Introduction
Multiple myeloma (MM) is a type of malignant tumor
that originates from B cell lines (1). The disease is characterized by an
increase in the number of abnormal plasma cells, which generate
monoclonal immunoglobulin, and a malignant proliferation within
bone marrow, causing fracture and bone marrow function failure,
which results in the clinical symptoms of MM, including bone pain,
anemia, hypercalcemia, infection and renal failure (1). Without treatment, patients with
progressive stages of MM have a median survival of only 6 months,
whilst following chemotherapy, the median survival is >3 years
(2,3).
Only 25% of patients with MM survive for >5 years; therefore, MM
has long been regarded as an incurable disease with an urgent
requirement for novel therapies to improve the prognosis of
patients (2,3).
Phosphoinositide 3-kinase (PI3K)/Akt/mammalian
target of rapamycin (mTOR) is an important signaling pathway that
affects the energy metabolism, size, cycle, proliferation, survival
and apoptosis of cells, and is closely associated with numerous
other signaling pathways (4,5). The administration of drugs that target
the PI3K-AKT-mTOR signaling pathway, combined with additional
therapy, is a promising approach for patients with MM (2).
Plumbagin, a natural naphthoquinone compound, is the
primary active component of the traditional Chinese medicine Baihua
Dan (6). Baihua Dan has been
administered clinically in China for centuries, and the effects of
plumbagin include anti-inflammatory (7), antiseptic (8) and anti-protozoa (9) effects. Plumbagin functions through a
variety of pathways to inhibit and kill tumor cells; it was
previously demonstrated that plumbagin induces apoptosis in human
lung cancer A549 cells by inhibiting the tissue plasminogen
activator (PA)-induced expression of matrix metalloproteinases and
urokinase PA (10). Subsequently the
metastasis of tumor cells is reduced. However, the anticancer
effect of plumbagin on MM and the precise molecular mechanisms
underlying its behavior remain unclear. Therefore, the present
study aimed to investigate the effects and molecular mechanisms of
plumbagin on the proliferation and apoptosis of MM cells.
Materials and methods
Chemicals and reagents
RPMI-1640, fetal bovine serum (FBS),
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
and lactate dehydrogenase (LDH) were all purchased from
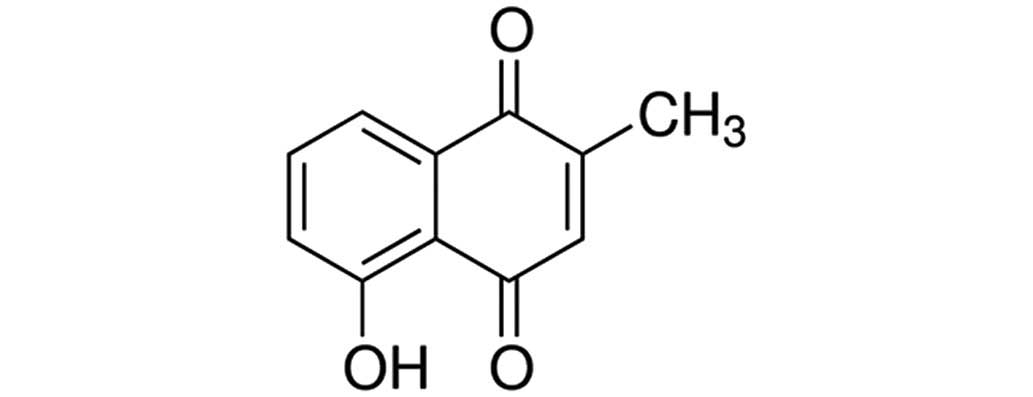
Sigma-Aldrich (St. Louis, MO, USA). The chemical structure of
plumbagin (purity, 98%; Sigma-Aldrich) is presented in Fig. 1. A Pierce™ BCA Protein Assay kit was
purchased from Thermo Fisher Scientific, Inc. (Waltham, MA, USA)
and the Caspase-3 Colorimetric Assay kit was purchased from
Beyotime Institute of Biotechnology, Inc. (Nanjing, China).
Cell lines and cell culture
Human MM OPM1 cells were provided by the Experiment
Center of The First Affiliated Hospital of Chengdu Medical College
(Chengdu, China). OPM1 cells were cultured in RPMI-1640 containing
10% FBS and 1% penicillin and streptomycin at 37°C in a humidified
atmosphere with 5% CO2.
MTT assay
OPM1 cells were seeded at a density of 8,000
cells/well into a 96-well culture plate. After 24 h, the OPM1 cells
were treated with plumbagin at doses of 0 (dimethyl sulfoxide
vehicle-only), 1, 5, 10, 20 and 50 µM (11), for 24 and 48 h. Following treatment,
10 µl MTT stock solution (5 mg/ml) was added to each well and
incubated for 4 h at 37°C in a humidified atmosphere with 5%
CO2. A total of 150 µl DMSO was added to each well to
dissolve the crystals, and cell viability was subsequently measured
at a wavelength of 450 nm.
LDH leakage
OPM1 cells were seeded at a density of 8,000
cells/well into a 96-well culture plate. After 24 h, the OPM1 cells
were treated with plumbagin (0, 10, 20 and 50 µM) for 24 and 48 h.
Following treatment, 100 µl LDH solution was added to each well and
incubated for 30 min at 37°C in a humidified atmosphere with 5%
CO2. Absorbance was measured using an enzyme-linked
immunosorbent assay reader at 490 nm.
