Introduction
The extracellular matrix (ECM) is composed of highly
variable and dynamic components, which regulate cell behavior and
fate (1). In breast cancer, a number
of ECM proteins are significantly deregulated and specific matrix
components promote tumor progression and metastasis (2). Fibronectin (FN) is a component of the
mammary mesenchymal compartment. FN is not expressed in normal
adult breast tissue, whereas increased mRNA and protein levels have
been reported in the stroma of breast tumors (3). A high level of FN expression is
associated with an increased risk of mortality of breast cancer,
and it may be a useful marker for predicting poor prognosis in
breast cancer patients (4). In
addition, increased FN expression is associated with an invasive
and metastatic breast cancer phenotype (5). Changes in the production and
organization of FN contribute to the ‘pre-metastatic niche’, which
facilitates the adhesion of bone marrow-derived cells to promote
tumor progression and metastasis (6,7).
An important event in the initiation of cancer
metastasis is epithelial-mesenchymal transition (EMT), which is a
process during which epithelial cells lose apical-basal polarity
and gain a mesenchymal phenotype (8).
During this transition, epithelial carcinoma cells acquire
phenotypic changes and become highly motile and invasive, which
facilitates the migration of cells from the originating site of the
tumor to distal sites (9). Recent
research demonstrates that FN induces an EMT response in MCF-10A
human mammary epithelial cells, via cooperation with Src kinase and
extracellular signal-regulated kinase (ERK)/mitogen-activated
protein kinase signaling, which is initiated by the type-I
transforming growth factor-β (TGF-β) receptor (10). However, the detailed mechanisms that
enable an EMT response in breast cancer cells with aberrant FN
stimulation remain to be elucidated.
The calpain family is a group of calcium-dependent
cysteine proteases and is involved in a variety of biological
activities by limited proteolysis of numerous substrates. Two
members of the calpain family µ-calpain (calpain-1) and m-calpain
(calpain-2) are ubiquitously expressed (11). Altered activity and expression of
calpains has been implicated in a number of disease states,
including cancer (12).
Investigations have demonstrated that calpain has a role in breast
cancer progression, prognosis and treatment response, thus it is
considered as a potential anticancer target (13). Furthermore, calpain activity is
aberrantly higher in breast cancer tissues compared with normal
breast tissues, and its expression correlates with metastatic
phenotypic characteristics and increased invasive properties of
tumors (14–16). Calpain also plays an essential role in
regulating cell migration and invasion by promoting focal adhesion
and invadopodia/podosome disassembly through cleavage of its
substrates, including talin (17),
focal adhesion kinase (FAK) (18),
cortactin (19) and ezrin (20). In addition, calpain is one of the key
downstream molecules required for growth factor-induced motility in
breast cancer (21). Notably, calpain
is also implicated in the EMT process in cancer cells. It has been
previously reported that calpain 2 is upregulated during
TGF-β-induced EMT in A549 lung adenocarcinoma cells (22). In addition, FN may induce cell
migration and invasion in A549 cells via ERK1/2-calpain-2 signaling
(23), indicating a potential role
for calpain in FN induced cellular response.
In view of the previously reported notable effects
of calpain on cell migration and invasion in breast cancer, the
present study sought to identify whether the upregulation and
activation of calpain play a role in FN induced migration and
invasion in breast cancer cells. This will enable a further
understanding of the role of calpain in the process of FN-induced
EMT.
