Introduction
Bladder cancer is the second most common
genitourinary malignancy worldwide (1). Over 90% of bladder cancer cases are
transitional cell carcinoma (TCC). Approximately 25% of cases are
diagnosed as muscle-invasive bladder cancer (MIBC), and ≤15% of
patients with non-muscle-invasive bladder cancer will develop an
invasive recurrent cancer within 1 year of treatment (2). Radical cystectomy is considered as the
gold standard treatment for patients with MIBC. Although the
techniques of surgery and chemoradiotherapy have improved, the
5-year survival rate of bladder TCC patients ranges from 48–66%
(3). Furthermore, once patients
develop distant metastases, the 5-year survival rate drops to only
6% (4). Better understanding of the
molecular mechanisms and identifying a novel prognostic marker of
bladder cancer may help to treat this disease.
Wip1 was first identified as a phosphatase that is
induced by p53 in response to ionizing radiation (5). Wip1 is encoded by protein phosphatase
magnesium-dependent 1 Δ, which is located on the 17q22/24 human
chromosomal region, and is a member of the protein phosphatase type
2C (PP2C) family. Previous studies have demonstrated that Wip1 is
overexpressed in various types of cancer and that Wip1 is
associated with cancer development, suggesting that this protein
may be a tumor biomarker and therapeutic target (6–9). However,
the expression and role of Wip1 in bladder cancer have not yet been
investigated.
The purpose of the present study was to determine
the levels of Wip1 expression in patients with bladder cancer and
the association between Wip1 expression and clinicopathological
features. The effects of Wip1 knockdown on proliferation, invasion
and migration in T24 (human bladder carcinoma cell line) cells and
the underlying signaling pathways were also evaluated.
Materials and methods
Patients and tissue samples
A total of 106 formalin-fixed paraffin-embedded
bladder TCC tissues and corresponding normal bladder tissues were
collected from patients at the Department of Urology, Beijing
Friendship Hospital, Capital Medical University (Beijing, China)
who underwent surgical resection between January 2009 and December
2011. All patients were diagnosed with TCC by postoperative
pathological examination. No patients were treated with
radiotherapy or chemotherapy prior to surgery. Clinical data
included age, gender, tumor size, pathological grade, lymph nodal
status, tumor-node-metastasis (TNM) classification stage and
patient survival time. Tumor staging was based on the seventh
edition of the TNM classification system (2009) (10). Follow-up information was obtained
using medical records and phone investigations. The date of the
last follow-up was October 1, 2015. The study protocol was approved
by the Ethics Committee of Beijing Friendship Hospital, Capital
Medical University and written informed consent was obtained from
all patients.
Immunohistochemistry
Paraffin-embedded tissue sections (4 µm thick) were
deparaffinized, rehydrated and subjected to antigen retrieval by
immersion in boiling citric acid buffer (pH 6.0; ZSGB-BIO, Beijing,
China) for 10 min. Endogenous peroxidase and non-specific
conjugation were blocked by 3% hydrogen peroxide and normal goat
serum (ZSGB-BIO, Beijing, China). Sections were incubated with
anti-Wip1 antibody (#ab31270; dilution 1:1,000; Abcam, Cambridge,
UK) at 4°C overnight. Subsequently, sections were incubated with
biotinylated anti-rabbit secondary antibody (#ZB-2010; dilution,
1:400; ZSGB-BIO) and streptavidin-horseradish peroxidase complex
(ZSGB-BIO) at room temperature for 10 min. Finally, sections were
stained with 3,3′-diaminobenzidine and counterstained with
hematoxylin, then dehydrated and mounted. An immunoreactivity score
was used to evaluate the staining of Wip1 expression in bladder TCC
tissues and normal bladder tissues. Four randomly selected fields
of each slide were counted using an inverted light microscope
(DMi1; Leica Microsystems GmbH, Wetzlar, Germany). Wip1 expression
was quantified according to the staining intensity and the
percentage of positive cells. The staining intensity was defined as
follows: 0 point, no staining; 1 point, weak staining; 2 points,
moderate staining; and 3 points, strong staining. The percentage of
positive cells was defined as follows: 1 point, ≤10%; 2 points,
11–50%; 3 points, 51–80%; and 4 points, ≥81%. The final points were
defined as the points of the staining intensity multiplied by those
of the percentage of positive cells. Wip1 expression was divided
into the following categories: No expression, 0–2 points; positive
expression, ≥3 points (low expression, 3–6 points and high
expression, >6 points). Two pathologists (Department of
Pathology, Beijing Friendship Hospital, Capital Medical University)
assessed these results independently.