Cellular apoptosis analysis by flow
cytometry
OPM1 cells were seeded at a density of
106 cells/well into a 6-well culture plate. After 24 h,
the OPM1 cells were treated with plumbagin (0, 10, 20 and 50 µM)
for 24 h. Following treatment, the cells were washed and fixed in
precooled PBS, resuspended with buffer solution and incubated at
room temperature with Annexin V-fluorescein isothiocyanate for 30
min in darkness. Subsequently, the cells were incubated with
propidium iodide for 30 min in darkness. Cell apoptosis was
enumerated using a Coulter® Epics XL™ flow cytometer
(Beckman Coulter, Inc., Brea, CA, USA).
Caspase-3 activity
OPM1 cells were seeded at a density of
106 cells/well into a 6-well culture plate. After 24 h,
the cells were treated with plumbagin (0, 10, 20 and 50 µM) for 24
h. Following treatment, the caspase-3 activity in fluorescence was
detected at a wavelength of 405 nm using a Caspase-3 Colorimetric
Assay kit (Beyotime Institute of Biotechnology), according to the
manufacturer's protocol.
Western blot analysis
OPM1 cells were seeded into a 6-well culture plate
at a density of 106 cells/well. After 24 h, the cells
were treated with plumbagin (0, 10, 20 and 50 µM) for 24 h.
Following treatment with plumbagin, the cells were harvested and
lysed in radioimmunoprecipitation assay buffer. Total protein was
determined using a Pierce™ BCA Protein assay kit. Equal amounts of
protein were loaded and separated by 7–12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and subsequently
transferred to a polyvinylidene fluoride membrane. The membrane was
then blocked with 5% skimmed milk and probed with the following
primary antibodies: Anti-PI3K (#sc-48637; dilution, 1:1,000),
anti-phosphorylated (p)-Akt1 (#sc-135650; dilution, 1:2,000),
anti-p-mTOR (#sc-101738; dilution, 1:1,000) and anti-β-actin
(#sc-130656; dilution, 1:1,000) antibodies (Santa Cruz
Biotechnology, Inc.) overnight at 4°C. The membrane was washed with
Tris-buffered saline with Tween-20, incubated with anti-rabbit
secondary antibody (#6401-05; dilution, 1:5,000; Amyjet Scientific,
Inc., Wuhan, China) at 4°C for 1 h, and visualized in an enhanced
chemiluminescence solution (GE Healthcare Life Sciences, Little
Chalfont, UK). Protein expression was then detected in a ChemiDoc
MP Imaging System (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Statistical analysis
Data are presented as the mean ± standard deviation
of three independent experiments and statistical analysis was
performed using SPSS software version 17.0 (SPSS, Inc., Chicago,
IL, USA). Statistical differences between the control and treatment
samples were determined by one-way analysis of variance. P<0.05
was considered to indicate statistically significant
differences.
Results
Plumbagin inhibits cell
proliferation
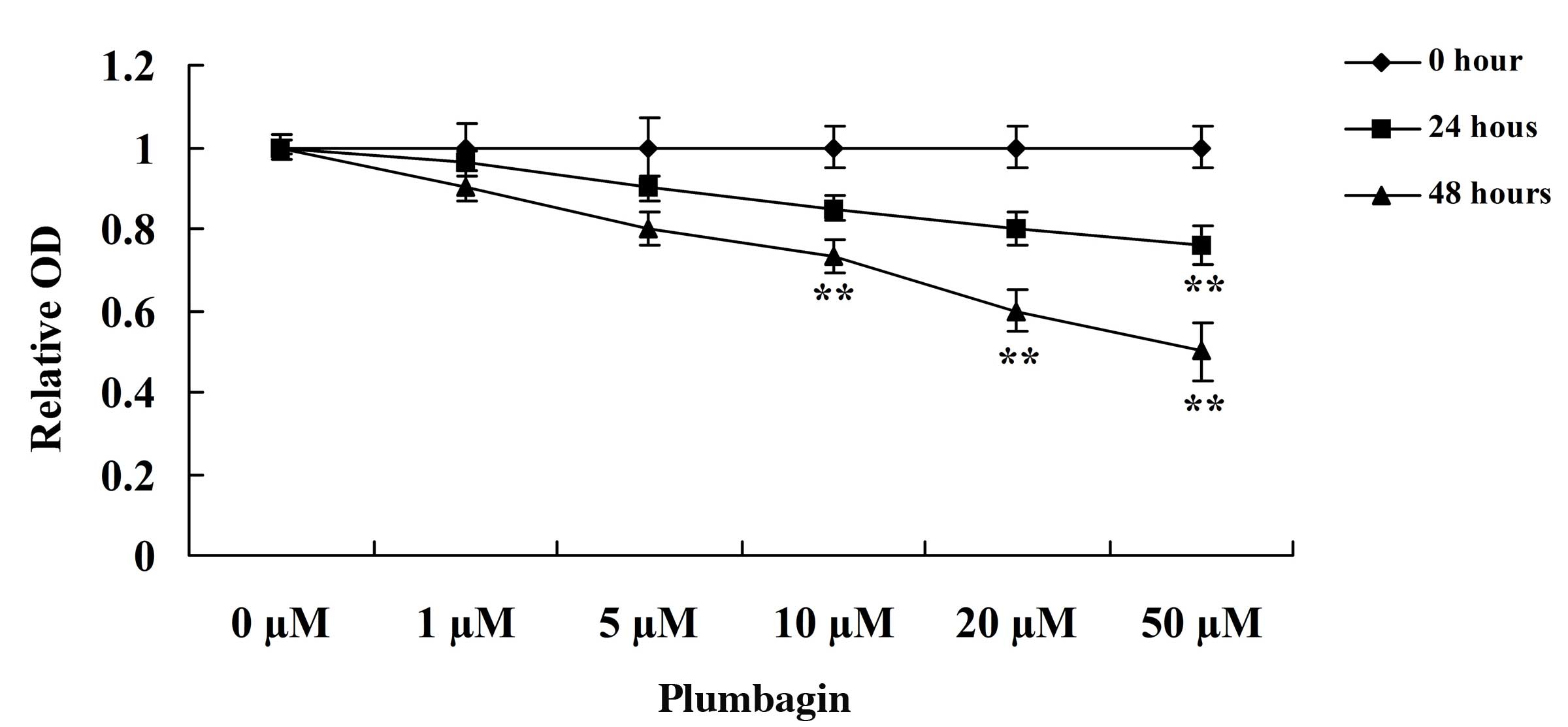
The present study assessed the anticancer effect of
plumbagin on OPM1 cell viability using MTT assay. Incubation of the
OPM1 cells with 0, 1, 5, 10, 20 and 50 µM plumbagin resulted in
significantly reduced cell viability (Fig. 2). Following treatment with 10, 20 and
50 µM plumbagin for 48 h, and 50 µM plumbagin for 24 h, the
viability of the OPM1 cells significantly decreased (P=0.0003).
