Introduction
Papillary thyroid microcarcinoma (PTMC), which is a
particular variant of papillary thyroid carcinoma, is defined as a
papillary thyroid cancer measuring <1.0 cm at its maximal
diameter and accounts for ~85% of thyroid tumors in the United
States (1). To date, the diagnosis of
PTMC has been highly reliant on high frequency ultrasound
examination. Clinical outcomes for patients with PTMC are
excellent; the 10-year survival rate for patients with PTMC is
>90% (2). However, the
preoperative diagnostic rate is low, and PTMC is often misdiagnosed
due to its slow growth rate, absence of specific symptoms or
clinical characteristics and potential co-occurrence with benign
thyroid diseases (3). Currently, the
most effective treatment for PTMC remains disputed. Certain
researchers propose a “wait and see” approach until the tumor
reaches a large size, while others propose early treatment
following diagnosis (4,5). Although there have been a number of
reports regarding the ultrasonic diagnosis of PTMC in China and
other countries (6,7), few cases of PTMC associated with a
thyroid abnormality (including primary hyperthyroidism, Hashimoto's
thyroiditis and nodular goiter) have been diagnosed using
ultrasonography (8–10).
In the present study, 38 nodules that were isolated
from the thyroid glands of patients with PTMC and coexisting benign
thyroid diseases between April 2010 and September 2013 were
retrospectively analyzed. Of these nodules, 21 coexisted with
Hashimoto's thyroiditis (HT), 10 with nodular goiter and 7 with
hyperthyroidism (PTH). Furthermore, ultrasonic images of 56 nodules
from patients with benign nodular goiter were used as controls. The
present study aimed to investigate the value of ultrasonography in
the diagnosis of PTMC coexisting with a thyroid abnormality, and to
improve the accuracy of PTMC diagnosis.
Materials and methods
Patients and nodule collection
The present study was approved by the Ethics
Committee of the Municipal Hospital of Taizhou University School of
Medicine (Taizhou, China), and written informed consent was
obtained from all patients. The present study retrospectively
analyzed 94 nodules collected from patients with thyroid disorders
at the Municipal Hospital of Taizhou University School of Medicine
between April 2010 and September 2013. The thyroid disorders had
been diagnosed with high frequency ultrasonography, and confirmed
by surgical resection and pathological examination. Of these
patients, 34 were men and 60 were women, and 38 were diagnosed with
PTMC and 56 with benign nodular goiter, which were considered as
the controls. The mean age of the patients was 45 years (range,
15–86 years). Patients with PTMC or benign thyroid disease who
could not tolerate, or were not willing to receive, surgery were
excluded from the present study.
Instruments and imaging
procedures
All ultrasound examinations were performed with the
Acuson Sequoia-512 (Siemens AG, Munich, Germany) or the Toshiba
SSA-770A Color Doppler instrument (Toshiba Corporation, Tokyo,
Japan) equipped with a 5–12 MHz linear probe. For ultrasound of the
thyroid nodules, the lobes and isthmus of the thyroid gland were
sectioned and examined to observe the shape, boundary, margin,
echotexture, microcalcification and intranodular or surrounding
vascularity.
According to the latest guidelines for Ultrasound on
Thyroid Gland in Chinese Physicians Branch of Ultrasound Doctor
(11), the following eight aspects of
the nodules were analyzed: i) Shape, in particular whether a nodule
was regular or irregular (a nodule was deemed regular when it was
ovoid or round); ii) aspect ratio, in particular, whether the
aspect ratio was more than or less than one (the aspect ratio was
defined as the ratio of the anteroposterior diameter to the
transverse diameter of the nodule); iii) boundary, in particular,
whether the boundary of a nodule was clear or unclear (a nodule had
a clear boundary when there was a definite boundary between the
nodule and peripheral tissue); iv) margin, in particular whether a
margin was smooth or rough (smoothness was defined when there was a
clear change between the nodule and peripheral tissue, whereas the
margin was rough when the nodule was angulated, lobulate, blurred
or obscured); v) echogenicity, specifically, whether a nodule
showed intranodular hypoechogenicity or non-hypoechogenicity (the
echogenicity of a nodule with respect to the adjacent tissue was
classified as hypoechoic, isoechoic, hyperechoic or mixed echoic);
vi) uniformity of the echotexture, in particular whether it was
homogeneous or heterogeneous (a heterogeneous nodule was defined as
having varying echoic levels); vii) the presence or absence of
microcalcification (microcalcification was deemed present when
there were several strong, punctate echogenic foci of ≤2 mm that
were distributed as clusters or scattering spots); and viii)
vascularity type, according to the distribution of blood flow in
the nodule (vascularity patterns were determined to be mixed type,
no vascular type, peripheral type or central type).
