Introduction
Boswellic acids (BAs) are gum resin extracts of
Boswellia serrata (Indian frankincense) and other
Boswellia species, which are mainly composed of volatile
oils (5–15%), pure resin (55–66%) and mucus (12–23%) (1,2). The gum
resin typically contains 30% BA (3);
however, gum resins from various Boswellia species contrast
in BA composition. For example, the gum resin of Boswellia
serrata contains similar amounts of 11-keto-β-boswellic acid
(KBA) and acetyl-KBA (AKBA) (3.0–4.7% and 2.2–2.9%, respectively);
whereas, the gum resin of Boswellia carterii Birdw (African
frankincense) contains decreased amounts of KBA (0.5%) compared
with AKBA (3.3%). BAs are pentacyclic triterpenes, which may exist
in an α- or β-configuration (2,4). Various
pharmacological studies indicate that β-configurated derivatives
exert stronger bioactivities compared with the respective α-isomers
(5). Numerous Boswellia
serrata products with varied compositions have been subject to
clinical investigation.
BAs have long been considered useful adjunct
pharmacological agents for the treatment of patients with malignant
gliomas (6) and, more recently, brain
metastasis (7). Two principal modes
of action associated with BAs have been postulated. One theory
suggests that BAs have anti-inflammatory properties, which are
useful for containing edema formation in the context of growing
brain tumors (6). The second theory
proposes that BAs possess intrinsic antitumor cell properties,
which may decrease the tumor burden. The latter assumption has been
largely based on cell culture studies, which includes a previous
study from the Laboratory of Molecular Neuro-Oncology, Department
of Neurology, University Hospital Zurich (Zurich, Switzerland)
(8) and one in vivo study on
the C6 rat model, published over a decade ago (9). BA-induced glioma cell death was
previously characterized as apoptotic by morphology, but appeared
not to involve caspase activation or be rescued by B-cell lymphoma
2 family proteins (8).
The present study examined the effects of selected
BA derivatives (α- and β-configuration), with a focus on human
glioma stem-like cells in vitro, and examined whether these
agents act in synergy with temozolomide (TMZ) or irradiation, which
are the standard choice of care in glioblastoma (10).
Materials and methods
Materials and cell lines
AKBA and β-BA were isolated from the resin of
Boswellia serrata and purified by the Alpinia Institute
(Alpinia Laudanum Institute of Phytopharmaceutical Sciences AG,
Walenstadt, Switzerland). α-BA was purchased from Fluka
(Sigma-Aldrich International GmbH, St. Gallen, Switzerland). Stock
solutions of AKBA, α-BA and β-BA were prepared in 100% ethanol (MSD
Merck Sharp & Dohme AG, Lucerne, Switzerland). Batch-specific
control of AKBA and β-BA was performed using high-pressure liquid
chromatography (Alpinia Laudanum Institute of Phytopharmaceutical
Sciences AG, Walenstadt, Switzerland). Aliquots were stored at 4°C.
Schering-Plough TMZ was provided by Merck (Kenilworth, NJ, USA). A
200 mM stock solution of TMZ was prepared in dimethyl sulfoxide
(Sigma-Aldrich, St. Louis, MO, USA) and stored at −20°C.
The human glioma U87MG and T-98 G cell lines were
purchased from the American Type Culture Collection (Manassas, VA,
USA). The other long-term cell lines (LTCs), including LN-18,
LN-428, D-247MG, LN-319, A-172, LN-308 and LNT-229, were kindly
provided by Professor N. de Tribolet (Lausanne, Switzerland). In
addition to established cell lines, the primary patient-derived
glioma initiating cells (GICs) T-269, T-325, S-24, ZH-161 and
ZH-305 were also used in the present study, which were isolated in
the Laboratory of Molecular Neuro-Oncology, Department of
Neurology, University Hospital Zurich, as previously described
(11). LTCs were cultured in
Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) containing 10% fetal calf serum
(FCS; Gibco; Thermo Fisher Scientific, Inc.) and 2 mM glutamine
(Gibco; Thermo Fisher Scientific, Inc.). GIC were maintained in
Neurobasal Medium® (Gibco; Thermo Fisher Scientific,
Inc.) supplemented with 2% B-27 supplement (Gibco; Thermo Fisher
Scientific, Inc.), 2 mM glutamine, 20 ng/ml epidermal growth factor
(PeproTech EC Ltd., London, UK), 20 ng/ml fibroblast growth factor
(PeproTech EC Ltd.) and 32 international units/ml heparin
(Sigma-Aldrich). For irradiation experiments, cells were irradiated
in a Co-radiation source (60-Co Thermal Energy Systems; Sulzer AG,
Winterthur, Switzerland) at 1, 3 and 9 Gray (Gy) prior to the
addition of BA derivatives for 72 h in serum-free medium (SFM;
Gibco; Thermo Fisher Scientific, Inc.).
