimally invasive surgery has been
increasingly employed for the resection and lymph node clearance of
urinary malignant tumors (22–24). The
therapeutic outcomes of laparoscopic surgery and open surgery are
similar in the treatment of tumors (25,26). The
development of port-site metastasis following laparoscopic tumor
resection is rare, and the cause remains elusive (15,17,18). In
1994, Stolla et al (27)
reported the first case of abdominal wall port-site metastasis
developing subsequent to laparoscopic pelvic lymph node clearance
in a patient with bladder urothelial cell carcinoma. However,
Micali et al (3) did not
identify a single case of port-site metastasis in a retrospective
review of the available clinical data of 2,604 patients that
underwent radical nephrectomies. Therefore, the incidence of
port-site metastasis remains unclear.
With regards to the incidence of local tumor
dissemination following laparoscopic resection of malignant tumors,
gynecological journals report an incidence of ~5%, while oncology
journals report an incidence of ~4% for colorectal cancer. The 3
cases of port-site metastasis discussed in the present study
accounted for an incidence of ~1.5% of all patients treated for
urological malignancies in The Second Affiliated Hospital of Dalian
Medical University. Certain researchers have argued that the
incidence of port-site metastasis following laparoscopic radical
resection of renal carcinoma is variable, and the incidence may be
as high as 21% (28). However, the
majority of researchers consider that port-site metastasis is rare,
and the incidence is <1% (2). In a
previous study, clinical data was collected from 19 urosurgery
centers, involving 10,912 patients who underwent laparoscopic
resection for urinary malignant tumors (3). It was reported that only 13 cases (0.1%)
developed post-operative tumor metastasis, including 10 cases of
port-site metastasis and 3 cases of retroperitoneal dissemination
(3). These 13 cases included
accidental detection of urothelial cell carcinoma following
laparoscopic nephrectomy in 4 cases, laparoscopic
nephroureterectomy for urothelial cell carcinoma in 3 cases,
laparoscopic resection of adrenal metastatic cancer in 4 cases,
laparoscopic pelvic lymph node clearance for penile squamous cell
carcinoma in 1 case and retroperitoneal lymph node clearance for
carcinoma of testis in 1 case. However, Micali et al
(3) analyzed 2,604 cases of
laparoscopic radical resection of renal carcinoma and did not
identify any case of port-site metastasis.
Certain researchers have maintained that it is
important to clarify the cause of tumor metastasis, since the
prognosis of port-site metastasis is unclear (29). The following hypotheses have been
largely supported as the possible causes of laparoscopic port-site
metastasis: i) Biological invasiveness of tumors; ii) local
traumatic factors; iii) host immune response; and iv) laparoscopic
surgical procedures (15,30). Based on these possible causes,
experience of the 3 present cases of port-site implantation
metastasis and review of the literature, the conclusions of the
current study are discussed below.
Port-site metastasis and tumor recurrence are
attributable to tumor invasiveness, which is known to depend on
tumor stage and Fuhrman grade classification (2,4). Previous
studies have reported that all cases of port-site metastasis
following laparoscopic radical resection of renal carcinoma were
high-grade tumors (Fuhrman grade >2), including 1 case
presenting with a sarcoma-like lesion whose pathological Fuhrman
grade was 4 (31–33). The pathology of case 1 in the present
study was confirmed as a high-grade, infiltrative, urothelial cell
carcinoma of the left renal pelvis and ureter, which was invading
the muscular layer. The pathology of case 2 in the present study
was confirmed as a high-grade, infiltrative, urothelial cell
carcinoma of the right renal pelvis, which was invading the renal
parenchyma. The pathology of case 3 in the present study was
confirmed as Fuhrman grade 3 renal clear cell carcinoma. Port-site
metastasis occurred 7, 1 and 3 months after laparoscopic surgery in
cases 1, 2 and 3, respectively. All 3 cases of port-site metastasis
were high-grade tumors, in which post-operative metastasis
developed rapidly with a poor prognosis. The present cases support
the hypothesis that port-site metastasis is associated with tumor
stage and Fuhrman grade classification.
