Introduction
Papillary thyroid carcinoma (PTC) is the most
prevalent type of malignancy of the thyroid gland and the endocrine
system (1). PTC incidence is
increased in the female population during the reproductive phase of
development (1). Although PTC
typically has a good prognosis, there is a group of patients
(<10%) that present with tumours exhibiting aggressive
characteristics (1). Therefore,
numerous previous studies have investigated the parameters that
have the potential to be independent prognostic factors of PTC
(1–5).
In the World Health Organisation Classification of Tumours of
Endocrine Organs, the prognosis of PTC depends on whether a patient
may be in the ‘risk group’, which is defined by the following
parameters: a) The patient being <45 years; b) the size of the
tumour is >4 cm; c) the presence of extrathyroidal extension; d)
the effectiveness of the surgery; e) the presence of distant
metastases (1). The tall cell and
columnar cell histological subtypes of PTC have a poor prognosis
compared with classical PTC (1). The
diffuse sclerosing variant of PTC may present with prominent
regional lymph node and lung metastases in <25% of patients
compared with the classical type of PTC, which also may present
with regional lymph node metastases in 25% of patients, but not
lung metastases (1). Toniato et
al (2) examined various factors
that potentially influenced tumour recurrence and the survival rate
in a group of 950 patients with PTC, with an average follow-up time
of 7.8 years. This previous study hypothesised that age at the time
of diagnosis (>45 years), an advanced disease stage (T4; distant
metastases; stage IV), the extent of thyroid surgery (subtotal
thyroid surgery) and the lack of administration of radioiodine
131-I therapy were effective and independent prognostic factors for
the recurrence of disease (2).
Spriano et al (3) studied the
pattern of regional lymph node metastases and potential prognostic
factors in differentiated thyroid carcinoma. This previous study
included 72 patients with PTC with regional lymph node metastases,
of which 18% developed recurrence after 6 years of disease-free
survival (3). The results from this
previous study identified that nodal metastases have a significant
prognostic impact, particularly nodal extracapsular spread, which
was established to be a potential predictive factor for distant
metastasis and the regional recurrence of PTC (3). Another previous study performed on 6,015
patients with PTC demonstrated that a tumour size >4 cm, the
presence of large extrathyroid extension, an age of ≥55, being of
the male gender and the presence of lymph node metastasis in the
lateral compartment are independent prognostic factors for reduced
tumour-specific survival (4). Jung
et al (5) investigated the
clinical features and prognostic factors associated with survival
in patients with poorly differentiated thyroid carcinoma and with
aggressive variants of PTC, including the tall cell, columnar cell,
solid and diffuse sclerosing variants of PTC. The results
identified that an age of >45 years, a tumour size >4 cm,
extrathyroidal invasion, cervical lymph node metastases, distant
metastasis, the absence of high-dose radioactive iodine therapy and
the tumour-node-metastasis (TNM) classification of malignant tumour
stages II, III and IV were significantly associated with poor
prognostic factors for survival using univariate analysis (5).
The variant of PTC identified as ‘papillary
microcarcinoma’ is defined as a tumour that is <1 cm in diameter
(1) and these tumours are frequently
identified incidentally. Within this type of tumour there is a
subgroup that presents with cervical lymph node metastasis at the
time of diagnosis (1). These tumour
tissues typically have specific immunohistochemical features
compared with incidentally identified microcarcinoma, which include
a loss of p27 and upregulation of cyclin D1 expression levels
reflecting a variation in biological characteristics and indicating
distinct clinical behaviour (1).
The non-metastatic expressed/non-metastatic 23
(nm23) gene has been established to be included in a group of
tumour suppressor genes (6). The
non-metastatic expressed/non-metastatic 23 nucleoside diphosphate
kinase 1 (nm23-H1) was the first identified member of the nm23 gene
family and its role in tumour suppression has been examined in
numerous types of malignant tumour (7–10).
However, the results from previous studies have not elucidated its
role in thyroid carcinogenesis (11–15).
Although a number of previous studies have indicated that high
levels of nm23-H1 expression in the advanced stages of papillary
and follicular thyroid carcinoma are indicative of its role in
proliferation (11,12), there are previous studies that have
presented contrasting results (13–15).
Notably, to the best of our knowledge there are no studies
demonstrating a statistically significant correlation between
nm23-H1 expression levels and the biological characteristics of
PTC, including the presence of lymph node metastases (13–17).
Prostaglandin-endoperoxide synthase 1 and 2 [also
termed cyclooxygenase (COX)-1 and COX-2] are key enzymes in the
synthesis of prostaglandins from arachidonic acid (18). The COX-1 enzyme is encoded by the gene
located on chromosome 9 at q33.2, and it is constitutively
expressed in almost all tissues (19,20). The
COX-2 enzyme is encoded by the gene located on chromosome 1 at
q31.1 and is an inducible isozyme primarily expressed at sites of
inflammation (21). The increased
expression of COX-2 has been detected in numerous types of
carcinoma (22,23). A number of studies demonstrated that
COX-2 expression is increased in the elderly (without papillary
cancer) but decreased in follicular variant of papillary cancer. By
contrast, other studies have detected increased expression of COX-2
in papillary cancer tissues compared with normal tissues without
significant statistical differences. However, the sample sizes in
these studies were small. COX-2 expression is decreased in patients
with papillary cancer, large tumor masses and advanced stage
(24–33). A number of previous studies have
demonstrated that COX-2 expression levels were significantly
increased in malignant nodular changes of the thyroid compared with
benign nodular changes of the thyroid (24–26). The
results of this previous study indicated that COX-2 may not be an
effective diagnostic marker of thyroid malignancy (28). Concordant with other previous studies,
the upregulation of COX-2 expression may significantly contribute
to the progression of PTC, particularly in early stages of the
disease (28–31). Previous studies have also established
that the expression levels of COX-2 in PTC and inflammatory thyroid
disease may provide a bridge for an association between
carcinogenesis and inflammation in the thyroid gland (28,32,33). Due
to the contradictory results in previous studies, the role of COX-2
in papillary cancer remains to be elucidated.
