Introduction
Cervical cancer is a common malignant tumor of the
female reproductive system, and is responsible for ~250,000 annual
mortalities (1). Neoadjuvant
chemotherapy is important in cervical cancer treatment, since
general chemotherapy drugs may promote tumor cell death by inducing
cell apoptosis, but tend to induce drug resistance (2). Autophagy is a process of self-digestion
by a cell through the action of enzymes originating within its
lysosome of the same cell (3).
Autophagy is often induced under conditions of stress that could
also lead to cell death (4–6). Abnormal regulation of autophagy is
closely associated with the incidence and development of tumors;
thus, autophagic cell death may be considered a novel target for
cancer treatment (6).
Beclin 1 is a 60-kDa coiled-coil protein that was
identified in rats with fatal Sindbis viral encephalitis in 1998
(7). Beclin 1 is a B-cell lymphoma 2
(Bcl-2) homology 3 domain-only protein that is essential for the
formation of double-membrane autophagosomes, which are required in
the initial step of autophagy (7–9). Previous
studies have reported that cell autophagy is closely associated
with tumor initiation and progression, and is important in cell
signal regulation in tumors (10–12).
However, the effects of beclin 1 on the biological behavior and
chemotherapy sensitivity of cervical cancer cells have not been
studied in detail thus far. In the present study, a beclin 1
expression vector was constructed and transfected into human
cervical cancer cells to investigate the effects of beclin 1
expression on the biological behavior and chemotherapy sensitivity
of HeLa cells.
Materials and methods
Materials
The human cervical cancer cell line HeLa was
obtained from the cell resource center of Shanghai Institutes for
Biological Sciences of Chinese Academy of Sciences (Shanghai,
China). TRIzol® and Lipofectamine® 2000 were
purchased from Invitrogen (Thermo Fisher Scientific, Inc., Waltham,
MA, USA). Reverse transcription-polymerase chain reaction (RT-PCR)
kit was purchased from Fermentas (Thermo Fisher Scientific, Inc.).
Taxol® (paclitaxel injection) was purchased from
Bristol-Myers Squibb (New York, NY, USA).
Cell transfection
HeLa cells were cultured in Gibco Dulbecco's
modified Eagle's medium (DMEM; Thermo Fisher Scientific, Inc.)
supplemented with 10% (v/v) heat-inactivated Gibco fetal bovine
serum (Thermo Fisher Scientific, Inc.) and penicillin-streptomycin
(100 IU/ml-100 µg/ml; Roche Applied Science, Penzberg, Germany) at
37°C in a humidified atmosphere of 5% CO2-95% air. Cells
at logarithmic phase were seeded at a density of 3×105
cells/well in a 6-well plate for 24 h prior to transfection. Beclin
1 expression plasmid was constructed as previously reported
(12). Lipofectamine® 2000
(4 µl diluted in 100 µl DMEM) was used for the transfection of 0.5
µg beclin 1 expression vector or empty vector diluted in 100 µl
DMEM, followed by incubation of the samples for 20 min at room
temperature. The plasmid DNA-Lipofectamine® 2000 complex
was then added into the cell medium, and incubated at 37°C in a
CO2 incubator for 8 h. Subsequently, the medium was
replaced, and the cells were incubated for 48 h prior to be used in
the corresponding experiments, which included a blank control group
(non-transfected HeLa cells); a negative control group (HeLa cells
transfected with mock-vehicle) and the experimental group (HeLa
cells transfected with beclin 1 expression vector). Transfected
cells were collected at 48 h post-transfection and used in the
subsequent experiments.
RT-PCR for the detection of beclin 1
expression
Cells were collected at 48 h post-transfection, and
total RNA was extracted using TRIzol® (Thermo Fisher
Scientific) for the detection of beclin 1 expression by RT-PCR. The
primers used for beclin 1 and the internal control glyceraldehyde
3-phosphate dehydrogenase (Sangon Biotech Co., Ltd., Shanghai,
China) are indicated in Table I. The
reaction was conducted using GeneAmp PCR System 9700 (Applied
Biosystems; Thermo Fisher Scientific) and performed at 37°C for 15
min, followed by 5 min at 98°C, then 35 cycles at 94°C for 20 sec,
53°C for 30 sec and 72°C for 40 sec. PCR products were evaluated on
2% agarose gels (Thermo Fisher Scientific, Inc.) containing 0.5
µg/ml ethidium bromide (Thermo Fisher Scientific, Inc.), and
photographed under an ultraviolet (UV) transilluminator (FR980;
Shanghai Furi Science & Technology Co., Ltd., Shanghai, China).