Acute viability assays
For LTCs, 10,000 cells/well were seeded in 96-well
plates (TPP; Sigma-Aldrich) in full medium (Gibco; Thermo Fisher
Scientific, Inc.) and allowed to attach for 24 h. The cells were
then exposed to the BA derivatives at 1.95–220.00 µM for 72 h in
SFM. GICs were seeded using the aforementioned method in neurobasal
medium (Gibco; Thermo Fisher Scientific, Inc.), incubated for 24 h
and then exposed to BA derivatives for 72 h. A
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay was used to determine the metabolic activity of LTC and GIC.
Briefly, MTT (Sigma-Aldrich) was dissolved in phosphate-buffered
saline (PBS). Sodium dodecyl sulfate (10%; Fluka; Sigma-Aldrich)
was used to dissolve the purple formazan. Absorbance was measured
using the Infinite M200 spectrophotometer (Tecan Schweiz AG,
Männedorf, Switzerland) at 540 nm. Solvent-treated cells (0.5%
ethanol; Merck) were used as a reference for comparison. In
addition, flow cytometry was used to assess the induction of
apoptotic vs. necrotic cell death. The cells were harvested with
accutase (Thermo Fisher Scientific, Inc.) and treated as indicated
in Fig. 3 in SFM, washed in PBS, and
resuspended in 10 mmol/l
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid/NaOH (pH 7.4),
140 mmol/l NaCl and 2.5 mmol/l CaCl2. Fluorescein
isothiocyanate (dilution, 1:100; Becton Dickinson AG, Allschwil,
Switzerland) or Pacific Blue-labelled Annexin V (AnxV; dilution,
1:100; BioLegend, Inc., San Diego, CA, USA) and propidium iodide
(PI; 50 µg/ml; Sigma-Aldrich) were added, and the fluorescence of
unfixed cells in a total of 10,000 events per condition was
recorded in a BD FACSVerse™ flow cytometer (Becton Dickinson AG).
AnxV- or PI-single or double-positive cells were quantified using
FlowJo Software (version 10.0.8; FlowJo LLC, Ashland, OR, USA).
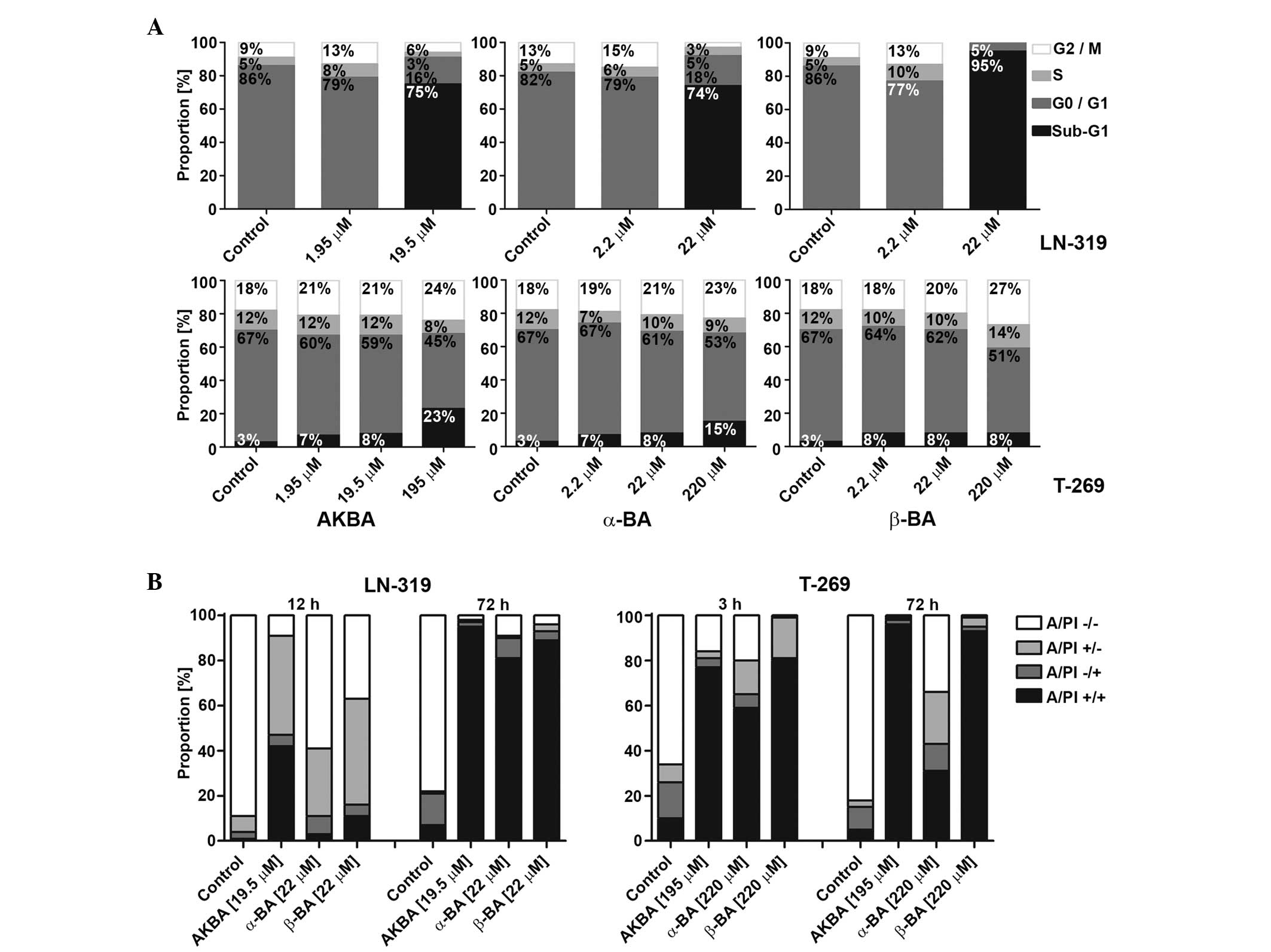 | Figure 3.Mode of glioma cell death induced by
AKBA, α-BA or β-BA. (A) LTC (LN-319) or GIC (T-269) were exposed to
AKBA at 1.95, 19.50 or 195.00 µM and α-BA or β-BA at 2.2, 22.0 or