Local traumatic factors may contribute to
implantation metastasis of cancer cells at the port-site (34). A previous study suggested that cancer
cells are more likely to implant during the early stage of wound
healing, possibly due to the attachment of cancer cell adhesion
protein fibers to the wound surface and wound healing (35). In addition, factors promoting wound
healing may be able to promote the growth of cancer cells (36). Considering these risk factors, a
previous study proposed that correct repair of the peritoneum
surrounding the laparoscopic port-site may be able to reduce the
risk of tumor metastasis (37).
However, in the present 3 cases, the retroperitoneal approach was
employed without rupturing the peritoneum; thus, the current study
suggests that local traumatic factors may also induce port-site
metastasis.
Furthermore, low immune function may also promote
tumor metastasis (38). A previous
study hypothesized that the host immune function of the patient may
be reduced as a result of certain media factors introduced during
the perioperative period (38). Media
factors may include the use of anesthetic agents, surgical trauma,
transfusion, temperature change, pain and psychological stress
(38,39). Previous studies on animal models have
demonstrated that surgical trauma may reduce the activity of
natural killer cells, thus promoting tumor metastasis (38,40).
Furthermore, 3 cases of port-site metastasis following laparoscopic
radical resection of renal carcinoma were reported to present
varying degrees of immune function impairment, including chronic
renal failure in 1 patient, alcoholic cirrhosis in another patient
and diabetes in the third patient (41,42). All 3
patients discussed in the present study suffered from renal
syndrome without receiving regular treatment, which may, to a
certain extent, have impaired holistic immune function during the
perioperative period and promoted tumor metastasis.
The performance of a laparoscopy is associated with
a number of additional factors that may provoke metastasis,
including the presence of pneumoperitoneum, contamination around
the port-site, incomplete tumor resection and the particular method
used to remove the specimen (30). A
previous study reported that air leakage around the port-site could
induces a ‘chimney effect’, suggesting that continuous air leakage
around the port would increase the number of cancer cells at the
port-site and promote metastasis (43). With regards to the present cases, all
ports were fixed in place and no pneumoperitoneal air leakage was
noted. A previous study stated that CO2 could stimulate the growth
of tumor cells, directly act on tumor cells or interfere with the
local defense mechanism (44). In
addition, repeated pulling and insertion of the instrument
contaminated by tumor rupture during the laparoscopic procedure may
result in transfer and invasion of tumor cells at the surgical
site, thus increasing the risk of port-site metastasis (45–47).
Injection of povidone-iodine into the incision may reduce the risk
of port-site metastasis (48). In the
current 3 cases, the tumors were excised completely, and no tumor
rupture occurred, as evidenced by cases 2 and 3, whose cut edges
were pathologically negative for cancer cells. However, the
instruments used in these cases were inserted repeatedly without
pretreatment with cytotoxic agents, nor was povidone-iodine used at
the port-sites, which may have increased the risk of metastasis.
Iwamura et al (7) and Chen
et al (9) reported cases of
port-site metastasis occurring as a result of not using a specimen
bag. Therefore, the majority of subsequent studies considered that
the use of specimen bags in laparoscopy could reduce port-site
contamination and implantation (15,49).
However, in the present study, impermeable specimen bags were
utilized in all 3 cases, and the bags were not ruptured during the
procedure. Therefore, it may be theorized that the occurrence of
port-site metastasis is due to multiple factors.