AlphaEaseFC 4.0 software (Genetic Technologies, Inc., Miami, FL,
USA) was used to semiquantitatively analyze the relative light
intensities of the bands. Triplicate experiments with triplicate
samples were performed.
 | Table I.Primer information. |
Table I.
Primer information.
| Gene | Primer sequence |
|---|
| Beclin 1 |
|
|
Forward |
5′-AGGAACTCACAGCTCCATTAC-3′ |
|
Reverse |
5′-AATGGCTCCTCTCCTGAGTT-3′ |
| GAPDH |
|
|
Forward |
5′-GCACCGTCAAGGCTGAGAA-3′ |
|
Reverse |
5′-AGGTCCACCACTGACACGTTT-3′′ |
Western blotting
Cells were harvested and cell lysates (30 µg
protein/lane) were fractionated by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (Invitrogen; Thermo
Fisher Scientific, Inc.) once quantified. The proteins were
electrotransferred onto polyvinylidene fluoride membranes (EMD
Millipore, Billerica, MA, USA) and blocked with 5% nonfat dry milk
(BBI Life Sciences Corporation, Shanghai, China) for 1 h at room
temperature. Next, the proteins were detected following incubation
for 1.5 h at room temperature with the following primary
antibodies: Anti-beclin 1 (1:5,000; rabbit monoclonal; catalog no.
ab51031; Abcam, Cambridge, MA, USA), anti-caspase-3 (1:1,000;
rabbit polyclonal; catalog no. 9662; Cell Signaling Technology,
Inc., Danvers, MA, USA), anti-Bcl-2 (1:1,000; catalog no. 2872;
rabbit polyclonal; Cell Signaling Technology, Inc.),
anti-Bcl-2-associated X protein (Bax; 1:1,000; rabbit polyclonal;
catalog no. 2772; Cell Signaling Technology, Inc.) and
anti-β-actin, (1:10,000; mouse monoclonal; catalog no. ab6276;
Abcam), which served as loading control. Following washes with
Tris-buffered saline containing 0.05% Tween 20 (Sinopharm Chemical
Reagent Co., Ltd., Shanghai, China), membranes were incubated with
horseradish peroxidase-conjugated goat anti-rabbit (1:1,000;
catalog no. ZB-5301) and goat anti-mouse (1:1,000; catalog no.
ZB-5305) secondary antibodies (Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd., Beijing, China) for 40 min at room
temperature. The bound antibodies were visualized using an enhanced
chemiluminescence reagent (EMD Millipore) and quantified by
densitometry using ChemiDoc™ XRS+ image analyzer (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Densitometric analyses of
the bands were adjusted with β-actin. Triplicate experiments with
triplicate samples were performed.
Hoechst 33258 staining
Apoptotic cells were evaluated by observing their
morphology using Hoechst 33258 staining (Sigma-Aldrich, St. Louis,
MO, USA). Cells of different groups were seeded in 6-well plates at
a density of 3×105 cells/well, and fixed with 3.7%
paraformaldelyde (Sigma-Aldrich) for 30 min at room temperature 24
h later. Next, cells were washed with PBS 3 times for 5 min each
and stained with Hoechst 33258 at 37°C for 30 min, prior to be
observed under a fluorescence microscope equipped with a UV filter
(Eclipse Ti; Nikon Corporation, Tokyo, Japan) at ×200
magnification.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay for the detection of cell proliferation
Following transfection, cells of different groups
were collected, seeded in 96-well plates (3×103
cells/well) and cultured for 1–7 days. In each group, the medium
was removed each day, and the wells were washed with
phosphate-buffered saline (Beijing Solarbio Science &
Technology Co., Ltd., Beijing, China). MTT assay was performed by
adding 20 µl of 5 mg/ml MTT (Sigma-Aldrich) for 4 h. The absorbance
of the solution was then measured at 570 nm on a ThermoMax
microplate reader (Molecular Devices, LLC, Sunnyvale, CA, USA).
Cell growth curves of each group were generated according the
results obtained. Triplicate experiments with triplicate samples
were performed.