Cell culture and small interfering RNA
(siRNA) transfection
Human bladder T24 cells were purchased from the Cell
Resource Center, Peking Union Medical College (Beijing, China) and
were cultured in Dulbecco's Modified Eagle's Medium (DMEM; Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 10%
fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) in
a humidified incubator at 37°C with 5% CO2. siRNA for
Wip1 and negative control siRNA were designed and synthesized by
Guangzhou RiboBio Co., Ltd. (Guangzhou, China), according to the
NM_003620 gene sequence in the National Center for Biotechnology
Information database (https://www.ncbi.nlm.nih.gov/nuccore/312434022/). The
sequences of Wip1-siRNA were as follows: Sense,
5′-AGGUGACACAGGACCAUAAdTdT-3′, antisense,
3′-dTdTUCCACUGUGUCCUGGUAUU-5′ and target sequence,
5′-AGGTGACACAGGACCATAA-3′. T24 cells were transfected using
Lipofectamine® 2000 Transfection Reagent (Invitrogen;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. When the cells reached 30–50% confluence in 6-well
plates, the medium was transferred to Opti-MEM®
Reduced-Serum medium (Invitrogen; Thermo Fisher Scientific, Inc.)
containing the complexes of Wip1-siRNA and Lipofectamine 2000.
Cells were incubated for 6 h, then the medium was switched to DMEM
supplemented with 10% FBS.
Reverse transcription
quantitative-polymerase chain reaction (RT-qPCR)
Total RNA was extracted with the RNeasy®
Mini kit (Qiagen Inc., Valencia, CA, USA) at 24 h
post-transfection, according to the manufacturer's protocol. Total
RNA (1 µg) was reverse transcribed into cDNA using a PrimeScript™
1st Strand cDNA Synthesis kit (Takara Biotechnology Co., Ltd.,
Dalian, China) on an ABI Veriti™ Thermal Cycler (Applied
Biosystems; Thermo Fisher Scientific, Inc.). RT-qPCR was conducted
in 20 µl reaction buffer with SYBR® Premix DimerEraser™
(Perfect Real Time) (Takara Biotechnology Co., Ltd., Dalian, China)
using the ABI® 7500 Fast Real-Time PCR system (Applied
Biosystems, Foster City, CA, USA). The following primer sequences
were used: Wip1 forward, 5′-GGAAGAAACTGGCGGAATG-3′ and reverse,
5′-TGGGAAGTTCTGGCTTATGG-3′; GAPDH, forward,
5′-CGGAGTCAACGGATTTGGTCGTAT-3′ and reverse,
5′-AGCCTTCTCCATGGTGGTGAAGAC-3′. GAPDH was used as the internal
control. RT-qPCR was performed under the following conditions: 95°C
for 30 sec, 40 cycles at 95°C for 3 sec, 60°C for 30 sec and 72°C
for 30 sec. The melting curve was analyzed for each sample. All
samples were performed in triplicate. The 2-ΔΔCq method was used to
calculate the relative mRNA levels of Wip1 (11).
Western blot analysis
Western blotting was performed at 48 h
post-transfection. The harvested T24 cells were washed with
ice-cold PBS and were lysed in radio immunoprecipitation assay
lysis buffer containing phenylmethanesulfonyl fluoride (Beyotime
Institute of Biotechnology, Haimen, China) at 4°C for 30 min. The
cells were subsequently centrifuged at 12,000 × g at 4°C for 10
min, and the supernatant was taken and protein concentration
detected using the BCA protein assay kit (Beyotime Institute of
Biotechnology). Total protein (40 µg) was separated by 10%
SDS-PAGE, then transferred to a nitrocellulose filter membrane.
Following blocking with 5% non-fat dry milk for 2 h at room
temperature, the membranes were incubated with the following
primary antibodies: Anti-Wip1 antibody (#ab31270; dilution, 1:800;
Abcam), anti-P53 antibody (#ab179477; dilution, 1:1,000; Abcam) and
anti-GAPDH antibody (#ab181602; dilution, 1:1,000; Abcam) at 4°C
overnight. Subsequently, the membranes were incubated with
peroxidase-conjugated affinipure goat anti-rabbit secondary
antibody (#ZB-5301; dilution 1:5,000; Zhongshan Golden Bridge
Biotechnology Co., Ltd., Beijing, China) at room temperature for 2
h. Following washing, protein bands were detected using the Bio-Rad
ChemiDoc™ XRS+ system (Bio-Rad Laboratories, Co., Ltd, Hercules,
CA, USA) using Image Lab™ software (Bio-Rad Laboratories, Co.,
Ltd.). GAPDH was used as the protein control and all experiments
were performed in triplicate.
MTT assay
The T24 cells were plated in 96-well plates at a
density of 2,000 cells/well in 100 µl DMEM supplemented with 10%
FBS. Following transfection with Wip1-siRNA or negative control
siRNA, the cells were cultured for 0, 24, 48 and 72 h. A total of
10 µl 5 mg/ml MTT was added to each well and the cells were
incubated at 37°C for 4 h. Next, 100 µl formazan solution was added
to each well and the cells were incubated at 37°C for an additional
4 h. The absorption value was measured at 570 nm using a
SpectraMax® M3 Multi-Mode Microplate Reader (Molecular
Devices, LLC, Sunnyvale, CA, USA). All reactions were performed in
triplicate.