Epstein-Barr virus (EBV)-infected cells have been
established to be an optimal model for investigating the
association between nm23-H1 and COX-2 (34). A previous study using these cells
suggested that COX-2 expression was promoted by the induction of
nm23-H1 expression via the nuclear factor-κB (NF-κB) signalling
pathway (34). Therefore, the current
study investigated the role of the expression levels of nm23-H1 and
COX-2 proteins in 130 samples of PTC, including 75 PTC cases and 55
microcarcinoma tumours.
Materials and methods
Patient information and tissue
samples
The archived tissue samples from patients with PTC
were fixed in 10% neutral-buffered formalin, embedded in paraffin,
sectioned, stained with haematoxylin and eosin and archived at the
Department of Pathology (University Hospital for Tumours, Zagreb,
Croatia) as described below. The tissues were from 130 patients who
underwent partial or total thyroidectomy with paratracheal lymph
node dissection at the Department of Head and Neck Surgery at the
University Hospital for Tumours between January 2000 and December
2007. These samples were subclassified using the World Health
Organisation classification (1) were
classified as follows: 67 papillary; 32 follicular; 13 sclerosing;
10 oxyphilic histological subtype; 8 classified as ‘other’ (1
macrofollicular, 1 insular, 1 clear-cell, 1 tall-cell and 4 tumours
with combined classification types, including 2
sclerosing-oxyphilic, 1 follicular-insular and 1
follicular-oxyphilic) (1).
All samples were subsequently analysed for the
following four parameters: a) The presence of lymphocytic
inflammatory infiltrate; b) the presence of intraglandular
dissemination; c) any additional benign changes in the thyroid
gland tissue; d) the presence of metastases in lymph nodes.
Tissue blocks were cut into 4 µm sections and were
dried onto slides for 12–24 h at >60°C. Tissue sections were
deparaffinised in xylene and rehydrated using graded alcohol and
deionised H2O. The sections were heated in a microwave
in a 0.3% citrate buffer, pH 6.0 (cat. no. S1699; Target Retrieval
Solution; DAKO; Agilent Technologies, Inc., Santa Clara, CA, USA) 4
times for 5 min each and washed with PBS twice for 5 min. Slides
were incubated with mouse monoclonal COX-2 (dilution, 1:100; cat.
no. sc-58344; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and
mouse monoclonal nm23-H1 (dilution, 1:200; cat. no. sc-56928; Santa
Cruz Biotechnology, Inc.) antibodies for 30 min at room
temperature. Immunohistochemical staining was performed with a Dako
Autostainer using the EnVision+ horseradish peroxidase
3,3′-Diaminobenzidine system (cat. no. K8000 Envision Flex; Dako;
Agilent Technologies, Inc.), which includes secondary antibody (30
min at room temperature) according to the manufacturer's protocol.
Subsequently, the sections were washed in H2O for 10
min, counterstained with Meyer's haematoxylin for 15–60 sec and
washed. The images were captured with a ZEISS Axiostar plus
microscope (Zeiss AG, Oberkochen, Germany). Paraffin blocks of
colorectal cancer established as being positive for COX-2
expression and ductal invasive breast cancer established as being
positive for nm23-H1 expression were used as a positive control.
The archived tissue samples from patients with colorectal and
breast cancer were fixed in 10% neutral-buffered formalin, embedded
in paraffin, sectioned, stained with haematoxylin and eosin and
archived at Department of Pathology (University Hospital for
Tumours, Zagreb, Croatia) (35–39).
Immunoreactivity scores (IRS) were obtained by
multiplying the scores (MS, multiplied score) of staining intensity
and the size of immunoreactive area. The intensity of stain was
scored as follows: 0, absent; 1, mild; 2, moderate; 3, strong. The
immunoreactive area was scored as follows: 0, negative; 1, <10%;
2, 10–50%; 3, >50% of cells stained. The IRS scores were
collated as follows: 0, MS 0; 1, MS 1–3; 2, MS 4 or 6; 3, MS 9.
Statistical analysis
Quantitative variables were presented as median
values and corresponding interquartile ranges and categorical
variables were presented as absolute frequencies and corresponding
percentages. The variation in IRS values for nm23-H1 and COX-2
expression levels and clinical (age, gender, tumour size and
presence of lymph node metastases) and histological variables
(histological subtype of tumour, lymphocytic infiltration and
presence of additional benign thyroid diseases) was assessed with
the χ2 test. The Kruskal-Wallis test was used to
determine statistically significant differences between the median
age, tumour size and nm23-H1 and COX-2 IRS values. SPSS Statistics
for Windows version 21 (IBM SPSS, Armonk, NY, USA) was used for all
statistical tests. P<0.05 was considered to indicate a
statistically significant difference.
Results
The present study examined samples from 130 patients
with PTC with the following data: Age, gender, localization and
size of the tumour, histological subtype, the presence of
lymphocytic infiltration and additional benign changes in the
thyroid gland, intraglandular spread of the tumour and the presence
of lymph node metastases.
In the current study, PTCs occurred in female
patients (107/130) that were aged between 20 and 80 years (median,
49.5) and primarily in the right lobe (74/130). The size of the
tumour varied from 0.3–6.5 cm (median, 1.2 cm) and 55 of the
tumours were ≤1 cm in diameter. From examining the tumour
morphology the following classifications were made: 67 papillary;
32 follicular; 13 diffuse sclerosing; 10 oxyphilic subtype; 8
classified as ‘other’. The latter group consisted of 1
macrofollicular, 1 insular, 1 clear-cell, 1 tall-cell; 4 of
combination subtypes, including 2 sclerosing-oxyphilic, 1
follicular-insular and 1 follicular-sclerosing PTC tumour.