Chemosensitivity assay
Cells of different groups at logarithmic phase were
collected and seeded in 96-well plates (5×103
cells/well). The medium of each group was removed overnight, and
the cells were exposed to increasing concentrations of
Taxol® (0, 0.1, 1, 10, 100, 500 and 1,000 µg/ml) for 48
h. The results of the above MTT assay were used to calculate the
inhibition rate and the half maximal inhibitory concentration
(IC50) values of Taxol®.
Statistical analysis
Data were expressed as the mean ± standard
deviation. Statistical software SPSS version 13.0 (SPSS, Inc.,
Chicago, IL, USA) was used for the assessment. Student's t test was
used to compare the means of two groups and one-way analysis of
variance was used for comparing the means of multiple samples.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression of beclin 1 at the
protein and messenger (m)RNA levels
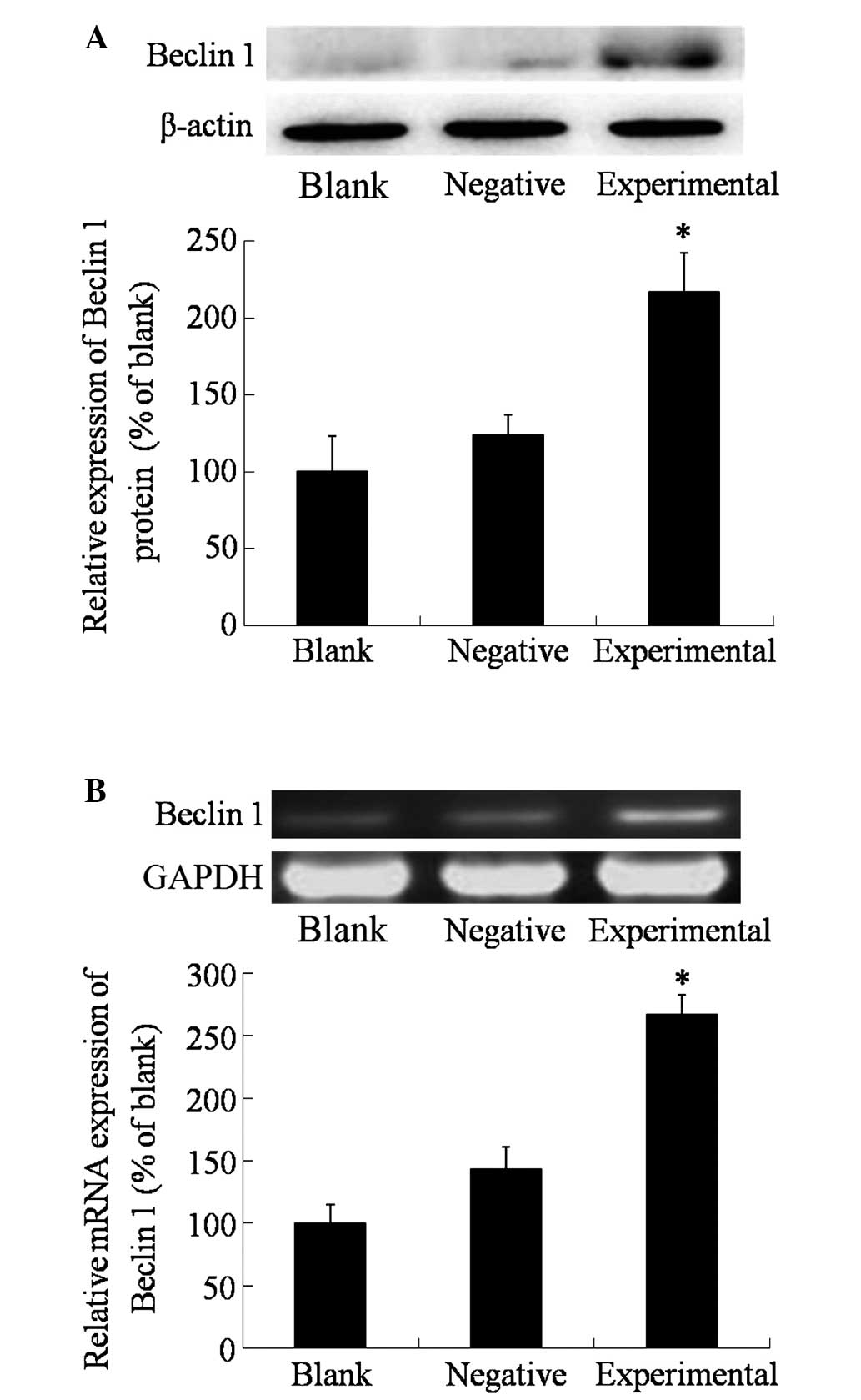
Upon transfection, the protein and mRNA expression
levels of beclin 1 in the different groups were measured using
western blotting and RT-PCR, respectively. As shown in Fig. 1, the expression of beclin 1 in the
blank and negative control groups was relatively low, and there was
no significant differences between the two groups (P>0.05).