Materials and methods
Reagents and antibodies
FN (Sigma-Aldrich; Merck Millipore, Darmstadt,
Germany) was dissolved in sterile distilled H2O as a
stock solution at 1 mg/ml and stored at −20°C. Calpeptin
(N-benzyloxycarbonyl-L-leucylnorleucinal) and calpain inhibitor IV
(Calbiochem; Merck Millipore) were dissolved in dimethylsulfoxide
(DMSO; 0.1 M) and stored at −20°C as stock solutions. Calpain
inhibitor I (ALLN; Calbiochem; Merck Millipore) was dissolved in
DMSO (0.1 M) and stored at 4°C. Matrigel was purchased from BD
Biosciences (San Jose, CA, USA). Antibodies to calpain-1 (catalog
no. sc-13990; 1:500; polyclonal rabbit anti-human), calpain-2
(catalog no. sc-373966; 1:500; polyclonal rabbit anti-human) and
FAK (catalog no. sc-557; 1:500; polyclonal rabbit anti-human) were
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Antibodies to E-cadherin (catalog no. 3195; 1:1,000; polyclonal
rabbit anti-human), ZO-1 (catalog no. 8193; 1:1,000; polyclonal
rabbit anti-human), N-cadherin (catalog no. 13116; 1:1,000;
polyclonal rabbit anti-human) and vimentin (catalog no. 5741;
1:1,000; polyclonal rabbit anti-human,) were obtained from Cell
Signaling Technology, Inc. (Danvers, MA, USA). Antibody to β-actin
(catalog no. AP0060; 1:3,000; polyclonal rabbit anti-human) was
purchased from Bioworld Technology, Inc. (St. Louis Park, MN,
USA).
Cell lines and cell culture
The MCF-7 human breast cancer cell line was
purchased from the Cell Bank at the Shanghai Institute of Cell
Biology (Shanghai, China). The cells were cultured in Dulbecco's
modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) at 37°C in a humidified incubator (5%
CO2) and supplemented with 10% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin
(Beyotime Institute of Biotechnology, Haimen, China) and 100 µg/ml
streptomycin (Beyotime Institute of Biotechnology).
Cell treatment
Cells were replated on FN (20 µg/ml) for 48 h prior
to analysis. When inhibitors were used, cells were incubated in
medium at 37°C containing the inhibitors for 1 h prior to being
replated on FN. The inhibitors were used as follows: ALLN (10 µM),
calpeptin (50 µM) and calpain inhibitor IV (25 µM).
Wound healing assay
The wound healing assay is a conventional method
used to study directional cell migration in vitro. Cells
were seeded into 6-well plates and grown to 90% confluence. The
monolayers were scraped with a micropipette tip and rinsed with
phosphate-buffered saline three times to remove any floating cells.
Representative images were captured at 0 and 48 h after scraping
(IX53; Olympus Corporation, Tokyo, Japan) and analyzed (cellSens;
version 1.14.14116.2; Olympus Corporation). The level of cell
migration was quantified as a percentage compared with the cells at
0 h of each group. The data shown were obtained from three
independent experiments.
Invasion assay
Invasive ability of the cells was measured by using
a Transwell chamber (EMD Millipore, Billerica, MA, USA) containing
membranes with pores (8 µm), which were initially coated with
Matrigel (40 µg/100 µl/chamber) as previously described (24). Following treatment with FN for 48 h or
ALLN (10 µM), calpeptin (50 µM) and calpain inhibitor IV (25 µM)
for 1 h prior to treatment with FN, the cells were suspended in
serum-free medium (5×105 cells/ml) and seeded into the
upper compartment, while the DMEM containing 10% FBS was added in
the lower compartment as a chemo-attractant. Following incubation
at 37°C for 24 h, the non-invasive cells on the upper side of the
membrane were removed with a cotton swab. The invasive cells on the
lower surface were fixed with 100% methanol and stained with 0.5%
crystal violet at room temperature for 20 min (Beyotime Institute
of Biotechnology). The invasive cells were quantified by manual
counting under an inverted microscope (CX51; Olympus Corporation)
at ×100 magnification. For each experimental group, 5 randomly
selected fields were analyzed.
Western blot analysis
Cells were collected and lyzed in lysis buffer
(Thermo Scientific Inc.). The lysates were clarified by
centrifugation at 4°C for 15 min at 13,000 × g. The protein
concentration in the supernatants was measured using bicinchoninic
acid assay kit (Thermo Scientific Inc.) with a microplate reader
(ELX808IU; BioTek Instruments, Inc., Winooski, VT, USA). Total
protein (30 µg/lane) was separated by 10% SDS-PAGE and transferred
to a polyvinylidene difluoride membrane (EMD Millipore, Billerica,
MA, USA). The membrane was blocked with 1% bovine serum albumin and
subsequently incubated with the appropriate primary antibodies as
described in the reagents and antibodies section overnight at 4°C.
