Introduction
Tumors possess a self-renewing ability that can
generate heterogeneous cells in tumor cells. Tumors are composed of
somatic mutations, each of which can be grown without restriction.
However, this does not explain the phenomenon that cancer cells
seem to have unlimited viability, and that not all tumor cells are
capable of unlimited growth. The characteristics of tumor cell
growth, metastasis and recurrence are similar to the basic
characteristics of stem cells. Therefore, the theory of tumor stem
cells (TSCs) has been suggested (1).
This theory provides a new direction and a visual angle to us to
gain a new understanding of the origin and nature of the tumor, as
well as the clinical treatment of cancer. In recent years, studies
conducted in China have increasingly focused on cancer stem cells.
The main reason leading to tumor is abnormality of stem cells,
leading to diseases, such as lung and colorectal cancer (1). Current studies have shown that malignant
tumor growth leads to the expression of stem cells in molecules
that play an important role in gene regulation (2).
Investigations regarding Nanog gene have
shown that it promotes cell induction, leading to analysis of
Nanog gene expression in tumor (3), although its expression is relatively
decreased in lung cancer (4). This
study primarily investigated the role of Nanog gene in the
two groups of patients with pulmonary adenocarcinoma and squamous
lung carcinoma.
Patients and methods
Patients
In total, 100 cases of tumor patients diagnosed with
lung cancer between April, 2010 and May, 2012 were selected for the
present study. Patient age was 22–76 years, with an average age of
58.43±10.44 years. The study included 50 men, aged 22–73 years,
with an average age of 59.12±9.06 years, and 50 women, aged 23–76
years, with an average age of 58.54±9.43 years. A CT scan, MRI,
chest X-ray, flexible bronchofiberscope examination and sputamentum
cell examination were performed on the patients, for confirmation
of lung cancer. In the 100 patients, there were 50 cases in group A
(pulmonary adenocarcinoma) for whom the diagnosed age for 17 cases
was <40 years, 16 cases were 40–60 years, and 17 cases were
>60 years. The tested diseases of this study were divided into 9
cases in phase I, 13 cases in phase II, 11 cases in phase III and
10 cases in phase IV. There were 21 patients with lymph node
metastasis, as indicated by test. In addition, 50 cases were
included in group B (squamous cell lung carcinoma). No significant
difference with regard to age, gender and diseases were observed,
compared with cases in group A.
Test methods of Nanog gene expression
in tumor stem cells
RT-PCR was used to quantify Nanog gene in
real-time. The mRNA agarose gel electrophoresis was used to test
100 cases, followed by 1 µg of RNA for reverse transcription.
PrimeScript RT was added to the 20 µl system and reverse
transcription was initiated initially at 42°C for 45 min, followed
by incubation at 70°C for 10 min, and cooling on ice to inactivate
reverse transcriptase. Subsequently, cDNA was synthesized. Primers
were designed from GeneBank data as follows: Nanog forward,
(5′-ATGCCTGCATTTTTCATCC-3′) and reverse,
(5′-GAGGCAGGTCTTCAGAGGAA-3′), with a product length of 189 bp.
β-actin was used as the internal control and its primers were:
Forward, (5′-CAGAGCAAGAGAGGCATCC-3′) and reverse,
(5′-CTGGGGTGTTGAAGGTCTC-3′), with a product length of 217 bp. PCR
reaction was prepared by using 2X SYBR Premix Ex Taq 10 µl, cDNA
template 2 µl, forward and reverse primers of 0.4 µl, and the total
volume was brought to 20 µl with autoclaved water. Clinical
SYBR-Green I fuel method was used for RT-PCR to amplify the genes.
Fully automatic fluorescent quantified PCR apparatus AB17500, and
Real-Time PCR system, were used and the temperature was set at 58°C
for 39 cycles. Following observation the results were recorded. The
relative CT value for β-actin was calculated in detail as indicated
in a previous study (5–8).
Observation index
The real-time quantified method was used to examine
age, gender, and any lymph node metastasis in the two groups of
patients. RT-PCR of tumor and adjacent normal tissue was used to
test the gene expression level of stem cells of the two groups of
patients. After obtaining the data, the gene expression amount of
groups A and B was analyzed to varying extent (7). PCR gel electrophoresis was used to test
Nanog expression in CD44+ cells, and the living curve
was used for statistics and to compare the survival rate of the
patients in five years. In addition, immunostaining was performed
to stain lung cancer cells in patients.
Statistical analysis
The data were presented as mean ± standard
deviation. Quantified data were expressed by cases (n) and
percentage. Data analysis was conducted using SPSS 15.0 software
(Chicago, IL, USA). A Student's t-test and χ2 test were
used to compare data. The ranked data were compared by a
non-parametric test. P<0.05 was considered to indicate a
statistically significant difference.