Statistical analysis
Statistical analyses were performed using SPSS 18.0
software (SPSS, Inc., Chicago, IL, USA). Student's t-tests
were used for comparing quantitative variables between PTMC and
benign control groups, and χ2-tests were used to compare
two-dimensional images. P<0.05 was considered to indicate a
statistically significant difference.
Results
Histopathological examination
The diagnosis of PTMC via histological examination
included 29 isolated nodules and 9 double focal nodules (3
unilateral and 6 bilateral multifocal nodules; in cases with
multiple lesions, the maximal lesion was investigated in the
present study). A total of 38 nodules derived from the thyroid
glands of patients with PTMC were associated with benign thyroid
diseases. Of these nodules, 21 were associated with HT, 10 with
nodular goiter and 7 with PTH.
Ultrasonic characteristics of all
nodules
A comparison of the ultrasonic results for PTMC and
benign nodules is shown in Table I.
The features that were significantly associated with PTMC included
an irregular shape, an aspect ratio of >1, a blurred boundary, a
spiculated margin, heterogeneous hypoechogenicity, the presence of
microcalcification and enlarged neck lymph nodes, as well as the
abundant distribution of blood flow. Furthermore, these features
showed a relatively high specificity and diagnostic accuracy
(P<0.05), although the presence of enlarged neck lymph nodes and
the abundant distribution of blood flow did not show a high
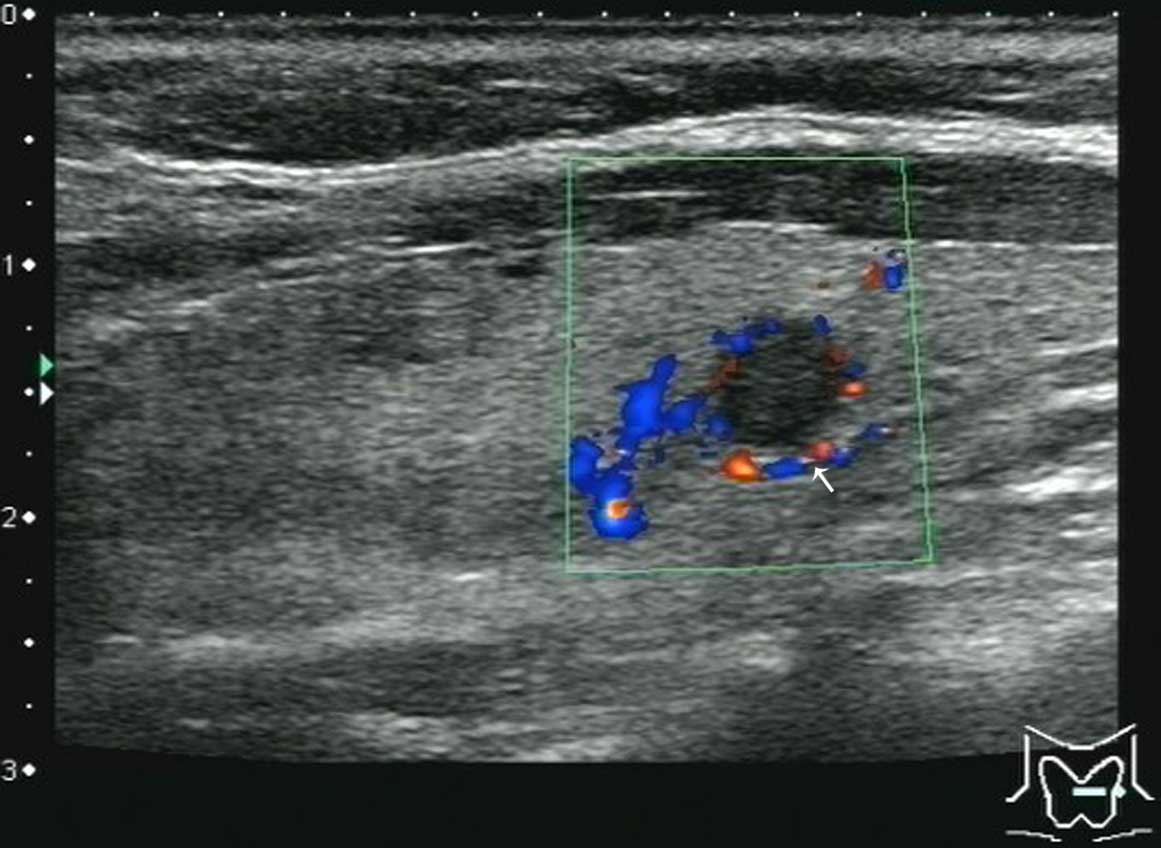
sensitivity. Figs. 1–7 reveal further information about the
nodules.
 | Table I.Comparison of the ultrasonic
characteristics of nodules between PTMC and thyroid disease. |
Table I.