Lymphocytic infiltration was present in 39/130 of tumour samples.
Intraglandular dissemination was identified in 37 samples and lymph
node metastases were present in 27 patients. Additional benign
changes in the surrounding tissue of the thyroid gland, including
follicular adenoma, lymphocytic thyroiditis, Hashimoto thyroiditis
and goitre were present in 65 patients. The most frequent type of
benign change was thyroiditis, which included Hashimoto and
lymphocytic thyroiditis and was present in 33 patients.
Immunohistochemical results (Table I) indicated that there was
moderate-strong to strong cytoplasmic staining for nm23-H1 and
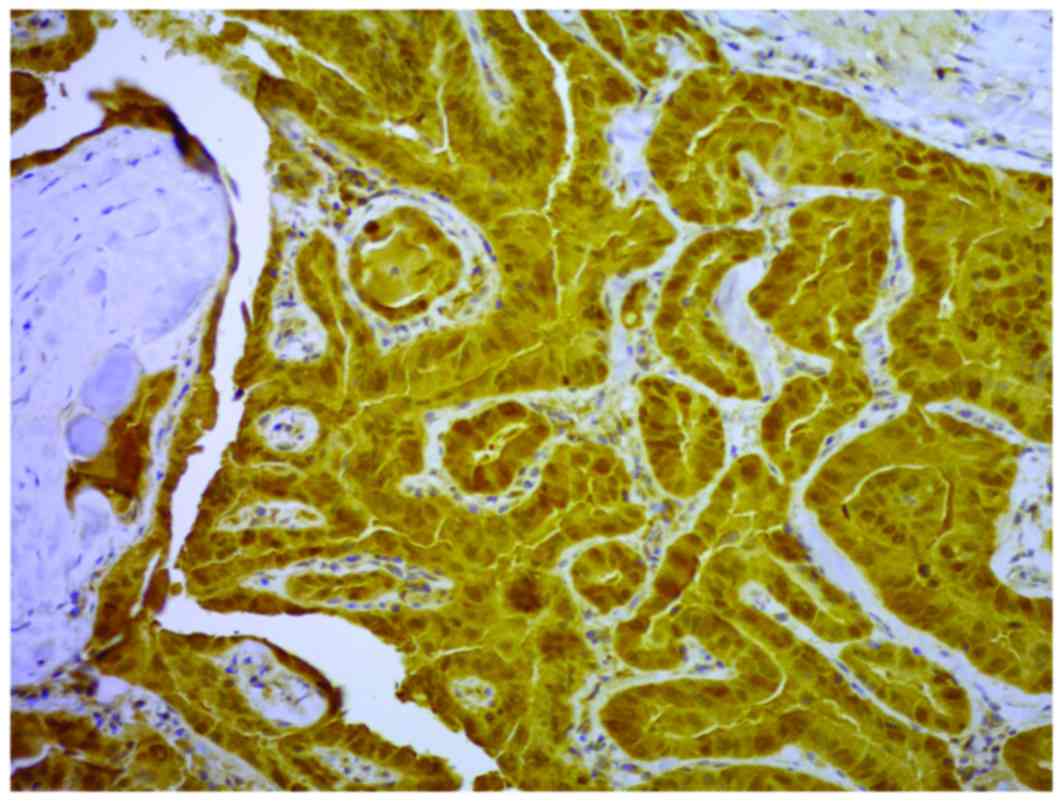
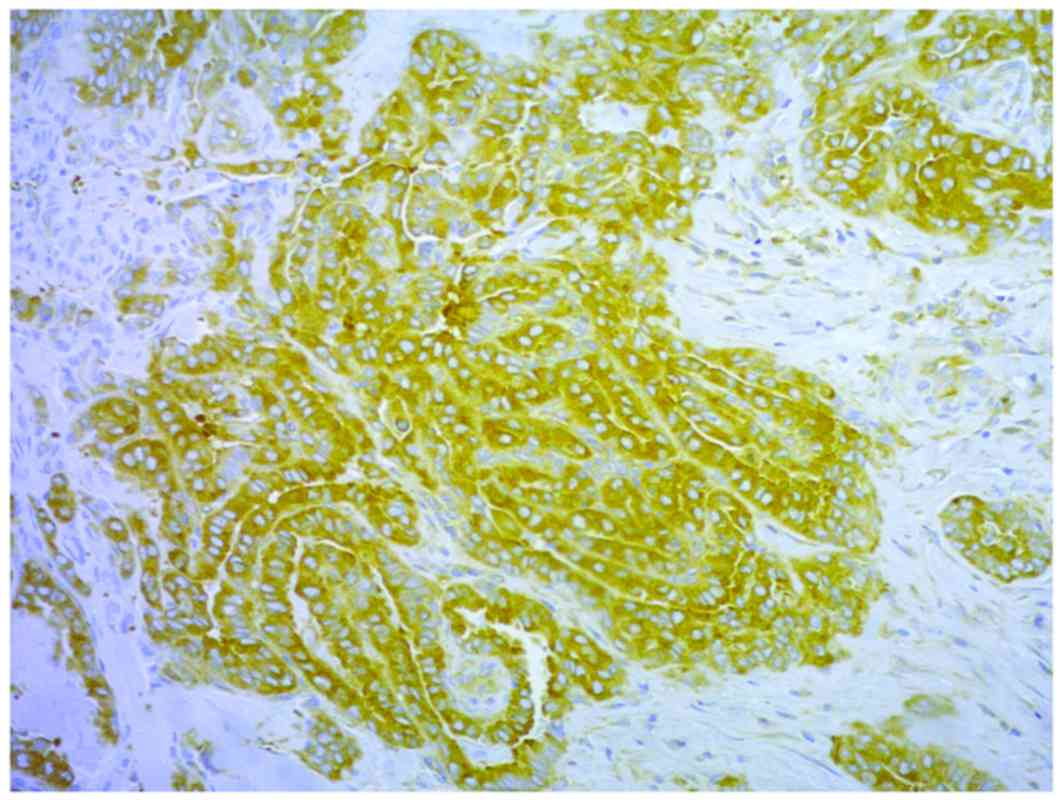
COX-2 (Figs. 1 and 2) in 118/130 and 88/130 of the samples,
respectively. COX-2 and nm23-H1 were coexpressed in all but two
tumours (P<0.001; Table II).
 | Table I.Distribution of patients with
papillary carcinoma. |
Table I.