These results indicate that plumbagin has a potent anticancer
effect on the proliferation of OPM1 cells.
Plumbagin increases cell
cytotoxicity
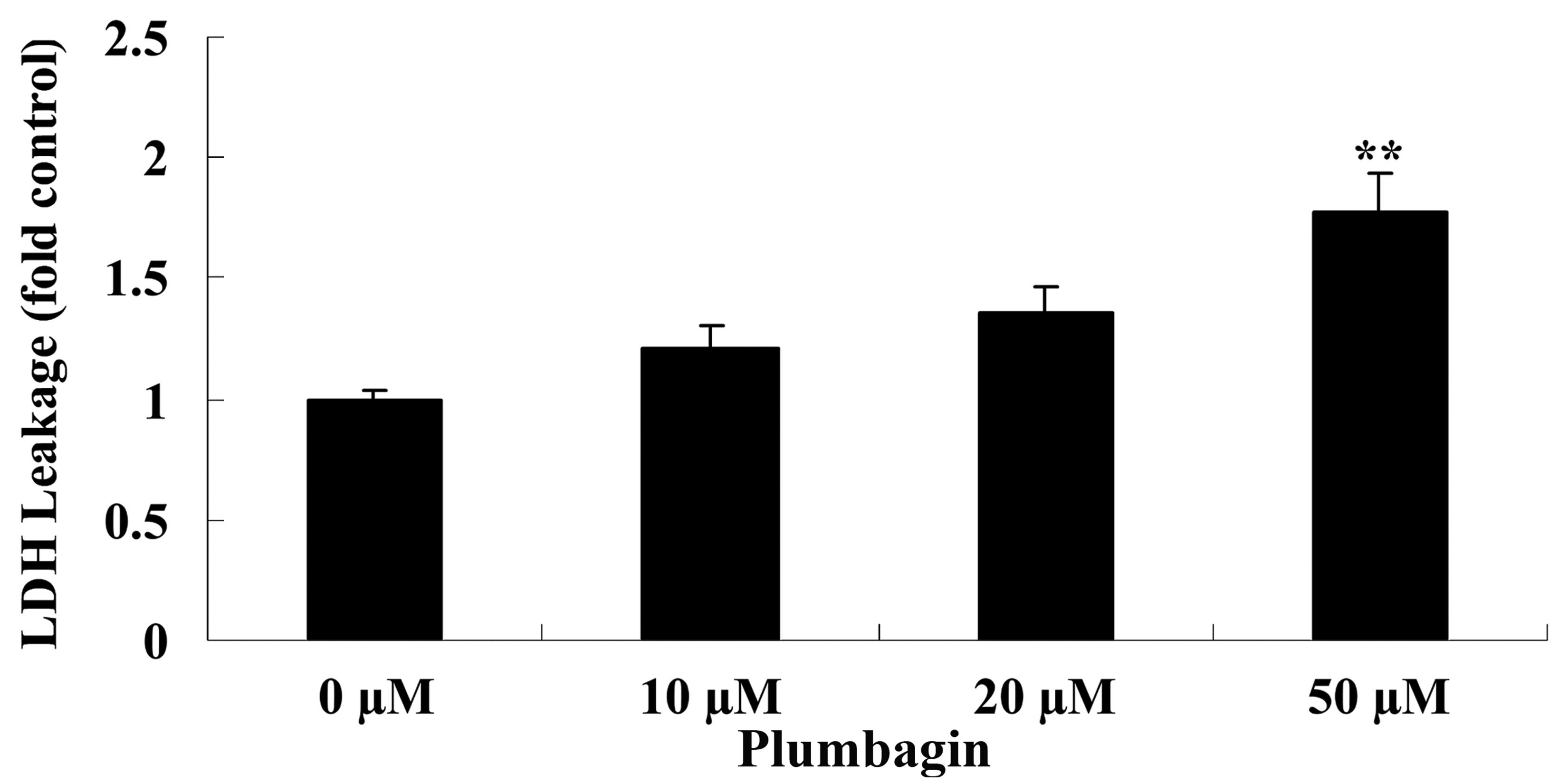
The current study subsequently examined the
anticancer effect of plumbagin on OPM1 cell cytotoxicity by
assessing LDH leakage. Treatment of the OPM1 cells with 50 µM
plumbagin resulted in significantly increased cell cytotoxicity at
24 h compared with cells treated with 0 µM plumbagin (P<0.0001;
Fig. 3).