Invasion and migration assays
The 24-well (8 µm pore) Transwell® cell
culture inserts (Corning Inc., Corning, NY, USA) were coated with
50 µl Matrigel™ Basement Membrane Matrix (BD Biosciences, San Jose,
CA, USA). Following transfection with Wip1-siRNA or negative
control siRNA, 1×105 cells were plated in the upper chamber, and
cultured with serum-free DMEM (Gibco; Thermo Fisher Scientific,
Inc.). DMEM containing 10% FBS (Gibco; Thermo Fisher Scientific,
Inc.) was placed in the lower chamber. Following incubation at 37°C
for 24 h, the cells and medium in the upper chamber were removed
with a cotton swab. Polyoxymethylene was added to the lower chamber
to fix the cells attached to the lower membrane surface, and 0.1%
crystal violet was added to the lower chamber to stain the cells.
Following this, crystal violet staining was decolorized using 33%
acetic acid. The absorption value was measured at 570 nm to
indicate cell invasion. Migration assays were performed using the
aforementioned method without Matrigel. All experiments were
performed in triplicate.
Statistical analysis
All statistical analyses were performed using SPSS
version 19.0 (IBM SSPS, Armonk, New York, USA). Values were
expressed as the mean ± standard deviation. For the clinical
characteristics, P-values were calculated using the χ2 test.
Survival outcomes were estimated with the Kaplan-Meier method and
then compared using the log-rank test. The Cox proportional hazards
regression model was used to define factors predicting prognosis
and the Student's t-test was used to evaluate the significance of
differences between two groups. P≤0.05 was considered to indicate a
statistically significant difference.
Results
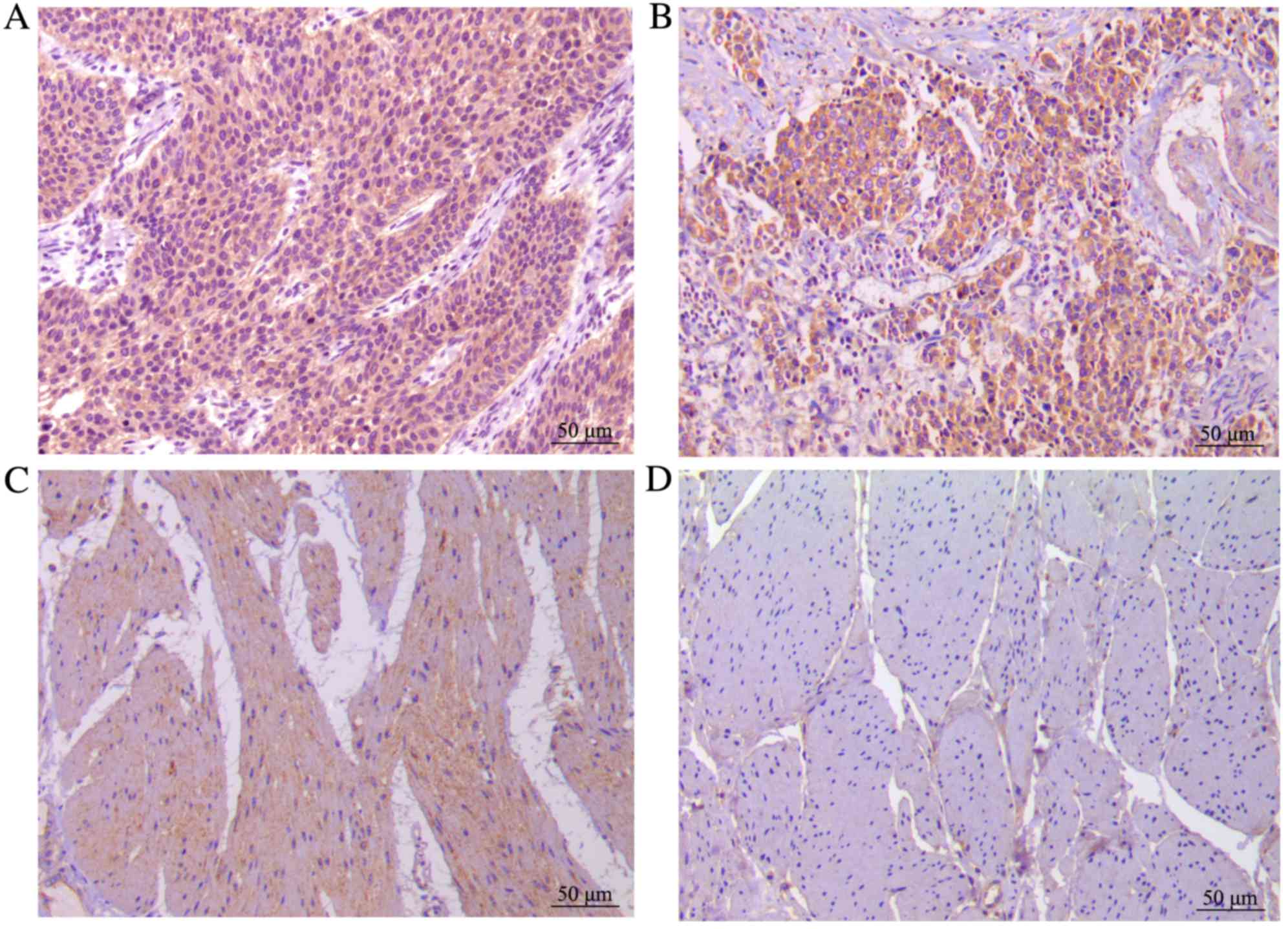
Wip1 is overexpressed in bladder TCC
tissues
In the present study, 106 bladder TCC tissues and
corresponding normal bladder tissues were analyzed using
immunohistochemistry. Wip1 expression was significantly higher in
the bladder TCC tissues (99/106, 93.4%) than in the normal tissues
(13/106, 12.3%; P<0.0001). In addition, the expression rate of
Wip1 in the low and high groups was 48.1 and 45.3%, respectively,
in the bladder TCC tissues. In normal tissues, Wip1 was expressed
at a low level or not expressed at all. Wip1 staining is presented
in Fig. 1.