Distribution of patients with
papillary carcinoma.
|
|
| nm23-H1 | COX-2 |
|---|
|
|
|
|
|
|---|
|
Characteristics | Total | Score 0, 1 | Score 2, 3 | Score 0, 1 | Score 2, 3 |
|---|
| Age, years |
|
≤45 | 49 | 4 | 45 | 12 | 37 |
|
>45 | 81 | 8 | 73 | 30 | 51 |
| Gender |
|
Male | 23 | 2 | 21 | 8 | 15 |
|
Female | 107 | 10 | 97 | 34 | 73 |
| Localization |
| Right
lobe | 74 | 7 | 67 | 21 | 53 |
| Left
lobe | 47 | 5 | 42 | 19 | 28 |
|
Isthmus | 9 | 0 | 9 | 2 | 7 |
| Tumour size,
cm |
| ≤1 | 55 | 5 | 50 | 19 | 36 |
|
>1 | 75 | 7 | 68 | 23 | 52 |
| Histological
type |
|
Classical | 67 | 6 | 61 | 21 | 46 |
|
Follicular | 32 | 1 | 31 | 6 | 26 |
|
Sclerosing | 13 | 3 | 10 | 8 | 5 |
|
Oxyphilic | 10 | 1 | 9 | 4 | 6 |
|
Other | 8 | 1 | 7 | 3 | 5 |
| Lymphocytic
infiltration |
|
Present | 39 | 5 | 34 | 13 | 26 |
|
Absent | 91 | 7 | 84 | 29 | 62 |
| Benign changes |
|
Present | 65 | 5 | 60 | 25 | 40 |
|
Absent | 65 | 7 | 58 | 17 | 48 |
| Intraglandular
spread |
|
Present | 37 | 4 | 33 | 11 | 26 |
|
Absent | 93 | 8 | 85 | 31 | 62 |
| Lymph node
metastasis |
|
Present | 27 | 1 | 26 | 7 | 20 |
|
Absent | 103 | 11 | 92 | 35 | 68 |
 | Table II.Coexpression of nm23-H1 and
COX-2. |
Table II.
Coexpression of nm23-H1 and
COX-2.
|
|
| nm23-H1 score |
|
|---|
|
|
|
|
|
|---|
|
|
| 1, 2 | 3, 4 | Total |
|---|
| COX-2 score |
| 1, 2 | n | 11 | 31 | 42 |
|
| % | 26.2 | 73.8 | 100 |
| 3, 4 | n | 1 | 87 | 88 |
|
| % | 1.1 | 98.9 | 100 |
| Total |
|
| n | 12 | 118 | 130 |
|
| % | 9.2 | 90.8 | 100 |
In the present study, the expression levels of
nm23-H1 and COX-2 were not associated with the patient's age,
gender, intraglandular dissemination of the tumour, tumour diameter
and the presence of metastatic regional lymph nodes. However,
statistically significant differences were identified between
histological subtypes. The nm23-H1 median expression levels were
significantly increased in the classical and follicular
histological subtype compared with other histological subtypes
(P=0.038). The COX-2 median expression levels were significantly
increased in patients with follicular histological subtype and
decreased in the diffuse-sclerosing subtype compared with patients
with follicular, papillary, oxyphilic and sclerosing papillary
cancer (P=0.006; Table III). The
subgroup ‘others’ was not included in this analysis as it
represents a number of distinct histological subtypes. The presence
of lymphocytic inflammatory infiltrate in the tumour was
significantly associated with thyroiditis in the surrounding
non-tumorous tissue (P<0.001; Table
IV), but this did not associate with the expression levels of
nm23-H1 and COX-2 in the tumour tissues (P=0.677 and P=0.649,
respectively).
 | Table III.nm23-H1, COX-2 and histological
subtype. |
Table III.
nm23-H1, COX-2 and histological
subtype.
|
|
|
|
|
| Median |
|
|---|
|
|
|
|
|
|
|
|
|---|
|
| Histological
subtype | n | Min. | Max. | 25th | 50th | 75th | P-value |
|---|
| nm23-H1 | Follicular | 32 | 1 | 3 | 2 | 3 | 3 | 0.038 |
|
| Classical | 67 | 0 | 3 | 2 | 3 | 3 |
|
|
| Oxyphilic | 10 | 1 | 3 | 2 | 2 | 3 |
|
|
| Sclerosing | 13 | 0 | 3 |
1.5 | 2 | 3 |
|
| COX-2 | Follicular | 32 | 0 | 3 | 2 | 3 | 3 | 0.006 |
|
| Classical | 67 | 0 | 3 | 1 | 2 | 2 |
|
|
| Oxyphilic | 10 | 1 | 3 | 1 | 2 | 3 |
|
|
| Sclerosing | 13 | 0 | 3 | 1 | 1 | 2 |
|
 | Table IV.Lymphocytic inflammatory infiltrate
in tumour and thyroiditis in surrounding thyroid tissue. |
Table IV.