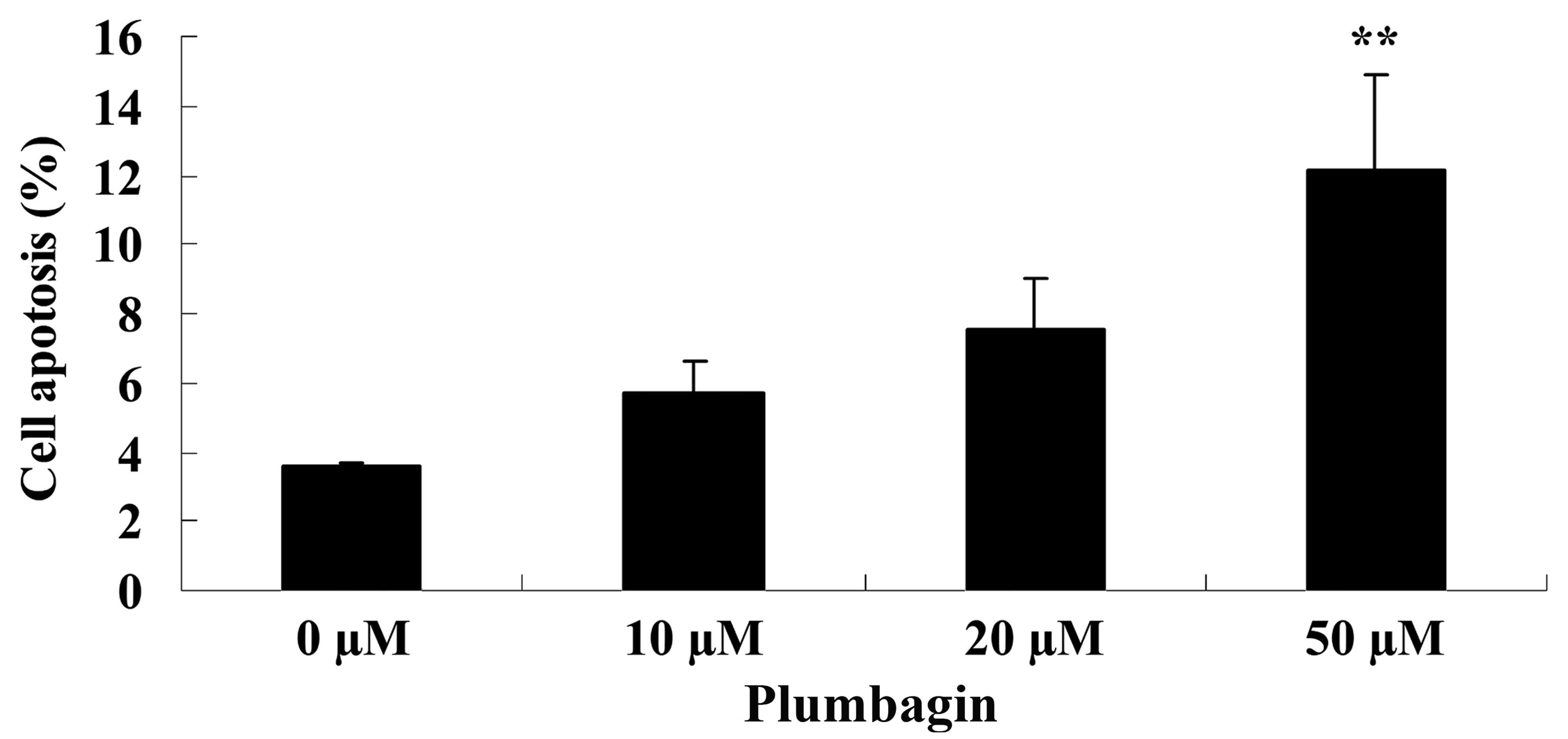
Plumbagin induces cell apoptosis
Next, the anticancer effect of plumbagin on OPM1
cell apoptosis was analyzed by flow cytometry. Following treatment
with 50 µM plumbagin for 24 h, it was observed that OPM1 cell
apoptosis significantly increased compared with cells treated with
0 µM plumbagin (P=0.0018; Fig.
4).