Wip1 expression is associated with
clinicopathological features and prognosis
To analyze the correlation between Wip1 expression
and clinical data, the patients were divided into low (including no
expression) and high expression groups based on immunohistochemical
points of bladder TCC tissues. High expression levels of Wip1 were
significantly associated with increased tumor size (P=0.002),
pathological grade (P=0.025), clinical T stage (P=0.001) and lymph
nodal metastasis (P=0.003), but not with gender and age (Table I). The Kaplan-Meier survival curves
and the log-rank tests demonstrated that the overall survival time
of patients in the high expression group was significantly lower,
compared with patients in the low expression group (P<0.0001;
Fig. 2). In the Cox proportional
hazards regression model analyses for prognosis, clinical T stage
(P=0.004), lymph nodal metastasis (P=0.001) and Wip1 expression
(P=0.025) were independent prognostic factors in patients with
bladder TCC (Table II).
 | Table I.Association between wild-type
p53-induced phosphatase expression levels and the
clinicopathological features of patients with bladder transitional
cell carcinoma. |
Table I.
Association between wild-type
p53-induced phosphatase expression levels and the
clinicopathological features of patients with bladder transitional
cell carcinoma.
| Clinicopathological
features | Total | Low | High | P-value |
|---|
| Gender |
|
|
| 0.144 |
| Male | 84 | 49 | 35 |
|
Female | 22 | 9 | 13 |
| Age, years |
|
|
| 0.526 |
| ≥60 | 74 | 39 | 35 |
|
<60 | 32 | 19 | 13 |
| Tumor size, cm |
|
|
| 0.002 |
| ≥3 | 51 | 20 | 31 |
|
<3 | 55 | 38 | 17 |
| Pathological
grade |
|
|
| 0.025 |
| G1 | 21 | 13 | 8 |
| G2 | 56 | 33 | 23 |
| G3 | 29 | 12 | 17 |
| Clinical T stage |
|
|
| 0.001 |
|
Ta+Tis+T1 | 43 | 32 | 11 |
|
T2-T4 | 63 | 26 | 37 |
| Lymph nodal
metastasis |
|
|
| 0.003 |
| N0 | 73 | 48 | 25 |
| N1 | 22 | 7 | 15 |
| N2 | 11 | 3 | 8 |
 | Table II.Cox proportional hazards regression
model analyses of Wip1 expression and other clinical prognostic
factors in patients with bladder transitional cell carcinoma. |
Table II.
Cox proportional hazards regression
model analyses of Wip1 expression and other clinical prognostic
factors in patients with bladder transitional cell carcinoma.