Lymphocytic inflammatory infiltrate
in tumour and thyroiditis in surrounding thyroid tissue.
|
|
| Lymphocytic
infiltration |
|
|---|
|
|
|
|
|
|---|
|
|
| Absent | Present | Total |
|---|
| Thyroiditis |
|
Absent | n | 76 | 21 | 97 |
|
| % | 78.4 | 21.6 | 100 |
|
Present | n | 15 | 18 | 33 |
|
| % | 45.5 | 54.5 | 100 |
| Total | n | 91 | 39 | 130 |
|
| % | 70 | 30 | 100 |
No single tumour was negative for nm23-H1 and COX-2.
However, a total of nine tumours were negative for one of the
proteins (nm23-H1, n=2; COX-2, n=7). The characteristics of these
tumours are presented in Table V.
From the nine samples, it may be hypothesized that the regulation
of COX-2 expression is complex and that, in the present study, a
direct link between nm23-H1 and COX-2 was not able to be
elucidated. It is also of note that the tumours from patients #83,
#87 and #115 exhibited the highest score for nm23-H1 expression
levels, but were negative for COX-2 expression.
 | Table V.Characteristics of tumours negative
for COX-2 and nm23-H1 protein. |
Table V.
Characteristics of tumours negative
for COX-2 and nm23-H1 protein.
| # | Gender | Age, years | Lobe | Histological
subtype | Diameter, cm | Intraglandular
dissemination | Lymph node | Other changes | Lymphocyte | nm23.H1 IRS | COX.2 IRS |
|---|
| 12 | F | 31 | Left | Classical |
1.4 | No | + | n/a | − | 2 | 0 |
| 23 | F | 43 | Right | Sclerosing |
1.2 | No | − | cht. | + | 0 | 1 |
| 41 | F | 40 | Right | Classical |
0.9 | No | − | n/a | − | 1 | 0 |
| 44 | F | 78 | Right | Follicular | 2 | No | − | n/a | − | 1 | 0 |
| 50 | F | 64 | Right | Classical | 1 | Yes | − | n/a | − | 0 | 3 |
| 83 | F | 54 | Isthmus | Sclerosing |
0.5 | No | − | Goitre | − | 3 | 0 |
| 87 | F | 44 | Right | Classical | 1 | No | − | Goitre | − | 3 | 0 |
| 115 | F | 53 | Left |
Sclerosing-oxyphilic | 1 | Yes | − | n/a | − | 3 | 0 |
| 127 | F | 32 | Right | Classical | 1 | No | − | Hash. | + | 1 | 0 |
The current study divided the tumour samples into
two groups depending on the tumour size (n=55, papillary
microcarcinoma; n=75, papillary carcinoma) and no statistically
significant difference was found in the expression levels of
nm23-H1 and COX-2 between the groups (P=0.924 and P=0.432). The
group of tumours with a diameter of ≤1 cm (microcarcinoma) were
divided into two distinct groups, which was due to the presence or
absence of lymph node metastases (n=9 and n=46, respectively). The
results of the current study indicated that papillary
microcarcinoma with lymph node metastases exhibited increased
expression levels of nm23-H1 and COX-2 (Tables VI and VII), but a statistically significant
difference was only established for the levels of COX-2 expression
(P=0.017).
 | Table VI.Expression of nm23-H1 in papillary
microcarcinoma with or without lymph node metastasis. |
Table VI.
Expression of nm23-H1 in papillary
microcarcinoma with or without lymph node metastasis.
|
|
| nm23-H1 score |
|
|---|
|
|
|
|
|
|---|
|
|
| 0, 1 | 2, 3 | Total |
|---|
| Lymph node
metastasis |
| Absent | n | 5 | 41 | 46 |
|
| % | 10.9 | 89.1 | 100 |
| Present | n | 0 | 9 | 9 |
|
| % | 0 | 100 | 100 |
| Total | n | 5 | 50 | 55 |
|
| % | 9.1 | 90.9 | 100 |
 | Table VII.Expression of COX-2 in papillary
microcarcinoma with or without lymph node metastasis. |
Table VII.
Expression of COX-2 in papillary
microcarcinoma with or without lymph node metastasis.
|
|
| COX-2 score |
|
|---|
|
|
|
|
|
|---|
|
|
| 0, 1 | 2, 3 | Total |
|---|
| Lymph node
metastasis |
|
Absent | n | 19 | 27 | 46 |
|
| % | 41.3 | 58.7 | 100 |
|
Present | n | 0 | 9 | 9 |
|
| % | 0 | 100 | 100 |
| Total | n | 19 | 36 | 55 |
|
| % | 34.5 | 65.5 | 100 |
Discussion
COX-2 is an enzyme that is expressed at sites of
inflammation and in numerous types of carcinoma, and this may be
associated with their aggressive characteristics (22,23). COX-2
is also expressed in benign and malignant thyroid tumours,
particularly in papillary carcinoma; however, the underlying
mechanistic role and potential association with distinct tumour
characteristics have yet to be been elucidated (24–33).
Russell et al (39)
demonstrated that cells containing the oncogenic fusion protein
RET/protein phosphatase 2C homolog 3 (PTC3) have increased nuclear
NF-κB activity, which promotes the secretion of myeloid growth and
chemotactic factors. RET/PTC3 thyrocytes are able to promote the
production of inflammatory mediators, including COX-2 (40).
The nm23-H1 kinase is established as a tumour
suppressor gene (6) and has been
indicated to have a tumour-suppressing function in a number of
types of malignant tumour; however the results of previous studies
evaluating the role of nm23-H1 in thyroid cancer are contradictory
and remain unclear (7–17).