Certain researchers regard the prognosis of
port-site metastasis to be unclear (32). In the current study, case 1 survived
for 10 months after the detection of port-site implantation, case 2
survived only for 2 months and case 3 remains in a stable condition
at present. Case 1 and 2 were diagnosed as high-grade urothelial
cell carcinoma complicated with bladder cancer. Case 1 received GC
chemotherapy for the port-site metastasis, whilst case 2 did not
receive any chemoradiation therapy, thus the life expectancy of
case 2 was shorter than that of case 1. Case 3 was diagnosed with
high-grade renal clear cell carcinoma and received sunitinib
targeted therapy following detection of the port-site metastasis,
although the treatment was discontinued 2 weeks later due to bone
marrow suppression. The condition of the patient remains stable at
present. However, as the patient has been observed for a relatively
short time, further follow-up observation is required. Overall,
port-site metastasis is more likely to occur in patients with
high-grade renal carcinoma or renal pelvis carcinoma compared with
patients with low-grade renal cell carcinoma, and the prognosis is
usually poor.
In conclusion, a review of the current literature
indicates that the occurrence of port-site metastasis subsequent to
laparoscopic radical resection of renal pelvis carcinoma and
nephron-sparing surgery is relatively rare, and its cause is
multifactorial. Although the exact cause remains unclear, the
occurrence of port-site metastasis may be considered attributable
to the combination of holistic and local factors. Measures to
reduce the occurrence of port-site metastasis include strict
abidance to the surgical guidelines for tumor resection, avoidance
of air leakage at the port-site, use of impermeable specimen bags
to remove the specimen under direct vision, irrigation of the
laparoscopic surgery instruments and incisional wound with
povidone-iodine when necessary, and enhancement of the body's
immunity. Post-operative follow-up observation and examination are
recommended, particularly in patients with high-grade renal
carcinoma or renal pelvis carcinoma, since timely detection of
tumor recurrence or metastasis and subsequent administration of
systemic chemotherapy are prerequisite for prolonging the life
expectance of patients.
References
|
1
|
Schneider C, Jung A, Reymond MA, Tannapfel
A, Balli J, Franklin ME, Hohenberger W and Köckerling F: Efficacy
of surgical measures in preventing port-site recurrences in a
porcine model. Surg Endosc. 15:121–125. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rassweiler J, Tsivian A, Kumar AV,
Lymberakis C, Schulze M, Seeman O and Frede T: Oncological safety
of laparoscopic surgery for urological malignancy: Experience with
more than 1,000 operations. J Urol. 169:2072–2075. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Micali S, Celia A, Bove P, De Stefani S,
Sighinolfi MC, Kavoussi LR and Bianchi G: Tumor seeding in
urological laparoscopy: An international survey. J Urol.
171:2151–2154. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kadi N, Isherwood M, Al-Akraa M and
Williams S: Port-site metastasis after laparoscopic surgery for
urological malignancy: Forgotten or missed. Adv Urol.
2012:6095312012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fentie DD, Barrett PH and Taranger LA:
Metastatic renal cell cancer after laparoscopic radical
nephrectomy: Long-term follow-up. J Endourol. 14:407–411. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Castilho LN, Fugita OE, Mitre AI and Arap
S: Port site tumor recurrences of renal cell carcinoma after
videolaparoscopic radical nephrectomy. J Urol. 165:5192001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Iwamura M, Tsumura H, Matsuda D, Kurosaka
S, Yoshida K and Baba S: Port site recurrence of renal cell
carcinoma following retroperitoneoscopic radical nephrectomy with
manual extraction without using entrapment sac or wound protector.
J Urol. 171:1234–1235. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Landman J and Clayman RV: Re: Port site
tumor recurrences of renal cell carcinoma after videolaparoscopic
radical nephrectomy. J Urol. 166:629–630. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen YT, Yang SS, Hsieh CH and Wang CC:
Hand port-site metastasis of renal-cell carcinoma following
hand-assisted laparoscopic radical nephrectomy: Case report. J
Endourol. 17:771–775. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dhobada S, Patankar S and Gorde V: Case
report: Port-site metastasis after laparoscopic radical nephrectomy
for renal-cell carcinoma. J Endourol. 20:119–122. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Castillo OA, Vitagliano G, Díaz M and
Sánchez-Salas R: Port-site metastasis after laparoscopic partial
nephrectomy: Case report and literature review. J Endourol.