220.0 µM for 72 h in serum-free (LTC) or neurobasal medium (GIC),
prior to flow cytometric cell cycle analysis. Cell distributions
are shown as bar graphs (black, sub-G1; dark grey, G0/G1; light
grey, S; white, G2/M). (B) Viability of LN-319 or T-269 was
determined by flow cytometry using A/PI staining following exposure
to 19.5 µM (LN-319) or 195.0 µM (T-269) AKBA and 22 µM (LN-319) or
220 µM (T-269) α-BA or β-BA for 3 or 12 and 72 h. Frequency plots
of double-negative (A/PI-/-), single-positive (A/PI-/+ or A/PI+/-),
or double-positive cells (A/PI+/+) are shown as bar graphs. BA,
boswellic acid; AKBA, acetyl-11-keto-β-boswellic acid; LTC,
long-term cell line; GIC, glioma stem-like culture; A/PI, Annexin
V/propidium iodide. |
Cell cycle analysis
The cells were treated as indicated in Fig. 3 in SFM, harvested with accutase, fixed
and permeabilized overnight in ice-cold 70% ethanol (Merck). The
cells were washed twice with PBS. RNA was digested with RNase A
(Gibco; Thermo Fisher Scientific, Inc.) and DNA was stained with PI
(50 µg/ml; Sigma-Aldrich) containing 0.1% Triton X-100
(Sigma-Aldrich) to permeabilize the cells. Fluorescence was
recorded in a BD FACSVerseTM flow cytometer (Becton Dickinson AG)
and data was analyzed using FlowJo Software (version 10.0.8). Cells
left of the G0/G1 peak were considered to have a DNA content of
<2n, indicative of cell death. Aggregated cells were gated
out.
Clonogenicity and spherogenicity
assays
For LTC, clonogenicity assays were performed by
seeding 200 cells per well in 96-well plates that were allowed to
adhere overnight in full medium. The cells were then exposed to BA
derivatives at increasing concentrations in SFM, as indicated in
Fig. 1. Subsequent to 24 h exposure,
FCS was added to each well to a final concentration of 10%, and the
assay was observed for at least 7 days. GICs were seeded at 200–400
cells per well in neurobasal medium and treated consecutively in
the aforementioned manner in neurobasal medium. The cell metabolism
of LTC and GIC was assessed by MTT assay.