A direct association between nm23-H1 and COX-2 has
been identified in EBV-infected lymphoblastoid cells (34). It was identified that COX-2
transcription may be promoted by nm23-H1 via the NF-κB signalling
pathway (34). The proteins nm23-H1
and COX-2 have each been investigated separately in a number of
previous studies, which included <110 PTC specimens. To the best
of our knowledge, the present study is the first investigation of
the coexpression levels of nm23-H1 and COX-2 in PTC tumours.
Immunohistochemistry was used to analyse the levels of nm23-H1 and
COX-2 protein expression in 130 PTC samples. Increased expression
levels of nm23-H1 were identified in 90% of the tumour tissues
(118/130). The proportion of tumours with increased expression
levels of COX-2 was lower at 67.6% (88/130) and 87 of the tumour
samples also exhibited an increased level of expression of nm23-H1
(P<0.001). Although these results are not able to elucidate the
underlying mechanisms of this observation, these data are
concordant with previous results from EBV-infected cells (34).
There was no statistically significant difference
between the expression levels of nm23-H1 and COX-2 according to the
age or the gender of the patients. Siironen et al (27) used immunohistochemistry to analyse
COX-2 protein expression levels in 108 samples from patients with
PTC, of which 49 were >45 years. The results revealed an
increase in the expression levels of COX-2 in this group of
patients, compared with the group of patients that were <45
(P=0.002) (27). Due to the results
of this previous study, the current study used two groups: Patients
who were <45 and patients who were >45 years at the time of
surgery. However the present study did not identify a statistically
significant difference in the expression levels of nm23-H1 and
COX-2 between these two age groups. Erdem et al (40) analysed the expression levels of COX-2
in 79 PTC tissues using immunohistochemistry and demonstrated
increased COX-2 staining in the group of patients who were >55
years old (P=0.05). In this previous study, patients were divided
into three age groups as follows: <35 years; 35–55 years; >55
years (40). García-González et
al (41) analysed COX-2
expression levels in normal, hyperplastic and neoplastic follicular
cells of the thyroid gland from 174 patients using
immunohistochemistry. The results did not detect an association
between COX-2 expression levels and the patient age or the presence
of lymph node metastases in 39 patients with PTC (41). Notably, it was identified that there
was a significant increase in the percentage of cases of papillary
microcarcinoma that exhibited an upregulation of COX-2 compared
with all the other types of PTC (41). Kim et al (30) analysed the COX-2 immunohistochemical
expression levels in 223 thyroid cancer samples, and they compared
the expression levels of COX-2 from clinical data of 168 cases (57
follicular carcinoma, 94 PTC and 17 anaplastic carcinoma). The PTC
group included 14 cases of papillary microcarcinoma (30). There was no significant correlation
identified between patient age, gender, presence of distant
metastasis and the expression levels of COX-2 (30). A positive expression level of COX-2
was inversely associated with tumour size, with papillary
microcarcinoma exhibiting the highest expression levels of COX-2
among the types of thyroid cancer examined in this previous study
(P=0.048) (30). Of the 168 thyroid
cancer samples, the tumours with lymph node metastases exhibited
reduced COX-2 staining compared with tumours that did not
metastasise to the lymph node, but there was no statistically
significant difference identified (30).
To investigate the potential association between
nm23-H1/COX-2 expression levels and specific types of biological
characteristic (primarily the metastatic potential), the current
study divided the tumour samples into two groups depending on the
tumour size. There was no statistically significant difference in
the expression levels of nm23-H1 and COX-2 between the groups
(P=0.924 and P=0.432). The 55 papillary microcarcinoma samples were
divided into metastatic and non-metastatic (n=9 and n=46,
respectively). The current study revealed that the COX-2 expression
levels were significantly increased in microcarcinoma tumours that
occurred with metastases (P=0.017), compared with those that
occurred without metastases. This result is concordant with the
hypothesis of a potential role for COX-2 in the aggressive
characteristics of papillary microcarcinoma. A previous study
indicated that the increased expression levels of COX-2 and reduced
expression levels of KAI-1/cluster of differentiation 68 in primary
cultures, tumour cell lines and tumour samples of PTC are
associated with an increase in the invasiveness of tumours
(31).
In the present study, the median expression levels
of nm23-H1 were significantly increased in the classical and
follicular histological subtypes of papillary carcinoma compared
with all other histological subtypes (P=0.038). The median
expression levels of COX-2 were significantly increased in the
follicular histological subtype, but were significantly decreased
in the diffuse-sclerosing subtype of PTC tumour (P=0.006). The
presence of lung metastases at the time of diagnosis is typical of
the diffuse-sclerosing subtype of PTC and this may be associated
with the tumour possessing aggressive characteristics (1). In the present study, 13 of the cases
were of this histological subtype and there were no lung metastases
present in the patients at the time of diagnosis. Therefore, these
results are not able to elucidate the role of COX-2 expression and
the pathogenicity of this subtype of PTC.
The association between chronic inflammation and
carcinogenesis via activation of COX-2 has been established
previously (42). In the present
study, the presence of lymphocyte inflammatory infiltrate in the
tumour was associated with the presence of thyroiditis in the
tissues surrounding the tumour (P<0.001), but it was not
associated with the expression levels of nm23-H1 and COX-2 in the
tumour tissue.
The results presented in the current study
demonstrate that the presence of COX-2 may be an important
diagnostic marker of thyroid papillary microcarcinoma, as its
expression levels demonstrated an association with lymph node
metastasis. The results also indicated that coexpression of COX-2
and nm23-H1 may be associated, with this coexpression being
associated with a distinct histological subtype of PTC. Although
the underlying mechanisms of the role of COX-2 and nm23-H1 in
thyroid carcinogenesis have yet to be elucidated, the results
presented in the current study may inform further investigations
into the origin and progression of thyroid papillary carcinoma and
microcarcinoma.