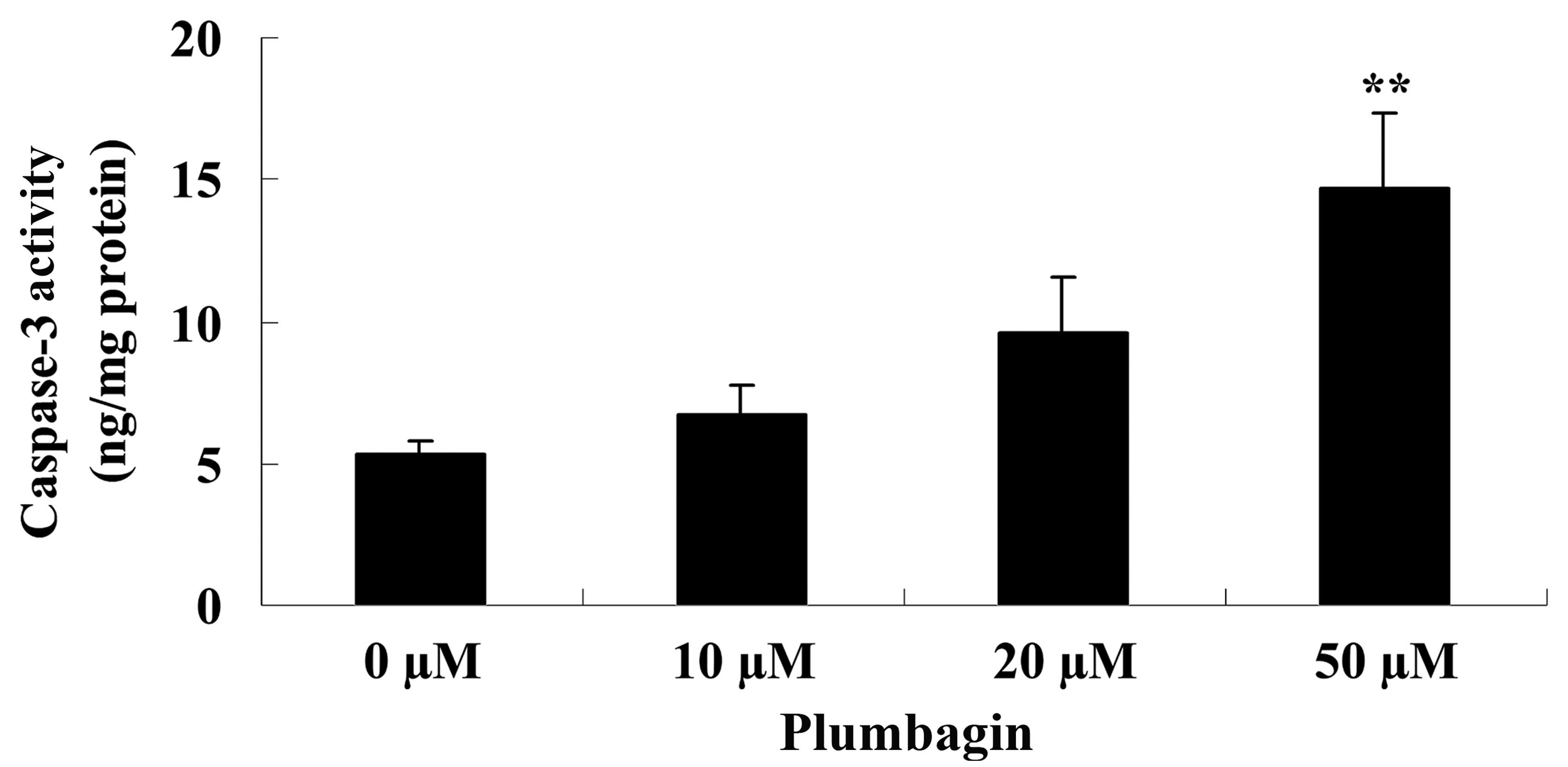
Plumbagin induces caspase-3
activity
The present study examined the anticancer effect of
plumbagin on caspase-3 activity in OPM1 cells following a 24 h
treatment time. The results demonstrated that incubation with 50 µM
plumbagin for 24 h significantly increased caspase-3 activity in
the OPM1 cells compared with cells treated with 0 µM plumbagin
(P<0.0001; Fig. 5).
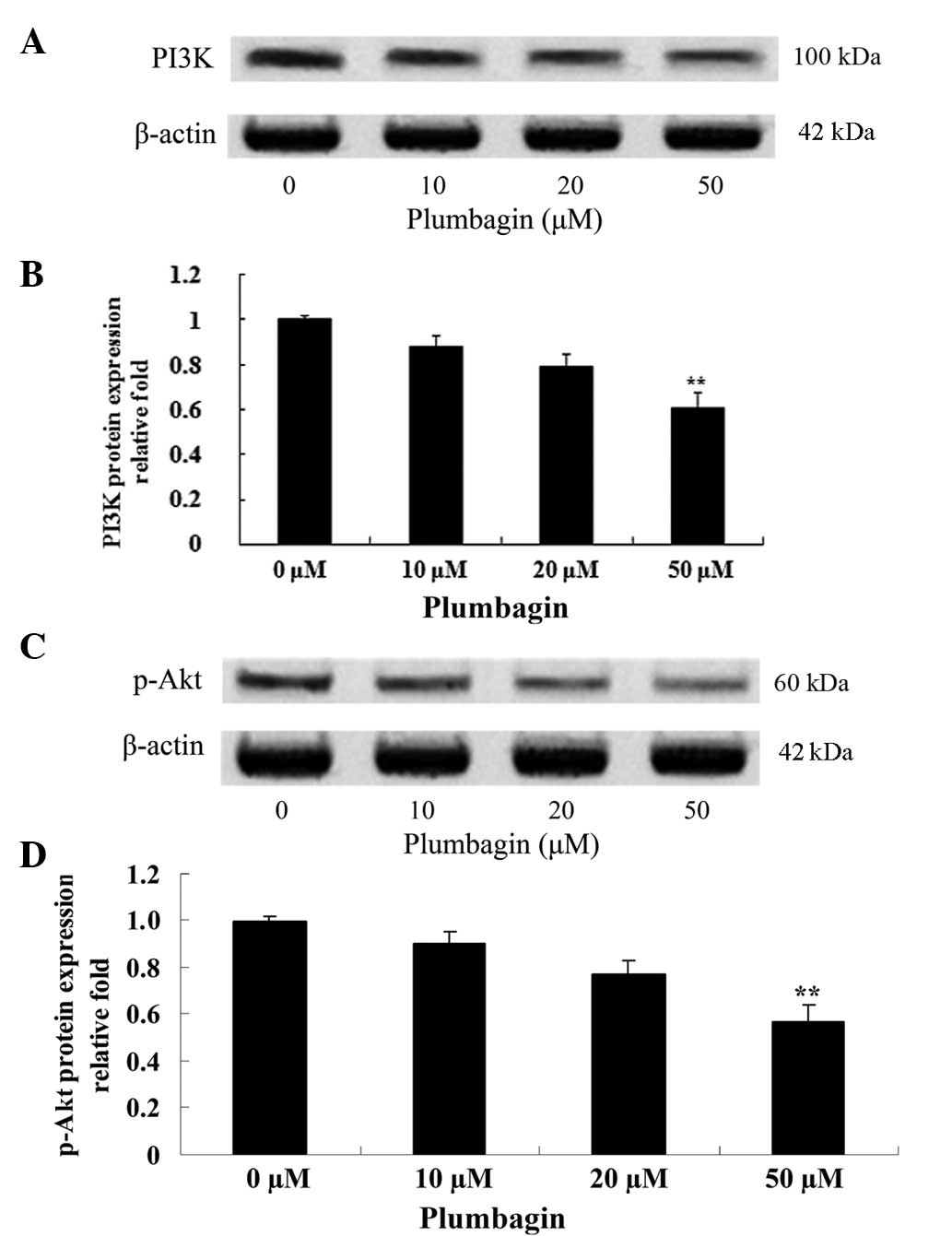
Plumbagin inhibits PI3K/Akt
The effect of plumbagin on the protein expression
levels of PI3K and p-Akt was evaluated using western blot analysis,