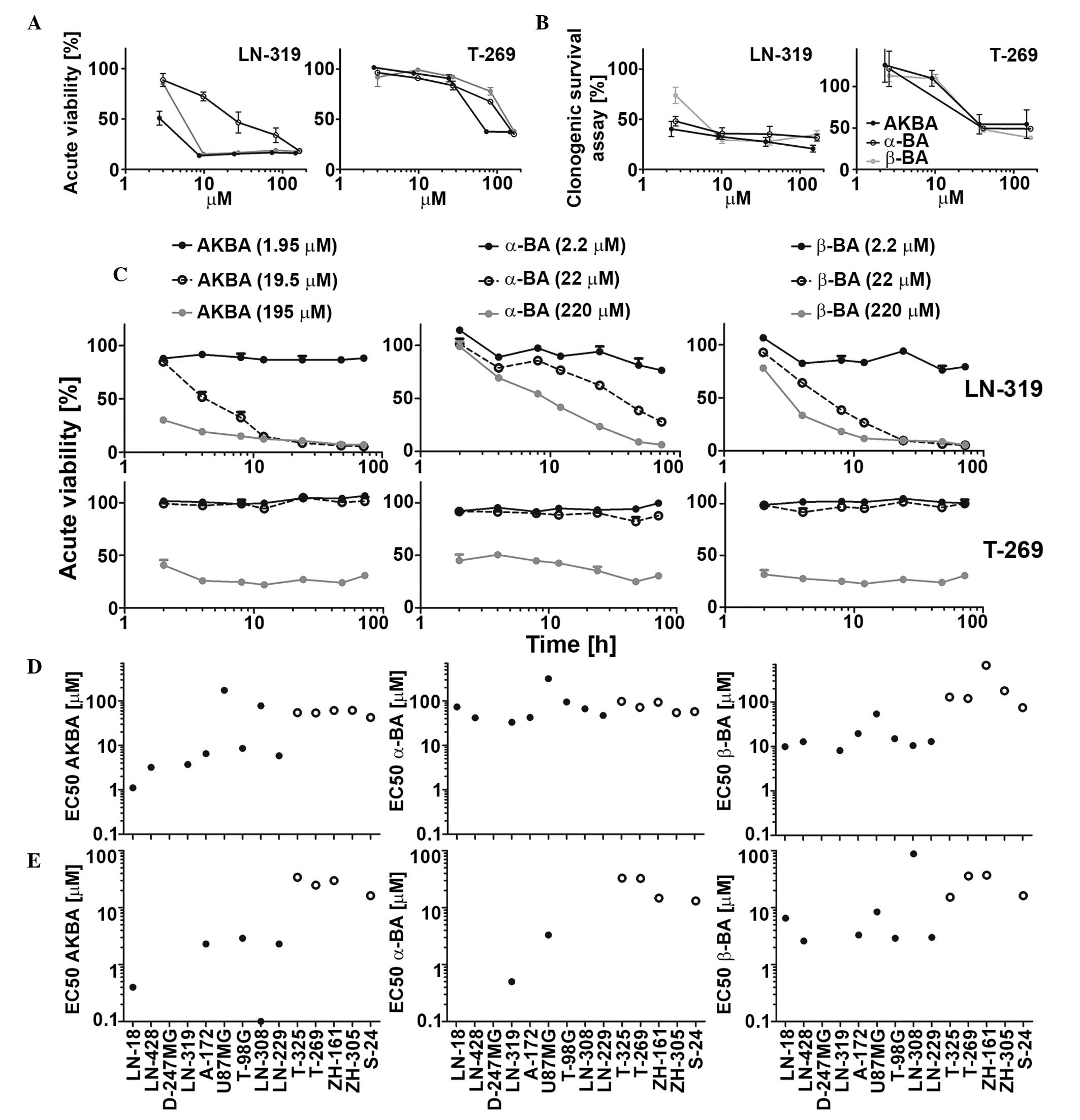 | Figure 1.Sensitivity of LTC and GIC to single
BA derivatives (AKBA, α-BA and β-BA) in acute viability and in
clonogenic survival assays. LN-319 LTCs or T-269 GICs were exposed
to increasing drug concentrations and metabolic activity was
assessed by MTT assay in (A) acute viability or (B) clonogenic
survival assays. (C) Cells were exposed to drugs (AKBA, 1.95, 19.50
or 195.00 µM; α-BA and β-BA, 2.2, 22.0 or 220.0 µM) for 2, 4, 8,
12, 24, 48 or 72 h and then subjected to MTT assays. Data are
expressed as mean and SEM (n=2). LTC (left, filled symbols) or GIC
(right, open symbols) were exposed to BA in a
concentration-dependent manner, and metabolic activity was assessed
by MTT assay in (D) acute viability or (E) clonogenicity assays.
EC50 values were calculated for each BA in every tested
cell line. Representative results of two independent experiments
are shown. The SEM was <15%. BA, boswellic acid; AKBA,
acetyl-11-keto-β-boswellic acid; LTC, long-term cell line; GIC,
glioma stem-like culture; MTT,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide;
EC50, half maximal effective concentration; SEM,
standard error of the mean. |
Data analysis
Data are representative of experiments performed
three times and the results were similar in all three experiments.
Statistical analysis was performed using GraphPad Prism 5 software
(GraphPad Software, Inc., La Jolla, CA, USA). The association
between the sensitivity of cell lines to BA and p53 and MGMT
expression was determined by Spearman's rank correlation
coefficient. Synergy of irradiation or TMZ and BA derivatives was
assessed by the fractional product method (12). Differences of 10% of observed vs.
predicted (additive) effect were considered synergistic. P<0.05
was considered to indicate a statistically significant difference.