21:404–407. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Greco F, Wagner S, Reichelt O, Inferrera
A, Lupo A, Hoda RM, Hamza A and Fornara P: Huge isolated port-site
recurrence after laparoscopic partial nephrectomy: A case report.
Eur Urol. 56:737–739. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Masterson TA and Russo P: A case of
port-site recurrence and locoregional metastasis after laparoscopic
partial nephrectomy. Nat Clin Pract Urol. 5:345–349.
2008.PubMed/NCBI
|
|
14
|
Lee BR, Tan BJ and Smith AD: Laparoscopic
port site metastases: Incidence, risk factors, and potential
preventive measures. Urology. 65:639–644. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Curet MJ: Port site metastases. Am J Surg.
187:705–712. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Clayman RV, Kavoussi LR, Soper NJ, Dierks
SM, Merety KS, Darcy MD, Long SR, Roemer FD, Pingleton ED and
Thomson PG: Laparoscopic nephrectomy. N Engl J Med. 324:1370–1371.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stewart GD and Tolley DA: What are the
oncological risks of minimal access surgery for the treatment of
urinary tract cancer? Eur Urol. 46:415–420. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Castillo OA and Vitagliano G: Port site
metastasis and tumor seeding in oncologic laparoscopic urology.
Urology. 71:372–378. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Highshaw RA, Vakar-Lopez F, Jonasch E,
Yasko AW and Matin SF: Port-site metastasis: the influence of
biology. Eur Urol. 47:357–360. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eng MK, Katz MH, Bernstein AJ, Shikanov S,
Shalhav AL and Zorn KC: Laparoscopic port-site metastasis in
urologic surgery. J Endourol. 22:1581–1585. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fuhrman SA, Lasky LC and Limas C:
Prognostic significance of morphologic parameters in renal cell
carcinoma. Am J Surg Pathol. 6:655–663. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Greene FL, Kercher KW, Nelson H, Teigland
CM and Boller AM: Minimal access cancer management. CA Cancer J
Clin. 57:130–146. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rajan P and Turna B: New trends in
minimally invasive urological surgery. Int Braz J Urol. 35:514–520.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu HY, Friedlander DF, Patel S and Hu JC:
The current status of robotic oncologic surgery. CA Cancer J Clin.
63:45–56. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu HY, Hevelone ND, Lipsitz SR, Kowalczyk
KJ and Hu JC: Use, costs and comparative effectiveness of robotic
assisted, laparoscopic and open urological surgery. J Urol.
187:1392–1398. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fairey AS, Kassouf W, Estey E, Tanguay S,
Rendon R, Bell D, Izawa J, Chin J, Kapoor A, Matsumoto E, et al:
Comparison of oncological outcomes for open and laparoscopic
radical nephroureterectomy: Results from the Canadian Upper Tract
Collaboration. BJU Int. 112:791–797. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stolla V, Rossi D, Bladou F, Rattier C,
Ayuso D and Serment G: Subcutaneous metastases after coelioscopic
lymphadenectomy for vesical urothelial carcinoma. Eur Urol.
26:342–343. 1994.PubMed/NCBI
|
|
28
|
Wexner SD and Cohen SM: Port site
metastases after laparoscopic colorectal surgery for cure of
malignancy. Br J Surg. 82:295–298. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Paolucci V, Schaeff B, Schneider M and
Gutt C: Tumor seeding following laparoscopy: International survey.
World J Surg. 23:989–997. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ramirez PT, Wolf JK and Levenback C:
Laparoscopic port-site metastases: etiology and prevention. Gynecol
Oncol. 91:179–189. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Minardi D, Lucarini G, Mazzucchelli R,
Milanese G, Natali D, Galosi AB, Montironi R, Biagini G and
Muzzonigro G: Prognostic role of Fuhrman grade and vascular
endothelial growth factor in pT1a clear cell carcinoma in partial
nephrectomy specimens. J Urol. 174:1208–1212. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Stephenson AJ, Chetner MP, Rourke K,
Gleave ME, Signaevsky M, Palmer B, Kuan J, Brock GB and Tanguay S:
Guidelines for the surveillance of localized renal cell carcinoma
based on the patterns of relapse after nephrectomy. J Urol.