Comparison of the ultrasonic
characteristics of nodules between PTMC and thyroid disease.
| Characteristics | PTMC | Thyroid disease | χ2 | P-value |
Sensitivitya |
Specificitya | Accuracya |
|---|
| Shape |
|
| 64.065 | <0.0001 | 35/38 (92.1) | 51/56 (91.0) | 86/94 (91.4) |
|
Irregular | 35 | 5 |
|
|
|
|
|
|
Regular | 3 | 51 |
|
|
|
|
|
| Aspect ratio |
|
| 26.284 | <0.0001 | 24/38 (63.1) | 49/56 (87.5) | 73/94 (77.7) |
| ≥1 | 24 | 7 |
|
|
|
|
|
|
<1 | 14 | 49 |
|
|
|
|
|
| Boundary/margin |
|
| 27.234 | <0.0001 | 28/38 (73.7) | 45/56 (80.4) | 73/94 (77.7) |
|
Blurred/spiculated | 28 | 11 |
|
|
|
|
|
|
Clear/smooth | 10 | 45 |
|
|
|
|
|
| Echo |
|
| 33.724 | <0.0001 | 34/38 (89.5) | 40/56 (71.4) | 74/94 (82.2) |
|
Heterogeneous/ | 34 | 16 |
|
|
|
|
|
|
hypoechogeneicity |
|
Other | 4 | 40 |
|
|
|
|
|
|
Microcalcification |
|
| 31.068 | <0.0001 | 25/38 (65.8) | 50/56 (89.3) | 75/94 (80.0) |
|
Present | 25 | 6 |
|
|
|
|
|
|
Absent | 13 | 50 |
|
|
|
|
|
| Enlarged neck lymph
node |
|
| 8.344 |
0.0050 | 10/38 (26.3) | 53/56 (94.7) | 63/94 (67.0) |
|
Present | 10 | 3 |
|
|
|
|
|
|
Absent | 28 | 53 |
|
|
|
|
|
| Blood flow
distribution |
|
| 17.742 | <0.0001 | 14/38 (36.8) | 54/56 (96.4) | 68/94 (72.3) |
| Grade
III | 14 | 2 |
|
|
|
|
|
| Grade
I–II | 24 | 54 |
|
|
|
|
|
Discussion
Ultrasonography, as the preferred imaging technique,
has been widely used to differentiate PTMC from benign nodules;
however, the majority of patients with PTMC have malignant nodules
(3). In the present study, the
ultrasonic features of malignant PTMC, compared with benign
nodules, were an irregular shape, an aspect ratio of >1, an
unclear boundary, a spiculated margin, heterogeneous
hypoechogenicity, microcalcification, the enlargement of neck lymph
nodes and the abundant distribution of blood flow. These
characteristics could easily differentiate PTMC from non-tumor
lesions and benign nodules. However, various types of PTMC have
been shown to coexist with other thyroid disease, rather than
independently. Therefore, the background ultrasonic images of the
thyroid parenchyma may effect the diagnosis of tumors. The
echogenicity of the normal thyroid parenchyma is intensive and
homogeneous compared with the isoechoic pattern of anterior muscle
and thyroid contours (10). Once the
echogenicity of the thyroid parenchyma becomes thickened,
heterogeneous and shows a nodular focus, diagnosing the disease
using ultrasonic images becomes a challenge (12). Due to a lack of understanding of PTMC,
primarily due to non-specific symptoms that may also indicate
benign thyroid disease, the accuracy rate of preoperative diagnosis
is very low. Therefore, investigating the ultrasonic features of
PTMC and the causes of misdiagnosis will improve the accuracy rate
of preoperative diagnosis.
HT and PTH are characterized by a reduced, thickened
and heterogeneous echogenicity of the thyroid parenchyma, which can
be classified into diffuse, nodular and restrictive types (13). Among these types, ultrasonic images of
the nodular pattern are complex and are easily mistaken for thyroid
malignant disease. Cases of nodular HT, PTH and PTMC coexisting
with HT or PTH nodules require differentiation from benign nodules
with HT or PTH. HT or PTH coexisting with a thyroid adenoma is
characterized by an intact envelope, a regular shape, peripheral
hypoechogenicity, an internal isoechoic or hyperechoic pattern and
the absence of microcalcification. When HT or PTH coexist with
nodular goiter, the majority of nodules are heterogeneous and
either cystic-solid, solid or cystic, and macrocalcification is
present. The nodules of patients with PTMC coexisting with HT or
PTH have an irregular shape, blurred margin, internal
hypoechogenicity, an aspect ratio of >1 and microcalcification.