The data are expressed as the mean ± standard error of the
mean.
Results
Intrinsic BA activity against LTC and
GIC glioma models
Three out of six major primary BA derivatives were
tested for acute growth inhibitory and anti-clonogenic properties
in a panel of nine LTC and five GIC models. Representative
concentration and time response curves for selected BA derivatives
are shown in Fig. 1A–C. The half
maximal effective concentration (EC50) values in acute
viability assays ranged between 1.1 and 314.4 µM in LTCs and
between 42.1 and 668.2 µM in GICs. AKBA showed the highest activity
in six out of nine LTCs and four out of five GICs (Fig. 1D). In clonogenic survival assays, the
EC50 values ranged between 0.1 and 88 µM in LTCs and
13.1 and 34.1 µM in GICs (Fig. 1E).
The EC50 values in acute viability and in clonogenic
assays were correlated (r=0.80, P=0.0025). Therefore, the results
of acute viability and clonogenic survival assays were similar
overall, suggesting that acute cytotoxicity is largely responsible
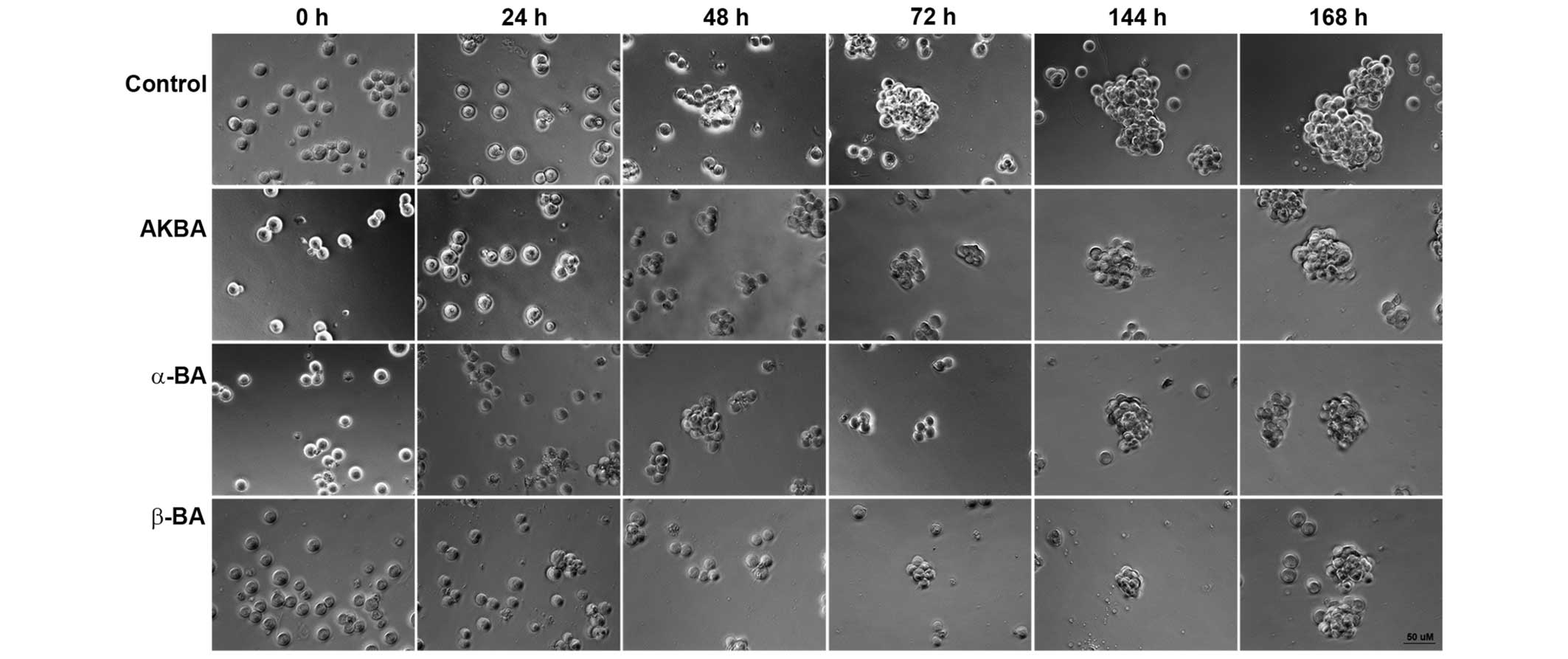
for the loss of clonogenicity or spherogenicity. Exposure to AKBA
(19.5 µM), α-BA (22.0 µM) or β-BA (22.0 µM) inhibited sphere
formation in ZH-161 (Fig. 2), T-269
or T-325 (data not shown).