which revealed that the anticancer effect of plumbagin is via the
PI3K/Akt signaling pathway in OPM1 cells. Following incubation with
50 µM plumbagin for 24 h, the OPM1 cells exhibited significantly
decreased expressions of PI3K and p-Akt (P<0.0001; Fig. 6).
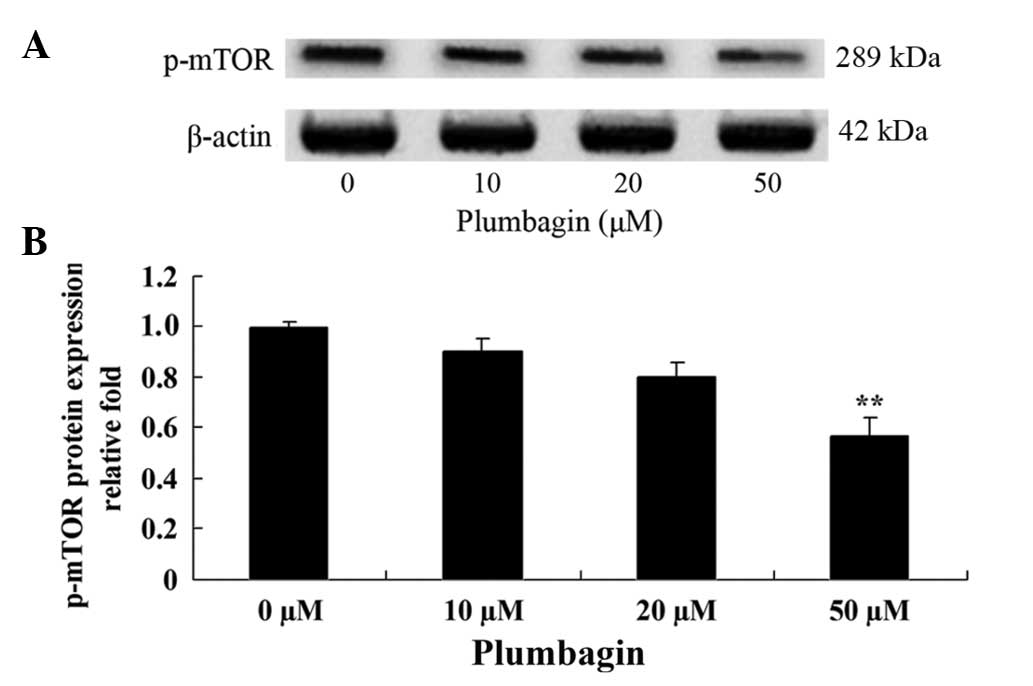
Plumbagin inhibits mTOR
The present study investigated the anticancer effect
of plumbagin on the mTOR signaling pathway in OPM1 cells, which
were treated with the plumbagin for 24 h. The results demonstrated
that treatment of the OPM1 cells with 5 µM plumbagin significantly
reduced the expression level of p-mTOR (P=0.0003 Fig. 7).