The membranes were washed three times with TBS containing Tween-20
buffer and incubated with the IRDyeTM800-conjugated secondary
antibody (catalog no. P/N 925-32211; 1:20,000; Rockland
Immunochemicals, Inc., Pottstown, PA, USA) at 37°C for 1 h,
followed by washing four times with phosphate-buffered saline.
Images of the membrane were captured with the Odyssey Infrared
Imaging System (LI-COR Inc., Lincoln, NE, USA). β-actin was used as
an endogenous control, and the relative expression of proteins was
normalized to the control group. Densities of signals on blots were
evaluated using ImageJ software (U.S. National Institutes of
Health, Bethesda, MD, USA).
Statistical analysis
All data obtained from at least three independent
experiments are expressed as the mean ± standard error.
Statistically significant differences were calculated by the
Student's t-test for comparing between two groups and
one-way analysis of variance for multiple-group comparisons,
followed by the Bonferroni post-hoc test. P<0.05 was considered
to indicate a statistically significant difference.
Results
FN stimulates cell migration and
invasion with changes in EMT marker expression in MCF-7 breast
cancer cells
Although FN has been previously demonstrated to
induce EMT in breast epithelial cells (10), whether it induces EMT in breast cancer
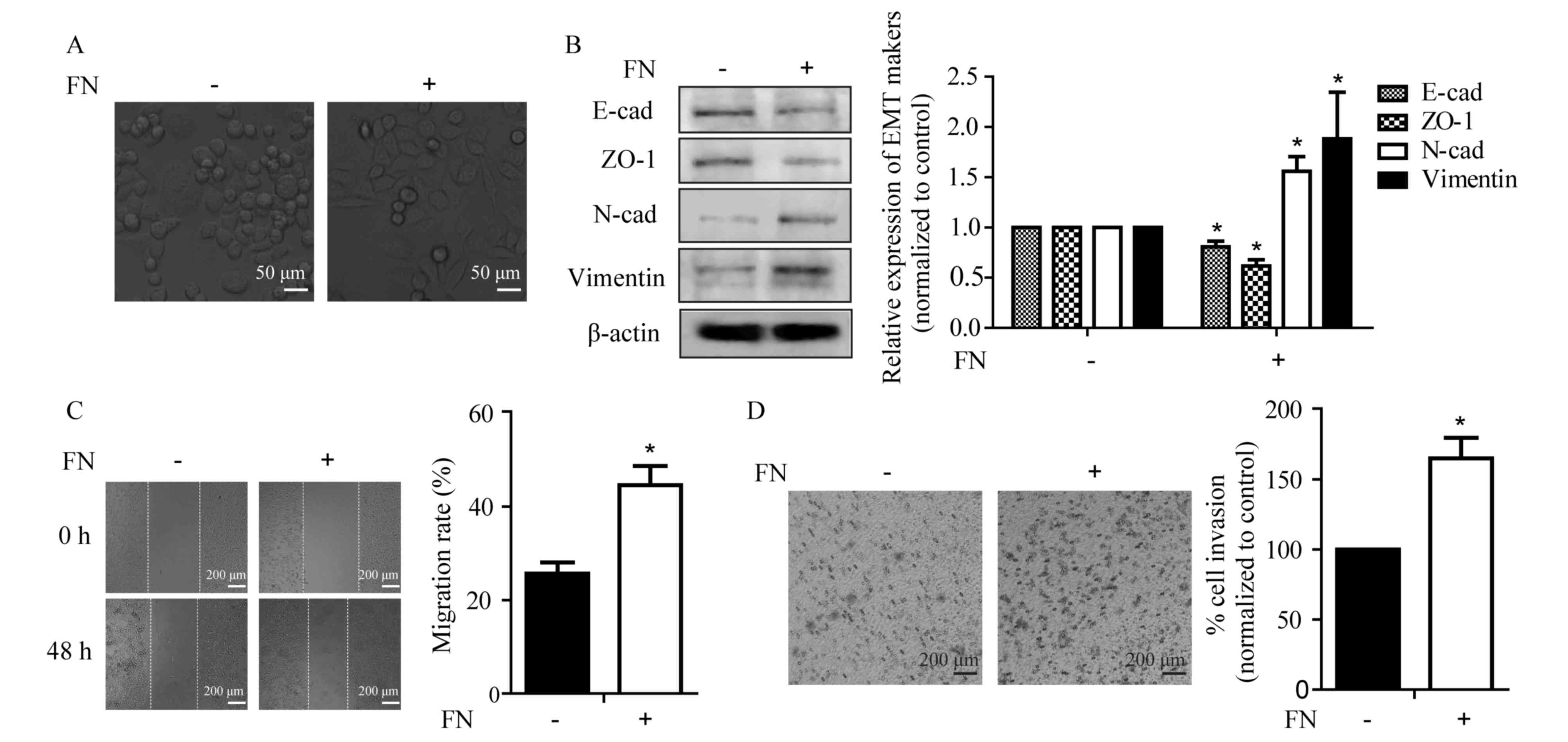
cells requires investigation. As shown in Fig. 1A, FN was capable of inducing an