Results
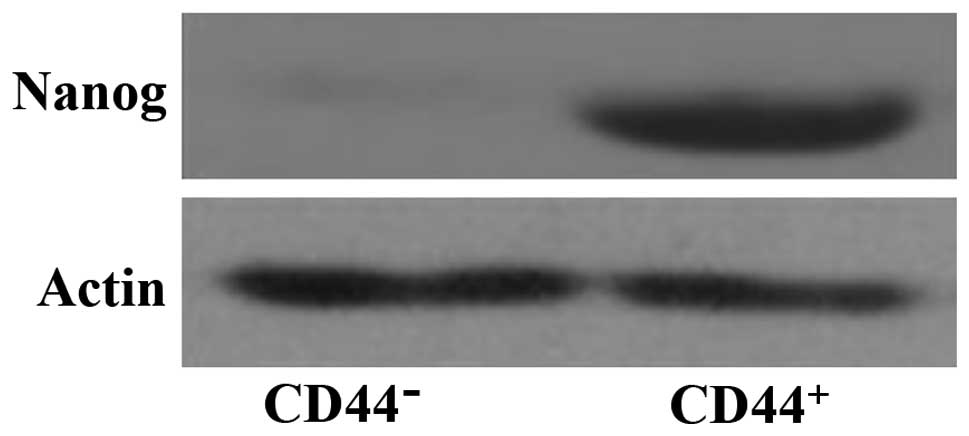
Nanog expression in CD44+
cells in lung cancer stem cell
As shown in Fig. 1,
the lung cancer stem cells CD44+ cells showed a higher
expression of Nanog, suggesting that it plays an important
role in lung cancer stem cells.
Nanog expression in adjacent normal
tissue and benign lesion cancer tissue
The results showed that Nanog was mainly
expressed in the nucleus of lung cancer cells, and in positive
control (spermatogenous cell). The expression was mainly in the
nucleus, and Nanog expression in lung cancer cells was
significantly higher than that of adjacent normal tissue and benign
lesion lung tissue (Fig. 2).
Nanog gene expression
We found that the expression level of phase I
patients was 1.30±0.29, phase II patients was 1.38±0.32, phase III
was 2.28±0.52 and phase IV was 2.47±0.63. The patients expression
levels had significant different extents of the improvement
(p<0.05). The Nanog gene expression in cancer tissues
significantly decreased, and the data showed that Nanog levels in
pulmonary adenocarcinoma and squamous lung carcinoma patients were
basically the same (Table I).
 | Table I.Nanog gene expression at
different phases in pulmonary adenocarcinoma and squamous lung
carcinoma patients. |
Table I.
Nanog gene expression at
different phases in pulmonary adenocarcinoma and squamous lung
carcinoma patients.
|
| Pulmonary
adenocarcinoma (n=50) | Squamous lung
carcinoma (n=50) |
|---|
|
|
|
|
|---|
| Phase | Cancer tissue | Adjacent normal
tissue | Cancer tissue | Adjacent normal
tissue |
|---|
| I phase |
1.30±0.29 |
0.34±0.13c,1 |
1.33±0.46 |
0.36±0.14c,5 |
| II phase |
1.38±0.32 |
0.36±0.14c,2 |
1.58±0.40 |
0.38±0.10c,6 |
| III phase |
2.28±0.52a,b,9 |
0.44±0.11c,3 |
2.33±0.58a,b,11 |
0.42±0.15c,7 |
| IV phase |
2.47±0.63a,b,10 |
0.43±0.15c,4 |
2.56±0.60a,b,12 |
0.47±0.13c,8 |
Nanog expression of cells in various
differentiation condition
A comparison of the Nanog detection rate in
differentiation cells in pulmonary adenocarcinoma and squamous lung
carcinoma yielded 33.5 and 37.8%, respectively. In middle- and
high-differentiation cells, the Nanog detection rate was
relatively significantly high, while in the no and low
differentiation cells, the expression was 89.1 and 70.2%,
respectively (p<0.05). In the present study, we found that
Nanog is basically the same in stem cells of pulmonary
adenocarcinoma and squamous lung carcinoma patients (Table II).
 | Table II.Nanog detection in adenocarcinoma and
squamous lung carcinoma patients under various differentiation
conditions. |
Table II.