Discussion
MM is a common malignant plasma cell proliferative
disease of the bone marrow, which accounts for 10–15% of all
malignant blood diseases (12). Even
though life expectancy and diagnostics are improving for this
disease, its incidence is increasing, and fractures and clinical
complications, including, renal failure, are also on the rise
(13,14). The results of the present study
demonstrated that treatment with plumbagin significantly reduced
cell viability, increased cell cytotoxicity, activated cell
apoptosis and promoted caspase-3 activity in OPM1 cells. In
addition, previous studies have reported that plumbagin suppresses
the growth of oral squamous cell carcinoma (15), colon cancer (16) and breast cancer (17) cells.
It has been previously demonstrated that the
overexpression and abnormal activation of PI3K and Akt is involved
in the occurrence and development of a number of tumors, including
ovarian, breast, pancreatic, stomach and non-small cell lung cancer
(3). Akt, a type of serine/threonine
protein kinase, is essential in PI3K signal transmission. The
pleckstrin homology domain of Akt binds to the activation products
of PI3K, and Akt becomes phosphorylated and activated.
Subsequently, active Akt regulates downstream target genes, which
are involved in cell proliferation, differentiation and apoptosis
(18). In the present study,
pretreatment with plumbagin significantly suppressed the expression
of PI3K and p-Akt in OPM1 cells. Similarly, Li et al
(19) reported that plumbagin induced
apoptosis in human non-small cell lung cancer cells through
inhibition of the PI3K/Akt/mTOR pathway. Furthermore, Wang et
al (11) observed that plumbagin
induced cell cycle arrest in human pancreatic cancer cells via the
PI3K/Akt/mTOR-mediated pathway.
The downstream effects of PI3K and Akt are often
identified in patients with cancer (3). mTOR is an important downstream molecule
of Akt, which is essential in tumorigenesis (20). In the PI3K/Akt-mTOR signaling pathway,
Akt negatively regulates two tumor suppressor genes: PTEN, which is
upstream of Akt, and Tuberous Sclerosis Complex 1 and 2, which are
located downstream of AKT and upstream of mTOR (21–23).
Downstream effects, conserved throughout protein evolution, of
forkhead box O (FOXO) transcription factors and mTOR are important
in tumorigenesis (22). In mammalian
cells, Akt is able to phosphorylate a number of proteins (24); FOXO transcription factors may be
directly phosphorylated and inactivated by Akt, and under normal
physiological conditions, FOXO suppresses mammalian cell
proliferation (25). By contrast,
mTOR, which promotes cell proliferation, is indirectly activated by
Akt (25). The results of the current
study indicated that treatment with plumbagin significantly reduced
the expression level of p-mTOR in OPM1 cells. Similarly, Chen et
al (26) reported that plumbagin
induced cell apoptosis and inhibited cell growth in human colon
cancer cells via the PI3K/Akt-mTOR signaling pathway.
In conclusion, the results of the present study
demonstrate that plumbagin inhibits cell proliferation and promotes
apoptosis of MM cells. In addition, the present study identified a
potential cellular mechanism of plumbagin in MM cells, which was
the PI3K/Akt/mTOR signaling pathway. Additional studies are
required to confirm the therapeutic effect of plumbagin for its use
in clinical trials in patients with MM.
References
|
1
|
Liu N, Zhou H, Yang G, Geng C, Jian Y, Guo
H and Chen W: Retrospective analysis of genetic abnormalities and
survival in 131 patients with multiple myeloma. Oncol Lett.
9:930–936. 2015.PubMed/NCBI
|
|
2
|
Tan E, Weiss BM, Mena E, Korde N, Choyke
PL and Landgren O: Current and future imaging modalities for
multiple myeloma and its precursor states. Leuk Lymphoma.
52:1630–1640. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang F, Zhang W, Guo L, Bao W, Jin N, Liu
R, Liu P, Wang Y, Guo Q and Chen B: Gambogic acid suppresses
hypoxia-induced hypoxia-inducible factor-1α/vascular endothelial
growth factor expression via inhibiting phosphatidylinositol
3-kinase/Akt/mammalian target protein of rapamycin pathway in
multiple myeloma cells. Cancer Sci. 105:1063–1070. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Deng M, Wang J, Chen Y, Zhang L and Liu D:
Combination of SF1126 and gefitinib induces apoptosis of
triple-negative breast cancer cells through the PI3K/AKT-mTOR
pathway. Anticancer Drugs. 26:422–427. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang H, Bajraszewski N, Wu E, Wang H,
Moseman AP, Dabora SL, Griffin JD and Kwiatkowski DJ: PDGFRs are
critical for PI3K/Akt activation and negatively regulated by mTOR.
J Clin Invest. 117:730–738. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Padhye S, Dandawate P, Yusufi M, Ahmad A
and Sarkar FH: Perspectives on medicinal properties of plumbagin
and its analogs. Med Res Rev. 32:1131–1158. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang T, Wu F, Jin Z, Zhai Z, Wang Y, Tu B,
Yan W and Tang T: Plumbagin inhibits LPS-induced inflammation
through the inactivation of the nuclear factor-kappa B and mitogen
activated protein kinase signaling pathways in RAW 264.7 cells.
Food Chem Toxicol. 64:177–183. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Inbaraj JJ and Chignell CF: Cytotoxic
action of juglone and plumbagin: A mechanistic study using HaCaT
keratinocytes. Chem Res Toxicol. 17:55–62. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Saowakon N, Lorsuwannarat N, Changklungmoa
N, Wanichanon C and Sobhon P: Paramphistomum cervi: The in vitro
effect of plumbagin on motility, survival and tegument structure.