However, in the experimental group, the expression of beclin 1 was
significantly higher than that in the blank and negative control
groups (P=0.0364), which indicated the success of the transfection
with the beclin 1 expression vector.
Effect of beclin 1 expression on the
proliferation and apoptosis of HeLa cells
The growth curve of HeLa cells in the different
groups was obtained 1–7 days post-transfection. The data indicated
that the HeLa cells in the blank and negative control groups were
in good condition and grew normally as a sigmoid curve, while the
proliferation of HeLa cells transfected with beclin 1 expression
vector in the experimental group was significantly inhibited
(P=0.0245; Fig. 2). As shown in
Fig. 2, the survival of the cells in
the experimental group started to decrease from day 4
post-transfection.
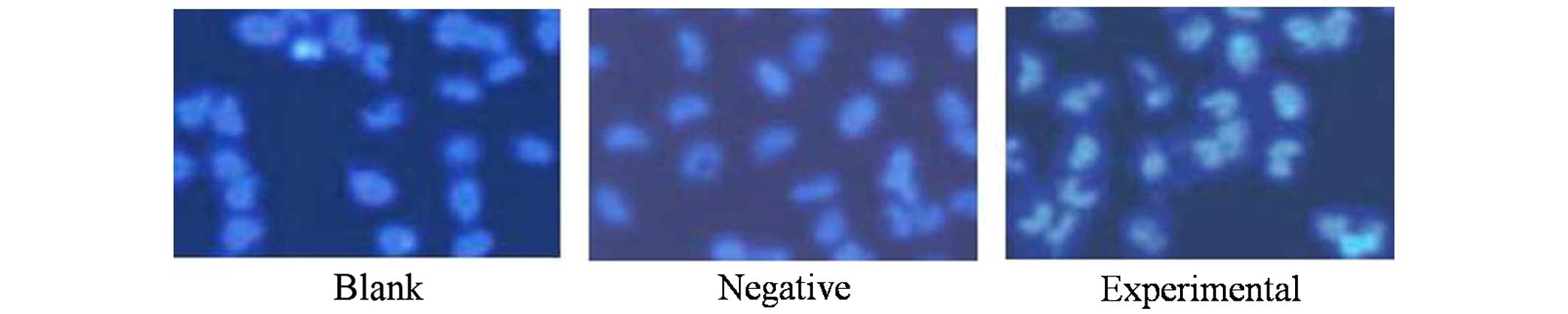
Hoechst 33258 staining for the detection of
morphological changes was employed to evaluate the effect of beclin
1 expression on HeLa cells. Following transfection, cells were
stained with Hoechst 33258, and apoptotic morphological changes
were observed in all the three groups. In the blank and negative
control groups, the nuclei of HeLa cells were round and
homogeneously stained (Fig. 3) with a
low rate of apoptosis, while the cells in the experimental group
exhibited evident apoptotic characteristics due to the increase in
the levels of beclin 1, including cell shrinkage, membrane
integrity loss or deformation, nuclear fragmentation and chromatin
compaction of late apoptotic appearance.
As detected by western blot (Fig. 4), the expression of the apoptotic
protein caspase-3 was elevated in the experimental cells that
exhibited high expression levels of beclin 1 (P=0.0163), which
indicated that beclin 1 may activate the caspase-dependent
apoptosis signaling pathway. In addition, the Bcl-2:Bax ratio was
decreased in HeLa cells overexpressing beclin 1, which further
confirmed that high expression of beclin 1 could activate cell
apoptosis.