Nanog detection in adenocarcinoma and
squamous lung carcinoma patients under various differentiation
conditions.
|
| Pulmonary
adenocarcinoma (n=50) | Squamous lung
carcinoma (n=50) |
|---|
|
|
|
|
|---|
| Phase | Detection
cases/cases | Detection rate
(%) | Detection
cases/cases | Detection rate
(%) |
|---|
| No
differentiation | 8/9 | 89.1 |
8/10 | 82.0 |
| Low
differentiation |
7/12 | 70.2 |
9/13 | 69.4 |
| Middle
differentiation |
4/12 | 33.5 |
4/11 | 36.6a |
| High
differentiation | 3/8 | 37.8 | 2/7 | 28.8a |
Investigation and observation of the
patients in the present study
The patients were divided into two groups according
to high or low expression of Nanog, and then followed-up for
the survival rate of the two groups. As shown in Fig. 3, a high expression level of
Nanog in patients had a lower meta-survival rate (44%), and
a low expression level of Nanog patients had a higher
meta-survival rate (60%), and χ2=4.69. P<0.05 was
considered to indicate a statistically significant difference.
Discussion
Clinical studies have found that cell heterogeneity
is an important cause of tumor development (8). Certain specificity cells in the human
body have a certain ability for self-renewal and differentiation
(9), and these types of cells have
anti-drug and drug resistance (10),
showing some characteristics of stem cells (11). Tumor stem cells are tumor cells that
occur clinically, and are capable of renewal and proliferation
(12). They can indirectly influence
tumor growth, and therefore are of great significance in the
control and prevention of tumor (13). Nanog expression is a
transcription factor of embryonic stem cells (ESCs), and is also a
type of primitive reproduction cell. It has been found that
Nanog exists in embryonic stem cell, reproduction stem cell
and other related tumor cells (14,15).
Relevant studies suggested that cancer cells in human body can
grows in an uncontrollable manner with low-differentiation. The
results of the present study suggest that Nanog intervention
can effectively regulate human body mechanism of tumor patients,
and Nanog plays an important role in the treatment process.
However, there is currently no evidence showing whether the
diseases are associated with Nanog (14). In the present study, we knocked out
Nanog gene, and found that the tumor was inhibited after the
knockout, suggesting Nanog can directly participate in human
body repair treatment (15). Previous
experiments found that except for repairing human stem cells, Nanog
(16) can self-renew and regulate as
well as differentiate. For instance, the higher the data, the
stronger the ability of low- and no-differentiation of the stem
cells (17).
Besides being expressed in reproductive cells and
malignant tumor, Nanog is also expressed in entity
tumor-like breast cancer, retinoblastoma and oral squamous cell
carcinoma (18). Nanog
pseudogene expression is found in cervix cancer and breast cancer
(19–21). From the data of 100 cases of lung
cancer in the present study, we found that Nanog gene
expression was significantly higher in lung cancer tissue than in
adjacent normal tissue (p<0.01). The data showed that there may
be Nanog gene in lung cancer stem cells (LCSCS) in tissue of
lung cancer patients (22). The main
factor promoting lung cancer cells in human is that it can
self-renew and proliferate in its LCSCS (23). When we examined the adjacent normal
tissue, we found Nanog is positive in 5 cases, demonstrating
this part of adjacent normal tissue may contain normal lung cancer
stem cells. The present study found that Nanog gene
expression is consistence with the differentiation extent of lung
cancer tissue and tumor, and a positive expression rate is evident
in low- and no-differentiation, but not in high-differentiation
(p=0.0112) (24). We found that the
Nanog gene and differentiation condition of tumor stem cells
are consistent. A high expression of Nanog can maintain the
low-differentiation condition of stem cells, and maintain
self-renewal and proliferation of the stem cells, which is crucial
in assistance to differentiation signals.
In summary, the present study found a correlation
between consistency of tumor and Nanog gene expression, showing
that when human cell differentiation reaches the lowest point,
Nanog gene was stronger. As a newly found specific marker,
the Nanog gene contributes to potential clinical prevention
of lung cancer.
References
|
1
|
Mak VC, Siu MK, Wong OG, Chan KK, Ngan HY
and Cheung AN: Dysregulated stemness-related genes in gynecological
malignancies. Histol Histopathol. 27:1121–1130. 2012.PubMed/NCBI
|
|
2
|
Kemmerling R, Alinger B, Dietze O,
Bösmüller HC, Ocker M, Wolkersdörfer GW, Berr F, Neureiter D and
Kiesslich T: Association of stem cell marker expression pattern and
survival in human biliary tract cancer. Int J Oncol. 41:511–522.