In conclusion, the current study demonstrates that
coexpression of nm23-H1 and COX-2 is typical of papillary thyroid
carcinoma. However, the levels of their expression may not be
associated with commonly examined clinical parameters, including
age, gender and the metastatic potential of the tumour. However,
the results identified a statistically significant increase in the
median expression levels of nm23-H1 in the classical and follicular
subtypes of PTC tissues compared with other tumours subtypes. The
median expression levels of COX-2 were also significantly increased
in follicular subtypes and decreased in diffuse-sclerosing
subtypes. Although the latter result demonstrated statistical
significance, there were only 13 tumours of this histological
subtype and therefore this trend requires further study.
References
|
1
|
DeLellis RA, Lloyd RV, Heitz PU and Eng C:
Papillary carcinomaWorld health organization classifications of
tumours: Pathology and genetics of tumours of endocrine organs.
IARC Press; Lyon: pp. 57–66. 2004
|
|
2
|
Toniato A, Boshin I, Casara D, Mazzarotto
R, Rubello D and Pelizzo M: Papillary thyroid carcinoma: Factors
influencing recurrence and survival. Ann Surg Oncol. 15:1518–1522.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Spriano G, Ruscito P, Pellini R,
Appetecchia M and Roselli R: Pattern of regional metastases and
prognostic factors in differentiated thyroid carcinoma. Acta
Otorhinolaryngol Ital. 29:312–316. 2009.PubMed/NCBI
|
|
4
|
Ito Y and Miyauchi A: Prognostic factors
and therapeutic strategies for differentiated carcinoma of the
thyroid. Endocr J. 56:177–192. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jung TS, Kim TY, Kim KW, Oh YL, Park DJ,
Cho BY, Shong YK, Kim WB, Park YJ, Jung JH and Chung JH: Clinical
features and prognostic factors for survival in patients with
poorly differentiated thyroid carcinoma and comparison to the
patients with the aggressive variants of papillary thyroid
carcinoma. Endocr J. 54:265–274. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yoshida BA, Skoloff MM, Welch DR and
Rinker-Schaeffer CW: Metastasis-supressor genes: A review and
perspective on an emerging field. J Natl Cancer Inst. 92:1717–1730.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rosengard AM, Krutzsch HC, Shearn A, Biggs
JR, Barker E, Marguilies IM, King CR, Liotta LA and Steeg PS:
Reduced Nm23/Awd protein in tumour metastasis and aberrant
Drosphila development. Nature. 342:177–180. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lacombe ML, Milon L, Munier A, Mehus JG
and Lambeth DO: The human Nm23/nucleoside diphosphate kinases. J
Bioenerg Biomembr. 32:247–258. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ouatas T, Salerno M, Palmieri D and Steeg
PS: Basic and translational advances in cancer metastasis: Nm23. J
Bioenerg Biomembr. 35:73–79. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee JH, Marshall JC, Steeg PS and Horak
CE: Altered gene and protein expression by Nm23-H1 in metastasis
suppression. Mol Cell Biochem. 329:141–148. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zou M, Shi Y, Al-Sedairy S and Farid NR:
High levels of Nm23 gene expression in advanced stage of thyroid
carcinomas. Br J Cancer. 68:385–388. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ferenc T, Lewiński A, Lange D,
Niewiadomska H, Sygut J, Sporny S, Włoch J, Sałacińska-Los E, Kulig
A and Jarzab B: Analysis of Nm23-H1 protein immunoreactivity in
follicular thyroid tumors. Pol J Pathol. 55:149–153.
2004.PubMed/NCBI
|
|
13
|
Luo W, Matsuo K, Nagayama Y, Urano T,
Furukawa K, Takeshita A, Nakayama T, Yokoyama N, Yamashita S and
Izumi M: Immunohistochemical analysis of expression of
nm23-H1/nucleoside diphosphate kinase in human thyroid carcinomas:
Lack of correlation between its expression and lymph node
metastasis. Thyroid. 3:105–109. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Farley DR, Eberhardt NL, Grant CS, Schaid
DJ, van Heerden JA, Hay ID and Khosla S: Expression of a potential
metastasis suppressor gene (nm23) in thyroid neoplasms. World J
Surg. 17:615–621. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Royds JA, Silcocks PB, Rees RC and
Stephenson TJ: Nm23 protein expression in thyroid neoplasms.
Pathologica. 86:240–243. 1994.PubMed/NCBI
|
|
16
|
Tabriz HM, Adabi Kh, Lashkari A, Heshmat
R, Haghpanah V, Larijani B and Tavangar SM: Immunohistochemical
analysis of nm23 protein expression in thyroid papillary carcinoma
and follicular neoplasm. Pathol Res Pract. 205:83–87. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shahebrahimi K, Madani SH, Fazaeli AR,
Khazaei S, Kanani M and Keshavarz A: Diagnostic value of CD56 and
nm23 markers in papillary thyroid carcinoma. Indian J Pathol
Microbiol. 56:2–5. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Maud JF, Alfin-Slater RB, Howton DR and
Popjak G: Prostaglandins, tromboxanes and prostacyclinLipids:
Chemistry, Biochemistry, and Nutrition. Springer US; New York, NY:
pp. 149–216. 1986, View Article : Google Scholar
|
|
19
|
Crofford LJ: COX-1 and COX-2 tissue
expression: Implications and predictions. J Rheumatol Suppl.