Effect of beclin 1 expression on the
chemosensitivity towards Taxol® of HeLa cells
The effect of beclin 1 expression on the
chemosensitivity of HeLa cells towards Taxol® was
evaluated using MTT assay, by measuring the proliferation
inhibitory effects of the different concentrations of
Taxol® tested in each group. As shown in Fig. 5, the inhibition rate of
Taxol® in the experimental group was higher than in the
other two groups at the same concentration. The IC50
values of Taxol® were 30.4, 118.0 and 125.5 µg/ml in the
experimental, blank control and negative control groups,
respectively. There were no significant differences between the
blank and negative control groups (P>0.05), while in the
experimental group, overexpression of beclin 1 significantly
reduced the IC50 value of Taxol®
(P=0.0148).
Discussion
Autophagy is a process of self-digestion of the
cytoplasm and organelles of a cell, in which cytoplasmic
constituents such as long-lived proteins, protein aggregates and
entire organelles are targeted to lysosomes for degradation by
means of double-membrane vesicles called autophagosomes (14). Autophagy is an evolutionarily
conserved mechanism of cell survival that usually occurs at a low
basal level under normal conditions in order to maintain cellular
homeostasis (15). Autophagy is
highly induced by multiple stimuli, including starvation and
metabolic stress (16). Dysfunction
of the autophagic pathway has been implicated in various diseases,
including cancer, obesity, cardiac disease, neurodegeneration,
aging, infection and inflammatory diseases (17–19).
However, the role of autophagy in association with cancer and
tumorigenesis is complex and highly context-dependent (20). Autophagy may be involved in cell cycle
regulation, apoptosis, angiogenesis and other aspects of tumor
initiation and progression (21).
Previous studies have reported that beclin 1 is absent or expressed
at very low levels in a wide variety of human tumors, and mutations
in the beclin 1 gene have been detected in numerous types of cancer
(2,13,22–25). A
previous study demonstrated that ectopic expression of beclin 1 in
MCF-7 cells activates autophagy, inhibits cellular proliferation
and clonogenicity, and suppresses tumorigenesis in mouse xenograft
models (26).
There are a number of studies reporting that beclin
1 expression is associated with chemosensitivity (27,28).
Beclin 1 may affect the chemosensitivity of cancer cells through
several mechanisms. For example, in a previous study on apoptosis
induced by etoposide in cervical cancer CaSki cells, overexpression
of beclin 1 increased the chemosensitivity and apoptosis rate of
CaSki cells (2,11,29). In
the present study, a beclin 1 expression vector was constructed and
transfected into HeLa cells in order to obtain HeLa cells with high
expression levels of beclin 1. The results indicated that,
following transfection with this beclin 1 expression vector, the
expression of beclin 1 was significantly increased at the mRNA and
protein levels. Overexpression of beclin 1 in HeLa cells resulted
in enhancement of the autophagy process, increased cell apoptosis
and inhibition of cell proliferation. By contrast, in the blank and
negative control groups, HeLa cells exhibited normal proliferation,
with an obvious sigmoid growth curve.
In addition, the chemosensivity of HeLa cells to
Taxol® was detected by MTT assay. The results indicated
that the IC50 value of Taxol® in normal HeLa
cells was 118.0 µg/ml, while in HeLa cells transfected with a blank
plasmid the IC50 value of Taxol® was 125.5
µg/ml, and in HeLa cells transfected with the beclin 1 expression
plasmid this value was reduced to 30.4 µg/ml. It was also observed
that the growth curve of HeLa cells overexpressing beclin 1 was
markedly shifted to the left, indicating that the chemotherapy
sensitivity of HeLa cells towards Taxol® was markedly
increased upon overexpression of beclin 1.
The autophagic gene beclin 1 regulates apoptosis in
different ways in different cells, and a close association exists
between autophagy and apoptosis (30). Bcl-2 and Bax are upstream proteins in
the mitochondria-mediated apoptotic pathway, and are important
regulatory factors of the permeability of the mitochondrial
membrane (31). Abnormal signaling of
Bcl-2/Bax may affect the release of cytochrome c and the
activation of the downstream protease caspase-3, thus further
mediating cell survival or cell death (32–34). In
the present study, overexpression of beclin 1 could reduce the
ratio of Bcl-2/Bax and enhance the expression of caspase-3,
indicating that in HeLa cells overexpressing beclin 1, the
Bcl-2/Bax and caspase-3 apoptosis signaling pathways were
activated.