In order to characterize the mode of growth
inhibition in the short-term assays, the cell cycle progression of
representative LTC (LN-319) or GIC (T-269) cells exposed to AKBA,
α-BA and β-BA were examined using flow cytometry at 72 h. In LTC,
within the concentration and time ranges of these experiments,
AKBA, α-BA and β-BA induced an increase of the sub-G1 fraction
associated with a strong decrease in G1, but only minor change in S
or G2/M cells, indicating cell death induction prior to or upon S
phase entry (Fig. 3A; upper panel).
In GIC, neither a cell cycle arrest nor an induction of cell death
prior or upon S phase entry was observed upon treatment with BA.
However, at the greatest drug concentrations, a trend towards
increased sub-G1 and G2/M fractions was observed (Fig. 3A; lower panel). In addition, flow
cytometry using AnxV/PI labeling for the early signs of apoptosis
and the assessment of primary or secondary necrosis confirmed the
induction of cell death, which evolved with early AnxV positivity
following 3 h (GIC) and 12 h (LTC) incubation and resulted in
secondary apoptotic events following 72 h incubation, consistent
with apoptotic cell death in LTC and GIC (Fig. 3B; left and right panels,
respectively). A possible association between tumor protein 53
(p53) or O6-methylguanine DNA methyltransferase
(MGMT) status and the sensitivity to BAs has also been
identified (13,14). Of the LTCs, U87MG, LNT-229, A-172 and
D247MG retain wild-type p53 function, determined by transcriptional
activity (13), whereas LN-18 and
T-98 G express MGMT (14). There was
no association between the sensitivity of the cell lines to BA and
p53 or MGMT status determined by the Spearman's rank
correlation coefficient (p53, r=−0.056, P=0.88; MGMT, r=0.089,
P=0.77) (13,14).
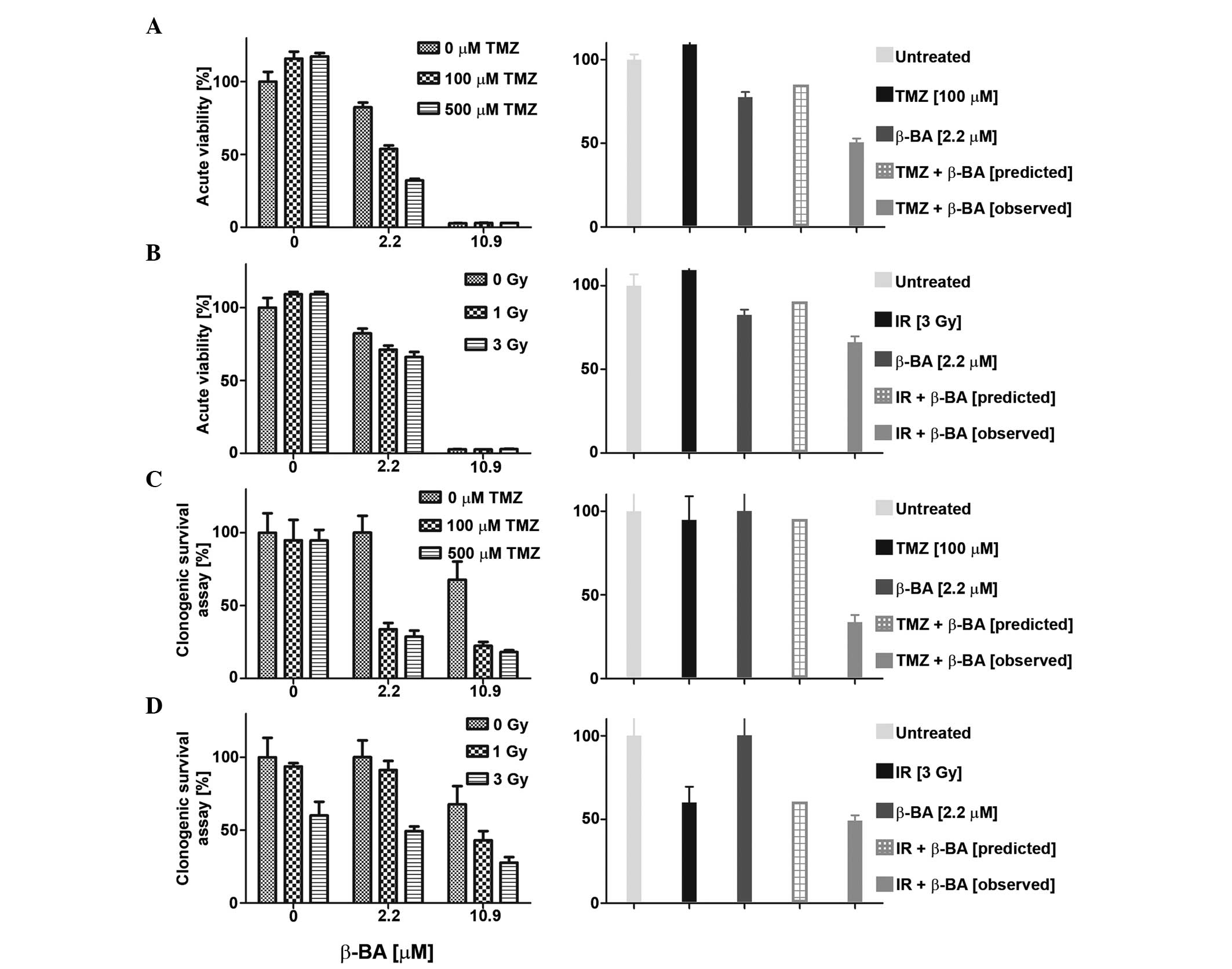
Synergistic activity of β-BA with
irradiation or TMZ
The sensitivity of glioma cell lines to combination
treatment with BA (AKBA, a-BA or β-BA) and TMZ (0, 100 or 500 µM)
or irradiation (1, 3 or 9 Gy) was also examined in acute viability
and clonogenic survival assays. Overall, the effects of combination
therapy were mostly additive and never antagonistic, as defined by
the fractional product method (data not shown) (12). Certain combinations of BA and TMZ or
irradiation met the criteria for synergistic inhibition, as
representatively shown in Fig. 4A–D.
For instance, co-treatment with β-BA and TMZ (Fig. 4A and C) or irradiation (Fig. 4B and D) revealed synergistic effects
at low BA concentrations (<EC50) in LN-319 cells in
the two experimental set-ups. No such synergistic effects were
observed for T-269 with either combination (data not shown).
Discussion
There is a great interest and requirement for novel
therapeutic options for glioblastoma, including phytotherapeutic
agents and agents targeting specifically tumor-associated edema
(6). BA derivatives have been used in
this indication for over a decade, mostly in central European
countries (2).
The present study confirms that BAs can exert
significant cytotoxic and antiproliferative activities on a panel