Exp Parasitol. 133:179–186. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shieh JM, Chiang TA, Chang WT, Chao CH,
Lee YC, Huang GY, Shih YX and Shih YW: Plumbagin inhibits
TPA-induced MMP-2 and u-PA expressions by reducing binding
activities of NF-kappaB and AP-1 via ERK signaling pathway in A549
human lung cancer cells. Mol Cell Biochem. 335:181–193. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang F, Wang Q, Zhou ZW, Yu SN, Pan ST, He
ZX, Zhang X, Wang D, Yang YX, Yang T, et al: Plumbagin induces cell
cycle arrest and autophagy and suppresses epithelial to mesenchymal
transition involving PI3K/Akt/mTOR-mediated pathway in human
pancreatic cancer cells. Drug Des Devel Ther. 9:537–560.
2015.PubMed/NCBI
|
|
12
|
Troeltzsch M, Oduncu F, Mayr D, Ehrenfeld
M, Pautke C and Otto S: Root resorption caused by jaw infiltration
of multiple myeloma: Report of a case and literature review. J
Endod. 40:1260–1264. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Que W, Li S and Chen J: NS-398 enhances
the efficacy of bortezomib against RPMI8226 human multiple myeloma
cells. Mol Med Rep. 7:1641–1645. 2013.PubMed/NCBI
|
|
14
|
Nakazato T, Sagawa M and Kizaki M:
Triptolide induces apoptotic cell death of multiple myeloma cells
via transcriptional repression of Mcl-1. Int J Oncol. 44:1131–1138.
2014.PubMed/NCBI
|
|
15
|
Ono T, Ota A, Ito K, Nakaoka T, Karnan S,
Konishi H, Furuhashi A, Hayashi T, Yamada Y, Hosokawa Y and Kazaoka
Y: Plumbagin suppresses tumor cell growth in oral squamous cell
carcinoma cell lines. Oral Dis. 21:501–511. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Eldhose B, Gunawan M, Rahman M, Latha MS
and Notario V: Plumbagin reduces human colon cancer cell survival
by inducing cell cycle arrest and mitochondria-mediated apoptosis.
Int J Oncol. 45:1913–1920. 2014.PubMed/NCBI
|
|
17
|
Dandawate P, Ahmad A, Deshpande J, Swamy
KV, Khan EM, Khetmalas M, Padhye S and Sarkar F: Anticancer
phytochemical analogs 37: Synthesis, characterization, molecular
docking and cytotoxicity of novel plumbagin hydrazones against
breast cancer cells. Bioorg Med Chem Lett. 24:2900–2904. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fuchs O: Targeting of NF-kappaB signaling
pathway, other signaling pathways and epigenetics in therapy of
multiple myeloma. Cardiovasc Hematol Disord Drug Targets. 13:16–34.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li YC, He SM, He ZX, Li M, Yang Y, Pang
JX, Zhang X, Chow K, Zhou Q, Duan W, et al: Plumbagin induces
apoptotic and autophagic cell death through inhibition of the
PI3K/Akt/mTOR pathway in human non-small cell lung cancer cells.
Cancer Lett. 344:239–259. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Koldehoff M, Beelen DW and Elmaagacli AH:
Inhibition of mTOR with everolimus and silencing by vascular
endothelial cell growth factor-specific siRNA induces synergistic
antitumor activity in multiple myeloma cells. Cancer Gene Ther.
21:275–282. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gay F, Oliva S, Petrucci MT, Conticello C,
Catalano L, Corradini P, Siniscalchi A, Magarotto V, Pour L,
Carella A, et al: Chemotherapy plus lenalidomide versus autologous
transplantation, followed by lenalidomide plus prednisone versus
lenalidomide maintenance, in patients with multiple myeloma: a
randomised, multicentre, phase 3 trial. Lancet Oncol. 16:1617–1629.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang Y, Zhou X, Xiao M, Hong Z, Gong Q,
Jiang L and Zhou J: Discovery of chrysoeriol, a PI3K-AKT-mTOR
pathway inhibitor with potent antitumor activity against human
multiple myeloma cells in vitro. J Huazhong Univ Sci Technolog Med
Sci. 30:734–740. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dong M, Yang G, Liu H, Liu X, Lin S, Sun D
and Wang Y: Aged black garlic extract inhibits HT29 colon cancer
cell growth via the PI3K/Akt signaling pathway. Biomed Rep.
2:250–254. 2014.PubMed/NCBI
|
|
24
|
Chen X, Yang C, Xu Y, Zhou H, Liu H and
Qian W: The microtubule depolymerizing agent CYT997 effectively
kills acute myeloid leukemia cells via activation of caspases and
inhibition of PI3K/Akt/mTOR pathway proteins. Exp Ther Med.
6:299–304. 2013.PubMed/NCBI
|
|
25
|
Jang J, Jeong SJ, Kwon HY, Jung JH, Sohn
EJ, Lee HJ, Kim JH and Kim SH, Kim JH and Kim SH: Decursin and
doxorubicin are in synergy for the induction of apoptosis via STAT3
and/or mTOR pathways in human multiple myeloma cells. Evid Based
Complement Alternat Med. 2013:5063242013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen MB, Zhang Y, Wei MX, Shen W, Wu XY,
Yao C and Lu PH: Activation of AMP-activated protein kinase (AMPK)
mediates plumbagin-induced apoptosis and growth inhibition in
cultured human colon cancer cells. Cell Signal. 25:1993–2002. 2013.
View Article : Google Scholar : PubMed/NCBI
|