2012.PubMed/NCBI
|
|
3
|
Chiu CG, Chan SK, Fang ZA, Masoudi H,
Wood-Baker R, Jones SJ, Gilks B, Laskin J and Wiseman SM:
Beta-catenin expression is prognostic of improved non-small cell
lung cancer survival. Am J Surg. 203:654–659. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu C, Xie D, Yu SC, Yang XJ, He LR, Yang
J, Ping YF, Wang B, Yang L, Xu SL, et al: β-Catenin/POU5F1/SOX2
transcription factor complex mediates IGF-I receptor signaling and
predicts poor prognosis in lung adenocarcinoma. Cancer Res.
73:3181–3189. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nayerossadat N, Maedeh T and Ali PA: Viral
and nonviral delivery systems for gene delivery. Adv Biomed Res.
1:272012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Levina V, Marrangoni A, Wang T, Parikh S,
Su Y, Herberman R, Lokshin A and Gorelik E: Elimination of human
lung cancer stem cells through targeting of the stem cell
factor-c-kit autocrine signaling loop. Cancer Res. 70:338–346.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Meng X, Wang X and Wang Y: More than 45%
of A549 and H446 cells are cancer initiating cells: evidence from
cloning and tumorigenic analyses. Oncol Rep. 21:995–1000.
2009.PubMed/NCBI
|
|
8
|
Huang D, Gao Q, Guo L, Zhang C, Jiang W,
Li H, Wang J, Han X, Shi Y and Lu SH: Isolation and identification
of cancer stem-like cells in esophageal carcinoma cell lines. Stem
Cells Dev. 18:465–473. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guzman ML, Swiderski CF, Howard DS, Grimes
BA, Rossi RM, Szilvassy SJ and Jordan CT: Preferential induction of
apoptosis for primary human leukemic stem cells. Proc Natl Acad Sci
USA. 99:16220–16225. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Houghton J, Stoicov C, Nomura S, Rogers
AB, Carlson J, Li H, Cai X, Fox JG, Goldenring JR and Wang TC:
Gastric cancer originating from bone marrow-derived cells. Science.
306:1568–1571. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Abbott BL: ABCG2 (BCRP): a cytoprotectant
in normal and malignant stem cells. Clin Adv Hematol Oncol.
4:63–72. 2006.PubMed/NCBI
|
|
12
|
Vermeulen L, De Sousa E, Melo F, van der
Heijden M, Cameron K, de Jong JH, Borovski T, Tuynman JB, Todaro M,
Merz C, Rodermond H, et al: Wnt activity defines colon cancer stem
cells and is regulated by the microenvironment. Nat Cell Biol.
12:468–476. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen D, Zhao M and Mundy GR: Bone
morphogenetic proteins. Growth Factors. 22:233–241. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim YS, Farrar W, Colburn NH and Milner
JA: Cancer stem cells: potential target for bioactive food
components. J Nutr Biochem. 23:691–698. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu S, Dontu G, Mantle ID, Patel S, Ahn
NS, Jackson KW, Suri P and Wicha MS: Hedgehog signaling and Bmi-1
regulate self-renewal of normal and malignant human mammary stem
cells. Cancer Res. 66:6063–6071. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lessard J and Sauvageau G: Bmi-1
determines the proliferative capacity of normal and leukaemic stem
cells. Nature. 423:255–260. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stovall DB, Wan M, Zhang Q, Dubey P and
Sui G: DNA vector-based RNA interference to study gene function in
cancer. J Vis Exp. 64:e41292012.PubMed/NCBI
|
|
18
|
Tsai LL, Yu CC, Chang YC, Yu CH and Chou
MY: Markedly increased Oct4 and Nanog expression correlates with
cisplatin resistance in oral squamous cell carcinoma. J Oral Pathol
Med. 40:621–628. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gu TT, Liu SY and Zheng PS: Cytoplasmic
NANOG-positive stromal cells promote human cervical cancer
progression. Am J Pathol. 181:652–661. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nagata T, Shimada Y, Sekine S, Hori R,
Matsui K, Okumura T, Sawada S, Fukuoka J and Tsukada K: Prognostic
significance of NANOG and KLF4 for breast cancer. Breast Cancer.
21:96–101. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu X, Mazur SJ, Lin T, Appella E and Xu Y:
The pluripotency factor nanog promotes breast cancer tumorigenesis
and metastasis. Oncogene. 33:2655–2664. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Primo MN, Bak RO and Mikkelsen JG:
Lentiviral vectors for cutaneous RNA managing. Exp Dermatol.
21:162–170. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cavazza A, Moiani A and Mavilio F:
Mechanisms of retroviral integration and mutagenesis. Hum Gene
Ther. 24:119–131. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rodriguez GP, Song JB and Crouse GF:
Transformation with oligonucleotides creating clustered changes in
the yeast genome. PLoS One. 7:e429052012. View Article : Google Scholar : PubMed/NCBI
|