of LTCs and GICs (Fig. 1). These
results extend the similar findings of previous studies on LTCs
(8) to GIC models that were not
available at the time. Sphere formation by GICs was inhibited in a
concentration-dependent manner (Fig.
2). The biochemical mode of cytotoxic action of these compounds
and associated proximate molecular targets remains unclear.
Furthermore, the profound induction of cell death in glioma cell
cultures may require certain concentrations of BA; whether the
concentrations can be achieved for prolonged time periods in an
in vivo dosing setting remains uncertain. Several studies
examining BA plasma and brain levels in rats following the oral
administration of Boswellia serrate gum resin extracts
(9,15,16)
revealed that the brain demonstrates availability of the major BAs,
with β-BA and α-BA exhibiting greatest and KBA and AKBA exhibiting
the lowest levels (15). However, the
achievable BA levels described by Gerbeth et al (15) were decreased compared with the
EC50 concentrations determined to be cytotoxic and
antiproliferative for a panel of LTCs and GICs in the present
study. This inconsistency may be due to the lack of radiological
partial or complete responses in glioblastoma patients exposed to
these agents.
From the current knowledge of glioma cells, it is
unlikely that the candidate biochemical targets of BAs, including
5-lipoxygenase, prostaglandin E synthase (PGES) or cathepsin G, are
sufficiently essential for glioma cell viability to represent a
lethal pharmacological target (2).
However, to formally prove or disprove this hypothesis, the targets
would require pharmacological or genetic deletion in order to
assess whether the toxic effect of BAs may be reproduced.
Alternatively, in an in vivo setting, the
targets of BA may be affected in glioma cells and in the
infiltrating host cell population, which contributes to tumor
growth and is now considered a promising co-target in the treatment
of gliomas (17). For example, the
inhibition of cyclooxygenase-2, which regulates the production of
prostaglandin E2 by PGES-1, was indicated to delay glioma
development in a murine glioma model by inhibiting the development
of myeloid derived suppressor cells (MDSCs) and the accumulation of
the cells in the tumor microenvironment (18).
Therefore, anti-glioma effects may be inhibited by
interfering with the infiltration of the tumor by MDSCs, microglial
cells and macrophages, and with proinflammatory or potentially
protumorigenic activities. To corroborate this assumption would
require the establishment of an in vivo paradigm of BA, the
control of glioma growth and the careful characterization of the
host cell composition and inflammatory and immunological activity.
The present data confirm that BAs possess intrinsic cytotoxic
properties at low micromolecular concentrations and may potentially
obtain synergy with irradiation and temozolomide. Therefore, BAs
should be considered for the treatment of glioblastoma, although
the proximate pharmacodynamic targets remain to be identified.