|
| Cox regression
analysis |
|---|
|
|
|
|---|
| Variable | HR (95% CI) | P-value |
|---|
| Gender
(male/female) | 0.74 (0.41–1.32) | 0.302 |
| Age (≥60/<60
years) | 1.06 (0.62–1.79) | 0.842 |
| Tumor size (≥3/<3
cm) | 0.91 (0.55–1.51) | 0.709 |
| Pathological grade
(G1+G2/G3) | 1.79 (0.92–3.48) | 0.087 |
| Clinical T stage
(Ta+Tis+T1/T2-T4) | 2.49 (1.33–4.64) | 0.004 |
| Lymph nodal
metastasis (N0/N+) | 3.34 (1.60–6.98) | 0.001 |
| Wip1 expression
(high/low) | 1.86 (1.02–3.18) | 0.025 |
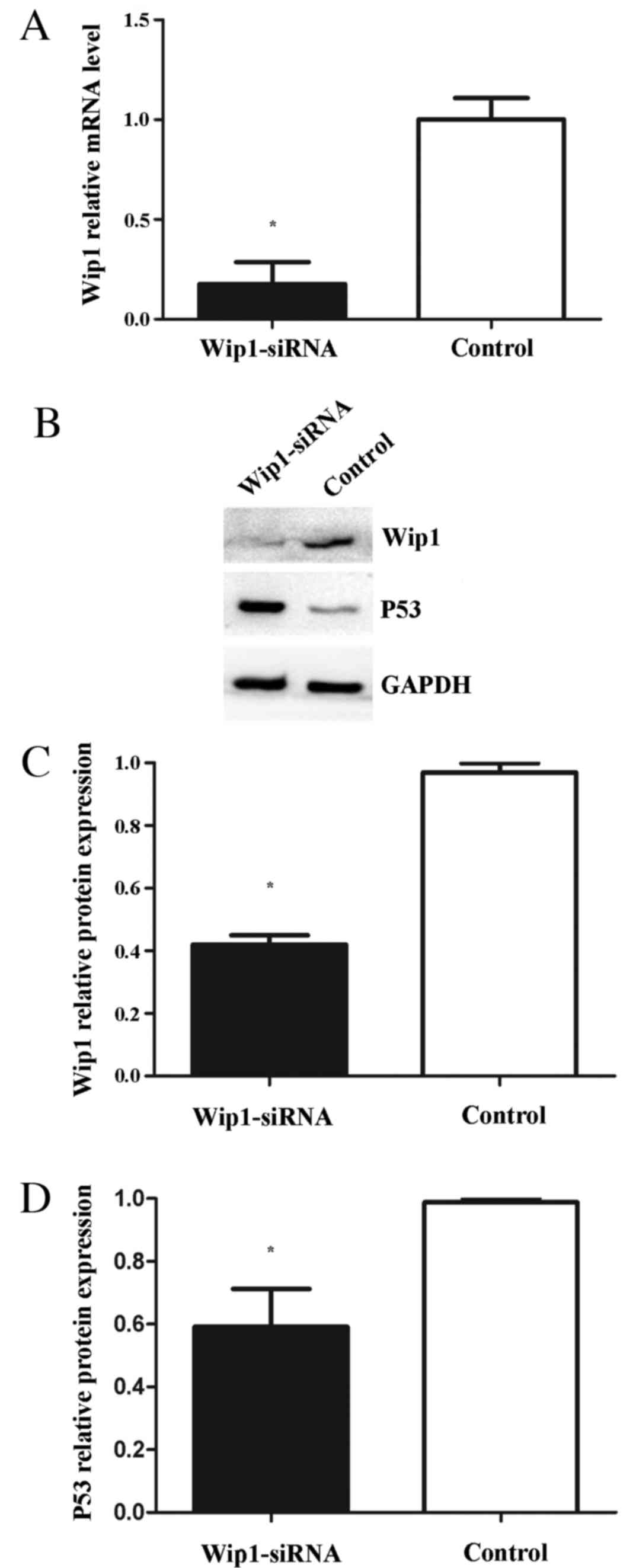
Downregulation of Wip1 decreases T24
cell proliferation, invasion and migration
To assess the effects of Wip1 on TCC cell
proliferation, invasion and migration, Wip1-siRNA or negative
control siRNA was transiently transfected into T24 cells. RT-qPCR
and western blot analyses demonstrated that Wip1 mRNA and protein
expression levels in the Wip1-siRNA-transfected T24 cells were
significantly lower compared with the control group (mRNA, P=0.019;
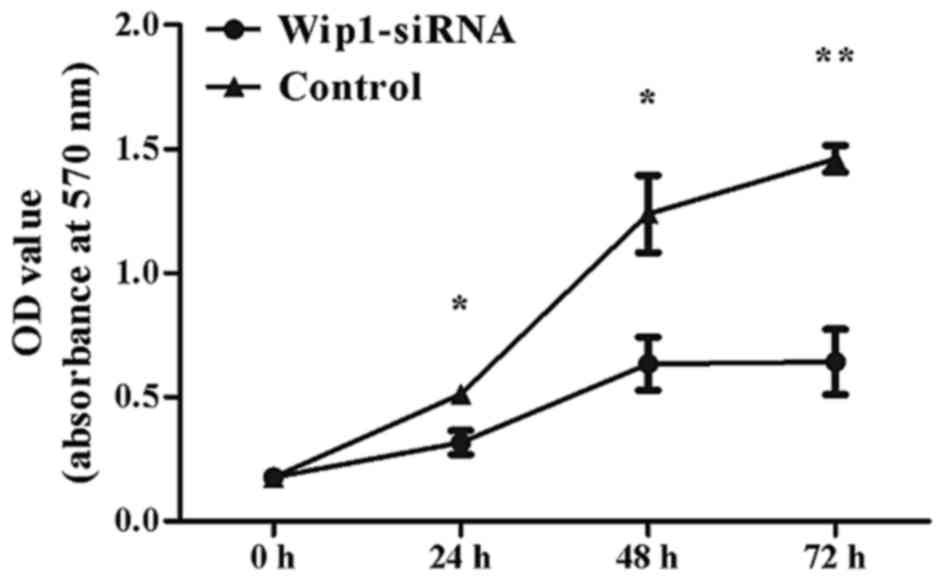
protein, P<0.001; Fig. 3A-C). MTT
assays indicated that Wip1-siRNA treatment significantly inhibited
T24 cell proliferation at 24 h (P=0.005), 48 h (P=0.002) and 72 h
(P<0.001) post-transfection (Fig.