49:15–19. 1997.PubMed/NCBI
|
|
20
|
NCBI Gene: PTGS1
prostaglandin-endoperoxide synthase 1 [Homo sapiens
(human)]. GENE ID: 5742. updated on 20-Feb-2017. https://www.ncbi.nlm.nih.gov/gene/5742
|
|
21
|
NCBI Gene: PTGS2
prostaglandin-endoperoxide synthase 2 [Homo sapiens
(human)]. GENE ID: 5743. updated on 26-Feb-2017. https://www.ncbi.nlm.nih.gov/gene/5743
|
|
22
|
Dubois RN, Abramson SB, Crofford L, Gupta
RA, Simon LS, van de Putte LB and Lipsky PE: Cyclooxygenase in
biology and disease. FASEB J. 12:1063–1073. 1998.PubMed/NCBI
|
|
23
|
Vane JR, Bakhle YS and Botting RM:
Cyclooxigenases 1 and 2. Annu Rev Pharmacol Toxicol. 38:97–120.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Specht MC, Tucker ON, Hocever M, Gonzalez
D, Teng L and Fahey TJ III: Cyclooxygenase-2 expression in thyroid
nodules. J Clin Endocronol Metab. 87:358–363. 2002. View Article : Google Scholar
|
|
25
|
Ji B, Liu Y, Zhang P, Wang Y and Wang G:
COX-2 expression and tumor angiogenesis in thyroid carcinoma
patients among northeast Chinese population-result of a
single-center study. Int J Med Sci. 9:237–242. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Krawczyk-Rusiecka K,
Wojciechowska-Durczynska K, Cyniak-Magierska A, Zygmunt A and
Lewinski A: Assessment of cyclooxygenase-1 and 2 gene expression
levels in chronic autoimmune thyroiditis, papillary thyroid
carcinoma and nontoxic nodular goitre. Thyroid Res. 7:102014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Siironen P, Ristimäki A, Nordling S,
Louhimo J, Haapiainen R and Haglund C: Expression of Cox-2 is
increased with age in papilary thyroid cancer. Histopathology.
44:490–497. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim SJ, Lee J, Yoon JS, Mok JO, Kim YJ,
Park HK, Kim CH, Byun DW, Suh KI and Yoo MH: Immunohistochemical
expression of COX-2 in thyroid nodules. Korean J Int Med.
18:225–229. 2003. View Article : Google Scholar
|
|
29
|
Ito Y, Yoshida H, Naakano K, Takamura Y,
Miya A, Kobayashi K, Yokozawa T, Matsuzuka F, Matsuura N, Kuma K
and Miyauchi A: Cyclooxygenase-2 expression in thyroid neoplasms.
Histopathology. 42:492–497. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim YA, Chang M, Park YJ and Kim JE:
Detection of survivin and COX-2 in thyroid carcinoma: Anaplastic
carcinoma shows overexpression of nuclear surviving and low COX-2
expression. Korean J Pathol. 46:55–60. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Scarpino S, Duranti E, Giglio S, Di Napoli
A, Galafate D, Del Bufalo D, Desideri M, Socciarelli F,
Stoppacciaro A and Ruco L: Papillary carcinoma of the thyroid: High
expression of COX-2 and low expression of KAI-1/CD82 are associated
with increased tumor invasiveness. Thyroid. 23:1127–1137. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cornetta AJ, Russell JP, Cunnane M, Keane
WM and Rothstein JL: Cyclooxygenase-2 expression in human thyroid
carcinoma and Hashimoto's thyroiditis. Laryngoscope. 11:238–242.
2002. View Article : Google Scholar
|
|
33
|
Nose F, Ichikawa T, Fujiwara M and Okayasu
I: Up-regulation of cyclooxygenase-2 expression in lymphocytic
thyroiditis and thyroid tumors: Significant correlation with
inducible nitric oxide synthase. Am J Clin Pathol. 117:546–551.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kaul R, Verma SC, Murakami M, Lan K,
Choudhuri T and Robertson ES: Epstein-Barr virus protein can
upregulate cyclo-oxygenase-2 expression through association with
the supressor of metastasis Nm23-H1. J Virol. 80:1321–1331. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Joo YE, Kim HS, Min SW, Lee WS, Park CH,
Park CS, Choi SK, Rew JS and Kim SJ: Expression of cyclooxigenase-2
protein in colorectal carcinomas. Int J Gastrointest Cancer.
31:147–154. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Soslow RA, Dannenberg AJ, Rush D, Woerner
BM, Khan KN, Masferrer J and Koki AT: COX-2 is expressed in human
pulmonary, colonic, and mammary tumors. Cancer. 89:2637–2645. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Barnes R, Masood S, Barker E, Rosengard
AM, Coggin DL, Crowell T, King CR, Porter-Jordan K, Wargotz ES and
Liotta LA: Low nm23 protein expression in infiltrating ductal
breast carcinomas correlates with reduced patient survival. Am J
Pathol. 139:245–250. 1991.PubMed/NCBI
|
|
38
|
Yamaguchi A, Ding K, Maehara M, Goi T and
Nakagawara G: Expression of nm23-H1 gene and Sialyl Lewis × antigen
in breast cancer. Oncology. 55:357–362. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Russell JP, Shinohara S, Melillo RM,
Castellone MD, Santoro M and Rothstein JL: Tyrosine kinase
oncoprotein, RET/PTC3, induces the secretion of myeloid growth and
chemotactic factors. Oncogene. 22:4569–4577. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Erdem H, Gündogdu C and Şipal S:
Correlation of E-cadherin, VEGF, COX-2 expression to prognostic
parameters in papillary thyroid carcinoma. Exp Mol Pathol.
90:312–317. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
García-González M, Abdulkader I, Boquete
AV, Neo XM, Forteza J and Cameselle-Teijeiro J: Cyclooxigenase-2 in
normal, hyperplastic and neoplastic follicular cells of the human
thyroid gland. Virchows Arch. 447:12–17. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Eschwège P, de Ledinghen V, Camilli T,
Kulkarni S, Dalbagni G, Droupy S, Jardin A, Benoît G and Weksler
BB: Arachidonic acid and prostaglandins, inflammation and oncology.
Presse Med. 30:508–510. 2001.(In French). PubMed/NCBI
|