In conclusion, the present study demonstrated that,
following transfection with beclin 1 expression plasmid, the
relative mRNA and protein expression levels of beclin 1 were
significantly enhanced, cell proliferation was significantly
reduced, cell apoptosis rate was increased and the
Bcl-2/Bax/caspase-3 apoptosis signaling pathway was activated in
HeLa cells. In addition, the IC50 of HeLa cells to
paclitaxel was significantly decreased, and its sensitivity to this
drug was improved, suggesting that in cervical cancer HeLa cells,
beclin 1 may not only regulate the process of autophagy, but it may
also regulate cell apoptosis and enhance the sensitivity of
cervical cancer cells to paclitaxel. The present results provide
novel targets and strategies for improving drug resistance and gene
therapy in cervical cancer.
References
|
1
|
Jia Y, Li S, Yang R, Zhou H, Xiang Q, Hu
T, Zhang Q, Chen Z, Ma D and Feng L: Knowledge about cervical
cancer and barriers of screening program among women in Wufeng
County, a high-incidence region of cervical cancer in China. PLoS
One. 8:e670052013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sun Y, Liu JH, Sui YX, Jin L, Yang Y, Lin
SM and Shi H: Beclin1 overexpression inhibitis proliferation,
invasion and migration of CaSki cervical cancer cells. Asian Pac J
Cancer Prev. 12:1269–1273. 2011.PubMed/NCBI
|
|
3
|
Zhang SK, Kang LN, Chang IJ, Zhao FH, Hu
SY, Chen W, Shi JF, Zhang X, Pan QJ, Li SM and Qiao YL: The natural
history of cervical cancer in Chinese women: Results from an
11-year follow-up study in China using a multistate model. Cancer
Epidemiol Biomarkers Prev. 23:1298–1305. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Deng Q, Wang Z, Wang L, Zhang L, Xiang X,
Wang Z and Chong T: Lower mRNA and protein expression levels of LC3
and Beclin1, markers of autophagy, were correlated with progression
of renal clear cell carcinoma. Jpn J Clin Oncol. 43:1261–1268.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang ZH, Li L, Peng ZL and Duan ZL: Effect
of autophagy gene Beclin 1 on the growth of cervical cancer HeLa
cells in vitro and in vivo. Zhonghua Zhong Liu Za Zhi. 33:804–809.
2011.(In Chinese). PubMed/NCBI
|
|
6
|
Denton D, Nicolson S and Kumar S: Cell
death by autophagy: Facts and apparent artefacts. Cell Death
Differ. 19:87–95. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liang XH, Kleeman LK, Jiang HH, Gordon G,
Goldman JE, Berry G, Herman B and Levine B: Protection against
fatal Sindbis virus encephalitis by beclin, a novel
Bcl-2-interacting protein. J Virol. 72:8586–8596. 1998.PubMed/NCBI
|
|
8
|
Oberstein A, Jeffrey PD and Shi Y: Crystal
structure of the Bcl-XL-Beclin 1 peptide complex: Beclin 1 is a
novel BH3-only protein. J Biol Chem. 282:13123–13132. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu B, Bao JK, Yang JM and Cheng Y:
Targeting autophagic pathways for cancer drug discovery. Chin J
Cancer. 32:113–20. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Meijer AJ and Codogno P: Regulation and
role of autophagy in mammalian cells. Int J Biochem Cell Biol.
36:2445–2462. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun Y, Liu JH, Jin L, Lin SM, Yang Y, Sui
YX and Shi H: Over-expression of the Beclin1 gene upregulates
chemosensitivity to anti-cancer drugs by enhancing therapy-induced
apoptosis in cervix squamous carcinoma CaSki cells. Cancer Lett.
294:204–210. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jin S and White E: Role of autophagy in
cancer: Management of metabolic stress. Autophagy. 3:28–31. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shin JY, Hong SH, Kang B, Minai-Tehrani A
and Cho MH: Overexpression of beclin1 induced autophagy and
apoptosis in lungs of K-rasLA1 mice. Lung Cancer. 81:362–370. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He C and Klionsky DJ: Regulation
mechanisms and signaling pathways of autophagy. Annu Rev Genet.
43:67–93. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Levine B and Kroemer G: Autophagy in the
pathogenesis of disease. Cell. 132:27–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kroemer G, Mariño G and Levine B:
Autophagy and the integrated stress response. Mol Cell. 40:280–293.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cheng Y, Ren X, Hait WN and Yang JM:
Therapeutic targeting of autophagy in disease: Biology and
pharmacology. Pharmacol Rev. 65:1162–1197. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kimmelman AC: The dynamic nature of
autophagy in cancer. Genes Dev. 25:1999–2010. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ravikumar B, Sarkar S, Davies JE, Futter
M, Garcia-Arencibia M, Green-Thompson ZW, Jimenez-Sanchez M,
Korolchuk VI, Lichtenberg M, Luo S, et al: Regulation of mammalian
autophagy in physiology and pathophysiology. Physiol Rev.