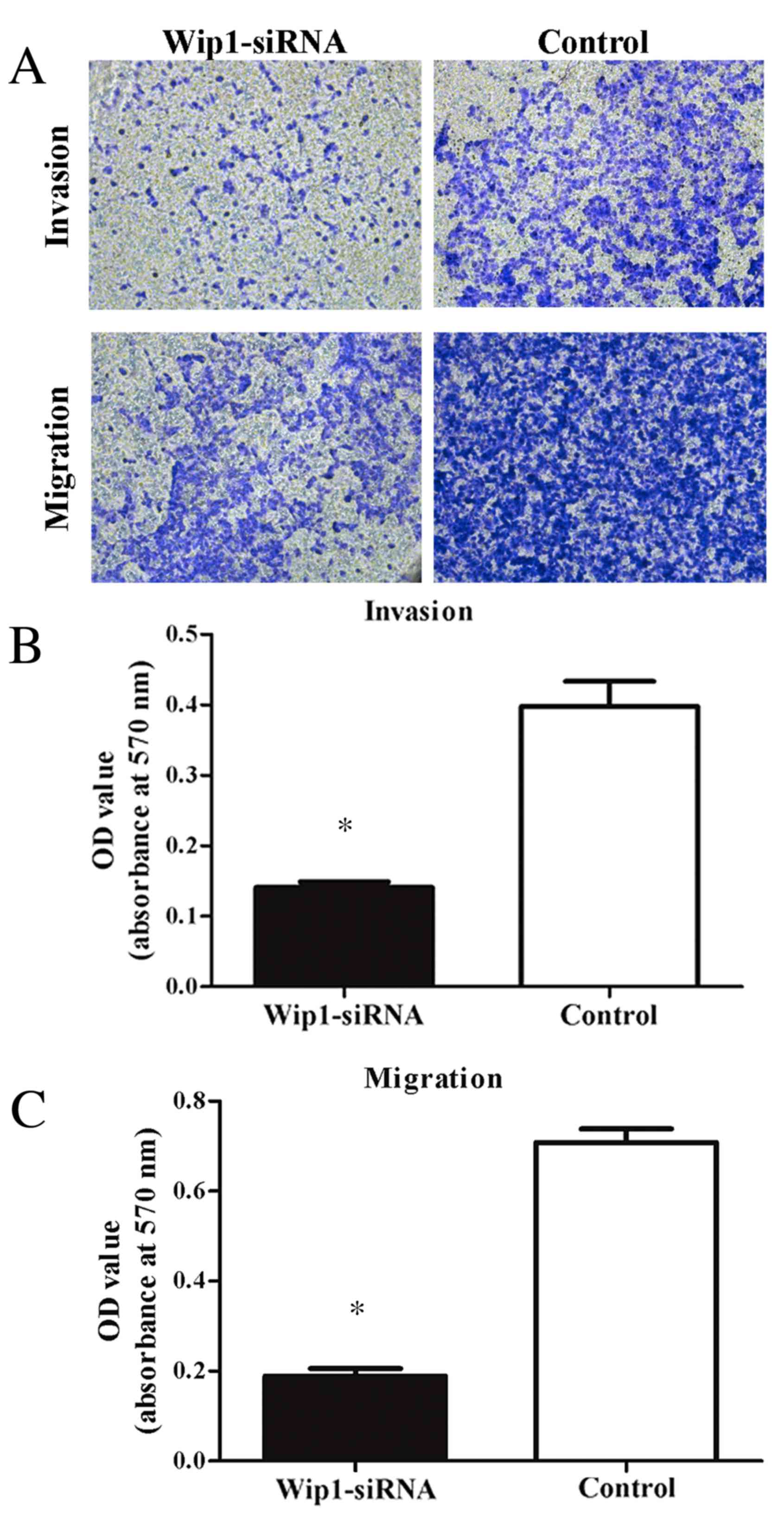
4). Furthermore, invasion and migration abilities were
significantly reduced in the Wip1-siRNA-transfected T24 cells when
compared with the controls (invasion and migration, P<0.001;
Fig. 5). To further examine the
potential signaling pathways mediating the aforementioned functions
of Wip1, the expression levels of p53 (a tumor suppressor) were
analyzed. Western blot analysis demonstrated that p53 protein
levels were significantly upregulated in the Wip1-siRNA-transfected
T24 cells (P=0.026; Fig. 3B and
D).
Discussion
Bladder cancer is a leading global cause of cancer
morbidity and mortality (1,12). In 2016, 76,960 new cases and 16,390
mortalities are predicted in the United States (4). However, the molecular mechanisms
underlying tumorigenesis and the progression of bladder cancer
remain to be elucidated.