EMT-like morphological change in MCF-7 cells, from a
cobblestone-like epithelial morphology to a spindle-like fibroblast
appearance. Corresponding with the changes in morphology, FN
treatment for 48 h led to alterations in the expression of
epithelial markers, including a significant decrease in E-cadherin
and ZO-1, as well as a significant increase in mesenchymal markers
N-cadherin and vimentin in MCF-7 cells (Fig. 1B). It has been well demonstrated that
EMT is associated with increased cellular motility (25). Thus, the motile phenotype of
FN-induced cells was evaluated by the wound healing and Transwell
invasion assays. FN treatment promoted wound closure from 25.6±2 to
44.6±4% and invasive ability of the MCF-7 cells from 100 to
164.9±15%. (Fig. 1C and D). These
results indicated that FN altered the expression of EMT markers and
facilitated cellular motility.
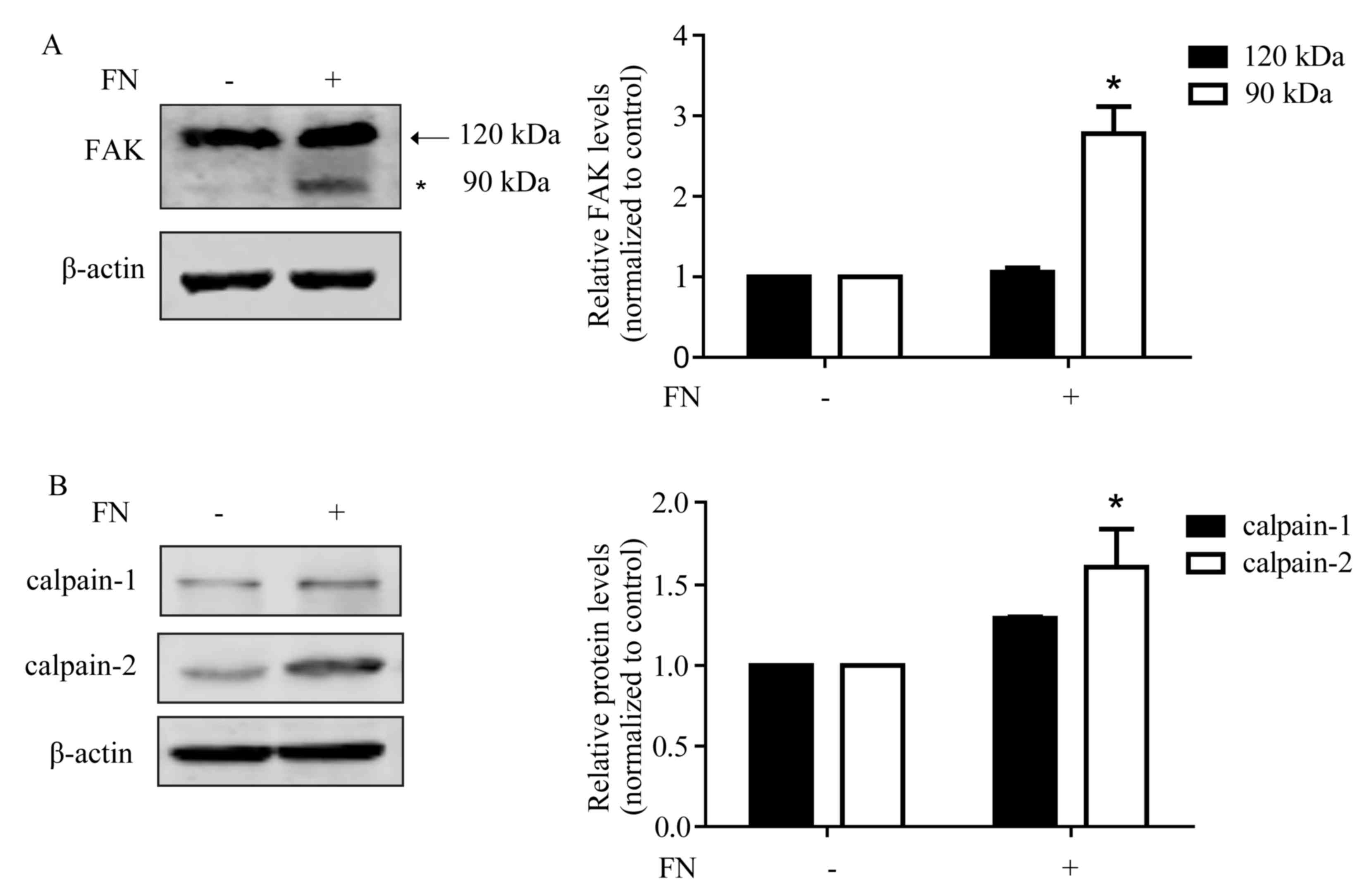
FN promotes FAK cleavage with an
increased expression of calpain-2
As a central component of focal adhesions, FAK can
interact with and phosphorylate several members of the focal
adhesion complex through multiple protein-binding domains (26). The cleavage of FAK leads to adhesion
complex turnover and increased cellular motility (27). As shown in Fig. 2A, following FN treatment for 48 h, FAK
was cleaved to the 90 kDa form. FAK is a sensitive substrate of