90:1383–1435. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ambjørn M, Ejlerskov P, Liu Y, Lees M,
Jäättelä M and Issazadeh-Navikas S: IFNB1/interferon-β-induced
autophagy in MCF-7 breast cancer cells counteracts its proapoptotic
function. Autophagy. 9:287–302. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Filippi-Chiela EC, Villodre ES, Zamin LL
and Lenz G: Autophagy interplay with apoptosis and cell cycle
regulation in the growth inhibiting effect of resveratrol in glioma
cells. PLoS One. 6:e208492011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wirawan E, Lippens S, Vanden Berghe T,
Romagnoli A, Fimia GM, Piacentini M and Vandenabeele P: Beclin1: A
role in membrane dynamics and beyond. Autophagy. 8:6–17. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li X, Yan J, Wang L, Xiao F, Yang Y, Guo X
and Wang H: Beclin1 inhibition promotes autophagy and decreases
gemcitabine-induced apoptosis in Miapaca2 pancreatic cancer cells.
Cancer Cell Int. 13:262013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao J, Wang ML, Li Z, Gao DM, Cai Y,
Chang J and Wang SP: Interferon-alpha-2b induces autophagy in
hepatocellular carcinoma cells through Beclin1 pathway. Cancer Biol
Med. 11:64–68. 2014.PubMed/NCBI
|
|
25
|
Duan ZL, Peng ZL, Wang ZH and Yan NH:
Correlation of autophagy gene Beclin1 to tumorigenesis and
development of epithelial ovarian cancer. Ai Zheng. 26:258–263.
2007.(In Chinese). PubMed/NCBI
|
|
26
|
Liang XH, Jackson S, Seaman M, Brown K,
Kempkes B, Hibshoosh H and Levine B: Induction of autophagy and
inhibition of tumorigenesis by beclin 1. Nature. 402:672–676. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu XY, Chen J, Cao QH, Dong M, Lin Q, Fan
XJ, Xia Q, Chen ZH, Liu Q and Wan XB: Beclin 1 activation enhances
chemosensitivity and predicts a favorable outcome for primary
duodenal adenocarcinoma. Tumour Biol. 34:713–722. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang TT, Cao QH, Chen MY, Xia Q, Fan XJ,
Ma XK, Lin Q, Jia CC, Dong M, Ruan DY, et al: Beclin 1 deficiency
correlated with lymph node metastasis, predicts a distinct outcome
in intrahepatic and extrahepatic cholangiocarcinoma. PLoS One.
8:e803172013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun Y, Jin L, Liu J, Lin S, Yang Y, Sui Y
and Shi H: Effect of autophagy on paclitaxel-induced CaSki cell
death. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 35:557–565.
2010.PubMed/NCBI
|
|
30
|
Niu TK, Cheng Y, Ren X and Yang JM:
Interaction of Beclin 1 with survivin regulates sensitivity of
human glioma cells to TRAIL-induced apoptosis. FEBS Lett.
584:3519–3524. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lindsay J, Esposti MD and Gilmore AP:
Bcl-2 proteins and mitochondria - specificity in membrane targeting
for death. Biochim Biophys Acta. 1813:532–539. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Du P, Cao H, Wu HR, Zhu BS, Wang HW, Gu
CW, Xing CG and Chen W: Blocking Bcl-2 leads to autophagy
activation and cell death of the HEPG2 liver cancer cell line.
Asian Pac J Cancer Prev. 14:5849–5854. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yao Q, Chen J, Lv Y, Wang T, Zhang J, Fan
J and Wang L: The significance of expression of autophagy-related
gene Beclin, Bcl-2, and Bax in breast cancer tissues. Tumour Biol.
32:1163–1171. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang W, Fan H, Zhou Y, Duan P, Zhao G and
Wu G: Knockdown of autophagy-related gene Beclin1 promotes cell
growth and inhibits apoptosis in the A549 human lung cancer cell
line. Mol Med Rep. 7:1501–1505. 2013.PubMed/NCBI
|