In the present study, 7 nodules were characterized by a clear
boundary and an isoechoic pattern, which could be confused with a
benign nodule. However, the ultrasonic features included
intranodular microcalcification and an abundant blood flow, which
could be used to differentiate from a benign nodule. A previous
study demonstrated that HT with coexisting thyroid cancer showed
much coarser calcification, but less microcalcification compared
with HT without co-occurrence of a malignant cancer (14). The results of the present suggested
that the incidence of coarse calcification is uncommon (2 cases of
HT nodules coexisting with PTMC) compared with microcalcification
(13 cases of HT nodules coexisting with PTMC). These results may
have been due to the small sample size and bias associated with the
selection of surgical patients.
PTMC is often clinically undetectable in cases of
PTMC coexisting with nodular goiter due to its small size and deep
localization in the thyroid gland; however, nodules with a diameter
of 2–3 mm can be detected with the use of high-resolution
transducers (3). In addition, certain
features of nodules make the diagnosis of PTMC a challenge,
including a lack of specific ultrasonic characteristics when
combined with nodular goiter, multifocal pattern (13.33% tumors of
multifocal nodules) and complex and irregular ultrasonic images.
Therefore, radiologists should assess every nodule in patients with
nodular goiter, in particular hypoechoic solid nodules, by
multi-section imaging. Furthermore, the following features of a
nodules may aid in the diagnosis of PTMC in patients with a
background of nodular goiter: i) The absence of a clear boundary
without an evident halo; ii) an aspect ratio of ≥1; iii) the
presence of microcalcification; iv) an abundant blood flow; and v)
an unexplained enlargement of the lymph node, in particular in the
nodes of the middle and lower neck, and paratracheal lymph node
(15).
In a previous study, the detection rate of PTMC was
significantly decreased when it coexisted with nodular goiter, HT
or PTH compared with PTMC alone, since atypical nodules may be
ignored when scanning multiple foci (16). Potentially the most important features
for determining a diagnosis of PTMC include: i) The size of the
PTMC nodule is small and the lesion appears during the early stages
of the disease. In addition, infiltration of tumor cells into the
adjacent gland is not evident, and certain PTMC nodules have a
regular shape (8.0% in the present study). ii) The internal blood
flow of PTMC is significantly reduced compared with thyroid cancer
with a diameter of >1.0 mm (15).
In the present study, the blood flow of PTMC nodules was
predominantly grade I–II according to the Adler grading system,
accounting for 24 (63.2%) of the nodules, whereas only 14 (36.8%)
of the nodules were grade III (17).
This difference may be due to the fact that the growth of PTMC
nodules is slow, novel blood vessels are few and the vascular
structure resembles normal vessels (18). However, given the high specificity,
the blood distribution in PTMC nodules may be considered an
important indicator for the diagnosis of PTMC. PTMC often coexists
with benign nodules and the pathogenesis of PTMC is not
well-elucidated; thus, benign nodules are more readily diagnosed
compared with PTMC nodules, leading to the misdiagnosis of
PTMC.
In conclusion, the present study demonstrated that
the ultrasonic characteristics of PTMC nodules include an irregular
shape, an aspect ratio of >1, a blurred boundary, a spiculated
margin, heterogeneous hypoechogenicity, the presence of
microcalcification, enlargement of neck lymph nodes and an abundant
distribution of blood flow. High-resolution ultrasound has an
important value in the diagnosis and preoperative assessment of
PTMC, and is able to provide high-quality images compared with
computed tomography or magnetic resonance imaging (3). The results of the present study suggest
that the presence of small nodules with one or more of the
aforementioned characteristics should be suspected as a malignant
nodule and PTMC. According to the guidelines proposed by the
American Thyroid Association, to prevent misdiagnosis an
ultrasound-guided fine-needle aspiration biopsy may be performed
(19). The sensitivities of enlarged
neck lymph nodes and the abundant distribution of blood flow are so
low that they may be considered as references to permit the
differentiation of PTMC from benign nodules.
References
|
1
|
Sugitani I, Kasai N, Fujimoto Y and
Yanagisawa A: A novel classification system for patients with PTC:
Addition of the new variables of large (3 cm or greater) nodal
metastases and reclassification during the follow-up period.