Wip1, an established oncogene, has been the subject
of a number of studies since it was identified in 1997 (5,13–15). It has been reported that Wip1
negatively regulates DNA damage response pathways by
dephosphorylating several key proteins, including p53, p16, ataxia
telangiectasia mutated, checkpoint kinase 1 and p38
mitogen-activated protein kinases (MAPKs), resulting in
tumorigenesis (16,17). In addition, the Wip1-p53 signaling
pathway is well researched in the DNA damage response system. The
tumor suppressor p53 serves an essential role in DNA damage
response pathways by regulating cell cycle arrest and apoptosis
(18). It is known that the
dysfunction of p53 has been associated with the progression,
prognosis and therapeutic response of tumors (19). Wip1 is not only able to directly
dephosphorylate p53 protein at serine 15, but also indirectly
inactivates p53 protein via p38, MAPK and Mdm2 (16,20,21).
Therefore, restoring p53 function by regulating Wip1 may be a
potential therapeutic approach. Furthermore, previous studies on
solid tumors demonstrated that Wip1 was associated with poor
prognosis in patients with nasopharyngeal carcinoma (8), liver and (9) prostate cancer (22) and lung adenocarcinoma (23). However, little is understood regarding
Wip1 expression levels in bladder cancer and its association with
clinicopathological features.
To the best of our knowledge, the present study is
the first to investigate the expression of Wip1 in patients with
bladder TCC. A total of 106 bladder TCC and corresponding normal
bladder tissues were analyzed by immunohistochemistry. The data
demonstrated that Wip1 was overexpressed in the bladder TCC
tissues, compared with the normal tissues. Furthermore, high
expression levels of Wip1 were positively associated with more
aggressive tumor characteristics, including tumor size,
pathological grade, clinical T stage and lymph nodal metastasis. To
determine the correlation between Wip1 expression and prognosis,
Kaplan-Meier survival curve and the Cox proportional hazards
regression model analyses were implemented. The results revealed
that high levels of Wip1 expression led to a shorter overall
survival time, and that Wip1 expression was an independent
prognostic factor. These results suggest that Wip1 expression may
be a potential prognostic marker for patients with bladder
cancer.
To assess the effects of Wip1 in bladder cancer,
further in vitro experiments were performed. Human bladder
T24 cells were transfected with Wip1-siRNA to downregulate mRNA and
protein expression levels of Wip1. The results demonstrated that
cell proliferation, invasion and migration were decreased in the
Wip1-siRNA-transfected T24 cells compared with the control group,
indicating that Wip1 may be a potential therapeutic target.
Furthermore, levels of p53 protein expression were upregulated in
the Wip1-siRNA-transfected T24 cells, similar to previous studies
(24,25). These results suggest that the
downregulation of Wip1 expression may inhibit bladder cancer cell
proliferation, invasion and migration via activation of the p53
pathway. However, mutations of p53 were observed in more than half
of patients with invasive bladder TCC (26). Whether Wip1 is able to restore
functional p53 protein requires elucidation in future studies.
In conclusion, the current study demonstrated that
Wip1 is overexpressed in patients with bladder TCC and that high
levels of Wip1 expression were positively correlated with more
aggressive tumor characteristics and poorer prognosis.
Downregulation of Wip1 expression inhibited bladder cancer cell
proliferation, invasion and migration by activating the p53 pathway
in the T24 cells. Therefore, these findings indicate that Wip1 may
serve as a potential prognostic marker and therapeutic target in
bladder cancer.
References
|
1
|
Burger M, Catto J, Dalbagni G, Grossman
HB, Herr H, Karakiewicz P, Kassouf W, Kiemeney LA, La Vecchia C,
Shariat S and Lotan Y: Epidemiology and risk factors of urothelial
bladder cancer. Eur Urol. 63:234–241. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Boccardo F and Palmeri L: Adjuvant
chemotherapy of bladder cancer. Ann Oncol. 17:(Suppl 5). v129–v132.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stein JP, Lieskovsky G, Cote R, Groshen S,
Feng AC, Boyd S, Skinner E, Bochner B, Thangathurai D, Mikhail M,
et al: Radical cystectomy in the treatment of invasive bladder
cancer: Long-term results in 1054 patients. J Clin Oncol.
19:666–675. 2001.PubMed/NCBI
|
|
4
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–301. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fiscella M, Zhang H, Fan S, Sakaguchi K,
Shen S, Mercer WE, Woude GF Vande, O'Connor PM and Appella E: Wip1,
a novel human protein phosphatase that is induced in response to
ionizing radiation in a p53-dependent manner. Proc Natl Acad Sci
USA. 94:6048–6053. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang L, Chen LH, Wan H, Yang R, Wang Z,
Feng J, Yang S, Jones S, Wang S, Zhou W, et al: Exome sequencing
identifies somatic gain-of-function PPM1D mutations in brainstem
gliomas. Nat Genet. 46:726–730. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Richter M, Dayaram T, Gilmartin AG, Ganji
G, Pemmasani SK, Van Der Key H, Shohet JM, Donehower LA and Kumar
R: Wip1 phosphatase as a potential therapeutic target in
neuroblastoma. PLoS One. 10:e01156352015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sun GG, Zhang J, Ma XB, Wang YD, Chen YJ
and Hu WN: Overexpression of wild-type p53-induced phosphatase1
confers poor prognosis of patients with nasopharyngeal carcinoma.