172:58–62. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pearlstone DB, Feig BW and Mansfield PF:
Port site recurrences after laparoscopy for malignant disease.
Semin Surg Oncol. 16:307–312. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee SW, Whelan RL, Southall JC and Bessler
M: Abdominal wound tumor recurrence after open and
laparoscopic-assisted splenectomy in a murine model. Dis Colon
Rectum. 41:824–831. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Murthy SM, Goldschmidt RA, Rao LN,
Ammirati M, Buchmann T and Scanlon EF: The influence of surgical
trauma on experimental metastasis. Cancer. 64:2035–2044. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hofer SO, Molema G, Hermens RA, Wanebo HJ,
Reichner JS and Hoekstra HJ: The effect of surgical wounding on
tumour development. Eur J Surg Oncol. 25:231–243. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Aoki Y, Shimura H, Li H, Mizumoto K, Date
K and Tanaka M: A model of port-site metastases of gallbladder
cancer: The influence of peritoneal injury and its repair on
abdominal wall metastases. Surgery. 125:553–559. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Vallejo R, Hord ED, Barna SA,
Santiago-Palma J and Ahmed S: Perioperative immunosuppression in
cancer patients. J Environ Pathol Toxicol Oncol. 22:139–146. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Snyder GL and Greenberg S: Effect of
anaesthetic technique and other perioperative factors on cancer
recurrence. Br J Anaesth. 105:106–115. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Eggermont AM, Steller EP and Sugarbaker
PH: Laparotomy enhances intraperitoneal tumor growth and abrogates
the antitumor effects of interleukin-2 and lymphokine-activated
killer cells. Surgery. 102:71–78. 1987.PubMed/NCBI
|
|
41
|
Wakim-Fleming J and Mullen KD: Long-term
management of alcoholic liver disease. Clin Liver Dis. 9:135–149.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Contin C, Pitard V, Delmas Y, Pelletier N,
Defrance T, Moreau JF, Merville P and Déchanet-Merville J:
Potential role of soluble CD40 in the humoral immune response
impairment of uraemic patients. Immunology. 110:131–140. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kazemier G, Bonjer HJ, Berends FJ and
Lange JF: Port site metastases after laparoscopic colorectal
surgery for cure of malignancy. Br J Surg. 82:1141–1142. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jacobi CA, Sabat R, Böhm B, Zieren HU,
Volk HD and Müller JM: Pneumoperitoneum with carbon dioxide
stimulates growth of malignant colonic cells. Surgery. 121:72–78.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gutt CN, Riemer V, Kim ZG, Jacobi CA,
Paolucci V and Lorenz M: Impact of laparoscopic colonic resection
on tumour growth and spread in an experimental model. Br J Surg.
86:1180–1184. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lee SW, Gleason NR, Bessler M and Whelan
RL: Port site tumor recurrence rates in a murine model of
laparoscopic splenectomy decreased with increased experience. Surg
Endosc. 14:805–811. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lee SW, Southall J, Allendorf J, Bessler M
and Whelan RL: Traumatic handling of the tumor independent of
pneumoperitoneum increases port site implantation rate of colon
cancer in a murine model. Surg Endosc. 12:828–834. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wittich P, Mearadji A, Marquet RL and
Bonjer HJ: Irrigation of port sites: Prevention of port site
metastases? J Laparoendosc Adv Surg Tech A. 14:125–129. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Iavazzo C and Gkegkes ID: Port-site
metastases in patients with gynecological cancer after
robot-assisted operations. Arch Gynecol Obstet. 292:263–269. 2015.
View Article : Google Scholar : PubMed/NCBI
|