Surgery. 135:139–148. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McHenry CR and Phitayakorn R: Follicular
adenoma and carcinoma of the thyroid gland. Oncologist. 16:585–593.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen HY, Liu WY, Zhu H, Jiang DW, Wang DH,
Chen Y, Li W and Pan G: Diagnostic value of contrast-enhanced
ultrasound in papillary thyroid microcarcinoma. Exp Ther Med.
11:1555–1562. 2016.PubMed/NCBI
|
|
4
|
Ito Y, Miyauchi A, Inoue H, Fukushima M,
Kihara M, Higashiyama T, Tomoda C, Takamura Y, Kobayashi K and Miya
A: An observational trial for papillary thyroid microcarcinoma in
Japanese patients. World J Surg. 34:28–35. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim HY, Park WY, Lee KE, Park WS, Chung
YS, Cho SJ and Youn YK: Comparative analysis of gene expression
profiles of papillary thyroid microcarcinoma and papillary thyroid
carcinoma. J Cancer Res Ther. 6:452–457. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li QS, Chen SH, Xiong HH, Xu XH, Li ZZ and
Guo GQ: Papillary thyroid carcinoma on sonography. Clin Imaging.
34:121–126. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moon WJ, Jung SL, Lee JH, Na DG, Baek JH,
Lee YH, Kim J, Kim HS, Byun JS and Lee DH: Thyroid Study Group,
Korean Society of Neuro- and Head and Neck Radiology: Benign and
malignant thyroid nodules: US differentiation-multicenter
retrospective study. Radiology. 247:762–770. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu X, Yu J, Kang WM, Ma ZQ and Ye X:
Surgical diagnosis and treatment of primary hyperthyroidism
complicated with occult thyroid carcinoma. Zhongguo Yi Xue Ke Xue
Yuan Xue Bao. 37:402–405. 2015.PubMed/NCBI
|
|
9
|
Konturek A, Barczyński M, Wierzchowski W,
Stopa M and Nowak W: Coexistence of papillary thyroid cancer with
Hashimoto thyroiditis. Langenbecks Arch Surg. 398:389–394. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gul K, Dirikoc A, Kiyak G, Ersoy PE, Ugras
NS, Ersoy R and Cakir B: The association between thyroid carcinoma
and Hashimoto's thyroiditis: The ultrasonographic and
histopathologic characteristics of malignant nodules. Thyroid.
20:873–878. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li F, Zhang J, Wang Y and Liu L: Clinical
value of elasticity imaging and contrast-enhanced ultrasound in the
diagnosis of papillary thyroid microcarcinoma. Oncol Lett.
10:1371–1377. 2015.PubMed/NCBI
|
|
12
|
Chaudhary V and Bano S: Thyroid
ultrasound. Indian J Endocrinol Metab. 17:219–227. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Khati N, Adamson T, Johnson KS and Hill
MC: Ultrasound of the thyroid and parathyroid glands. Ultrasound Q.
19:162–176. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ohmori N, Miyakawa M, Ohmori K and Takano
K: Ultrasonographic findings of papillary thyroid carcinoma with
Hashimoto's thyroiditis. Intern Med. 46:547–550. 2006. View Article : Google Scholar
|
|
15
|
Anil G, Hegde A and Chong FH: Thyroid
nodules: Risk stratification for malignancy with ultrasound and
guided biopsy. Cancer Imaging. 11:209–223. 2011.PubMed/NCBI
|
|
16
|
Ito Y, Miyauchi A, Kihara M, Higashiyama
T, Kobayashi K and Miya A: Patient age is significantly related to
the progression of papillary microcarcinoma of the thyroid under
observation. Thyroid. 24:27–34. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Adler DD, Carson PL, Rubin JM and
Quinn-Reid D: Doppler ultrasound color flow imaging in the study of
breast cancer: Preliminary findings. Ultrasound Med Biol.
16:553–559. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pettersson A, Nagy JA, Brown LF, Sundberg
C, Morgan E, Jungles S, Carter R, Krieger JE, Manseau EJ, Harvey
VS, et al: Heterogeneity of the angiogenic response induced in
different normal adult tissues by vascular permeability
factor/vascular endothelial growth factor. Lab Invest. 80:99–115.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
American Thyroid Association (ATA)
Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid
Cancer, . Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL,
Mandel SJ, Mazzaferri EL, McIver B, Pacini F, et al: Revised
American Thyroid Association management guidelines for patients
with thyroid nodules and differentiated thyroid cancer. Thyroid.
19:1167–1214. 2009. View Article : Google Scholar : PubMed/NCBI
|