Pathol Oncol Res. 21:283–291. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li GB, Zhang XL, Yuan L, Jiao QQ, Liu DJ
and Liu J: Protein phosphatase magnesium-dependent 1δ (PPM1D) mRNA
expression is a prognosis marker for hepatocellular carcinoma. PLoS
One. 8:e607752013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sobin L, Gospodarowicz M and Wittekind C:
TNM classification of malignant tumorsUICC International Union
Against Cancer. 7th. Wiley-Blackwell; pp. 262–265. 2009
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Witjes JA, Compérat E, Cowan NC, De Santis
M, Gakis G, Lebret T, Ribal MJ, Van der Heijden AG and Sherif A:
European Association of Urology: EAU guidelines on muscle-invasive
and metastatic bladder cancer: Summary of the guidelines. Eur Urol.
65:778–792. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shreeram S, Demidov ON, Hee WK, Yamaguchi
H, Onishi N, Kek C, Timofeev ON, Dudgeon C, Fornace AJ, Anderson
CW, et al: Wip1 phosphatase modulates ATM-dependent signaling
pathways. Mol Cell. 23:757–764. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lu X, Nannenga B and Donehower LA: PPM1D
dephosphorylates Chk1 and p53 and abrogates cell cycle checkpoints.
Genes Dev. 19:1162–1174. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fujimoto H, Onishi N, Kato N, Takekawa M,
Xu XZ, Kosugi A, Kondo T, Imamura M, Oishi I, Yoda A and Minami Y:
Regulation of the antioncogenic Chk2 kinase by the oncogenic Wip1
phosphatase. Cell Death Differ. 13:1170–1180. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu X, Nguyen TA, Moon SH, Darlington Y,
Sommer M and Donehower LA: The type 2C phosphatase Wip1: An
oncogenic regulator of tumor suppressor and DNA damage response
pathways. Cancer Metastasis Rev. 27:123–135. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lowe J, Cha H, Lee MO, Mazur SJ, Appella E
and Fornace AJ Jr: Regulation of the Wip1 phosphatase and its
effects on the stress response. Front Biosci (Landmark Ed).
17:1480–1498. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Marcel V, Dichtel-Danjoy ML, Sagne C,
Hafsi H, Ma D, Ortiz-Cuaran S, Olivier M, Hall J, Mollereau B,
Hainaut P and Bourdon JC: Biological functions of p53 isoforms
through evolution: Lessons from animal and cellular models. Cell
Death Differ. 18:1815–1824. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pfister C, Flaman JM, Dunet F, Grise P and
Frebourg T: p53 mutations in bladder tumors inactivate the
transactivation of the p21 and Bax genes, and have a predictive
value for the clinical outcome after bacillus Calmette-Guerin
therapy. J Urol. 162:69–73. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Crescenzi E, Raia Z, Pacifico F, Mellone
S, Moscato F, Palumbo G and Leonardi A: Down-regulation of
wild-type p53-induced phosphatase 1 (Wip1) plays a critical role in
regulating several p53-dependent functions in premature senescent
tumor cells. J Biol Chem. 288:16212–16224. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu X, Ma O, Nguyen TA, Jones SN, Oren M
and Donehower LA: The Wip1 phosphatase acts as a gatekeeper in the
p53-Mdm2 autoregulatory loop. Cancer Cell. 12:342–354. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jiao L, Shen D, Liu G, Jia J, Geng J, Wang
H and Sun Y: PPM1D as a novel biomarker for prostate cancer after
radical prostatectomy. Anticancer Res. 34:2919–2925.
2014.PubMed/NCBI
|
|
23
|
Satoh N, Maniwa Y, Bermudez VP, Nishimura
K, Nishio W, Yoshimura M, Okita Y, Ohbayashi C, Hurwitz J and
Hayashi Y: Oncogenic phosphatase Wip1 is a novel prognostic marker
for lung adenocarcinoma patients survival. Cancer Sci.
102:1101–1106. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun GG, Wang YD, Liu Q and Hu WN:
Expression of Wip1 in kidney carcinoma and its correlation with
tumor metastasis and clinical significance. Pathol Oncol Res.
21:219–224. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang HY, Liu ZS, Qiu L, Guo J, Li YF,
Zhang J, Wang TJ and Liu XD: Knockdown of Wip1 enhances sensitivity
to radiation in HeLa cells through activation of p38 MAPK. Oncol
Res. 22:225–233. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wagner U, Sauter G, Moch H, Novotna H,
Epper R, Mihatsch MJ and Waldman FM: Patterns of p53, erbB-2, and
EGF-r expression in premalignant lesions of the urinary bladder.
Hum Pathol. 26:970–978. 1995. View Article : Google Scholar : PubMed/NCBI
|