Acknowledgements
The present study was supported by the Alpinia
Institute (Walenstadt, Switzerland).
References
|
1.
|
Kreck C and Saller R: Indischer Weihrauch
und seine Zubereitungen einschliesslich H15 als traditionelles und
modernes Therapeutikum. Internist Prax. 38:857–872. 1998.
|
|
2.
|
Abdel-Tawab M, Werz O and
Schubert-Zsilavecz M: Boswellia serrata: An overall
assessment of in vitro, preclinical, pharmacokinetic and
clinical data. Clin Pharmacokinet. 50:349–369. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Basch E, Boon H, Davies-Heerema T, Foppo
I, Hashmi S, Hasskarl J, Sollars D and Ulbricht C:
Boswellia: An evidence-based systematic review by the
Natural Standard Research Collaboration. J Herb Pharmacother.
4:63–83. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Gerbeth K, Meins J, Kirste S, Momm F,
Schubert-Zsilavecz M and Abdel-Tawab M: Determination of major
boswellic acids in plasma by high-pressure liquid
chromatography/mass spectrometry. J Pharm Biomed Anal. 56:998–1005.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Poeckel D and Werz O: Boswellic acids:
Biological actions and molecular targets. Curr Med Chem.
13:3359–3369. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Streffer JR, Bitzer M, Schabet M, Dichgans
J and Weller M: Response of radiochemotherapy-associated cerebral
edema to a phytotherapeutic agent, H15. Neurology. 56:1219–1221.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Kirste S, Treier M, Wehrle SJ, Becker G,
Abdel-Tawab M, Gerbeth K, Hug MJ, Lubrich B, Grosu AL and Momm F:
Boswellia serrata acts on cerebral edema in patients
irradiated for brain tumors: A prospective, randomized,
placebo-controlled, double-blind pilot trial. Cancer.
117:3788–3795. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Glaser T, Winter S, Groscurth P, Safayhi
H, Sailer ER, Ammon HP, Schabet M and Weller M: Boswellic acids and
malignant glioma: Induction of apoptosis but no modulation of drug
sensitivity. Br J Cancer. 80:756–765. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Winking M, Sarikaya S, Rahmanian A,
Jödicke A and Böker DK: Boswellic acids inhibit glioma growth: A
new treatment option? J Neurooncol. 46:97–103. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Weller M, van den Bent M, Hopkins K, Tonn
JC, Stupp R, Falini A, Cohen-Jonathan-Moyal E, Frappaz D,
Henriksson R, Balana C, et al: EANO guideline for the diagnosis and
treatment of anaplastic gliomas and glioblastoma. Lancet Oncol.
15:e395–e403. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Günther HS, Schmidt NO, Phillips HS,
Kemming D, Kharbanda S, Soriano R, Modrusan Z, Meissner H, Westphal
M and Lamszus K: Glioblastoma-derived stem cell-enriched cultures
form distinct subgroups according to molecular and phenotypic
criteria. Oncogene. 27:2897–2909. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Webb JL: Enzyme and Metabolic Inhibitors.
1:New York, NY: Academic Press. 55–79, and pp 488–512. 1963.
|
|
13.
|
Wischhusen J, Naumann U, Ohgaki H,
Rastinejad F and Weller M: CP-31398, a novel p53-stabilizing agent,
induces p53-dependent and p53-independent glioma cell death.
Oncogene. 22:8233–8245. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Hermisson M, Klumpp A, Wick W, Wischhusen
J, Nagel G, Roos W, Kaina B and Weller M: O6-methylguanine DNA
methyltransferase and p53 status predict temozolomide sensitivity
in human malignant glioma cells. J Neurochem. 96:766–776. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Gerbeth K, Hüsch J, Fricker G, Werz O,
Schubert-Zsilavecz M and Abdel-Tawab M: In vitro metabolism,
permeation and brain availability of six major boswellic acids from
Boswellia serrata gum resins. Fitoterapia. 84:99–106. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Reising K, Meins J, Bastian B, Eckert G,
Mueller WE, Schubert-Zsilavecz M and Abdel-Tawab M: Determination
of boswellic acids in brain and plasma by high-performance liquid
chromatography/tandem mass spectrometry. Anal Chem. 77:6640–6645.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Charles NA, Holland EC, Gilbertson R,
Glass R and Kettenmann H: The brain tumor microenvironment. Glia.
59:1169–1180. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Fujita M, Kohanbash G, Fellows-Mayle W,
Hamilton RL, Komohara Y, Decker SA, Ohlfest JR and Okada H: COX-2
blockade suppresses gliomagenesis by inhibiting myeloid-derived
suppressor cells. Cancer Res. 71:2664–2674. 2011. View Article : Google Scholar : PubMed/NCBI
|