calpain and can be hydrolyzed to form the low molecular weight
isoforms via calpain activation. Furthermore, the generated ~90 kDa
cleavage fragment of FAK was identical in size to a previously
identified calpain-mediated cleavage product of FAK (28). Therefore, whether the expression of
calpain is changed following FN stimulation was assessed. It was
observed that the expression of calpain-1 increased following FN
stimulation, although this increase was not significant. The
expression of calpain-2 was significantly upregulated (Fig. 2B).
Inhibition of calpain reverses
FN-induced alteration of EMT markers
As shown in Figs. 1
and 2, upregulation of calpain-2 was
observed in the process of FN-induced EMT in MCF-7 cells. To
investigate whether activated calpain is a requisite or concomitant
in FN-induced EMT, three specific calpain inhibitors, ALLN,
calpeptin and calpain inhibitor IV were used. Compared with
non-treated cells, FN treatment led to downregulation of E-cadherin
and ZO-1, but upregulation of N-cadherin and vimentin. The changes
of E-cadherin, ZO-1 and vimentin expression induced by FN were
markedly suppressed by ALLN. Calpeptin or calpain inhibitor IV also
attenuated FN-induced upregulation of E-cadherin and ZO-1, and
downregulation of vimentin and N-cadherin (Fig. 3A). In addition, calpain inhibitors
calpeptin and calpain inhibitor IV also reversed the FN-induced
EMT-like morphological change in MCF-7 cells (Fig. 3B).
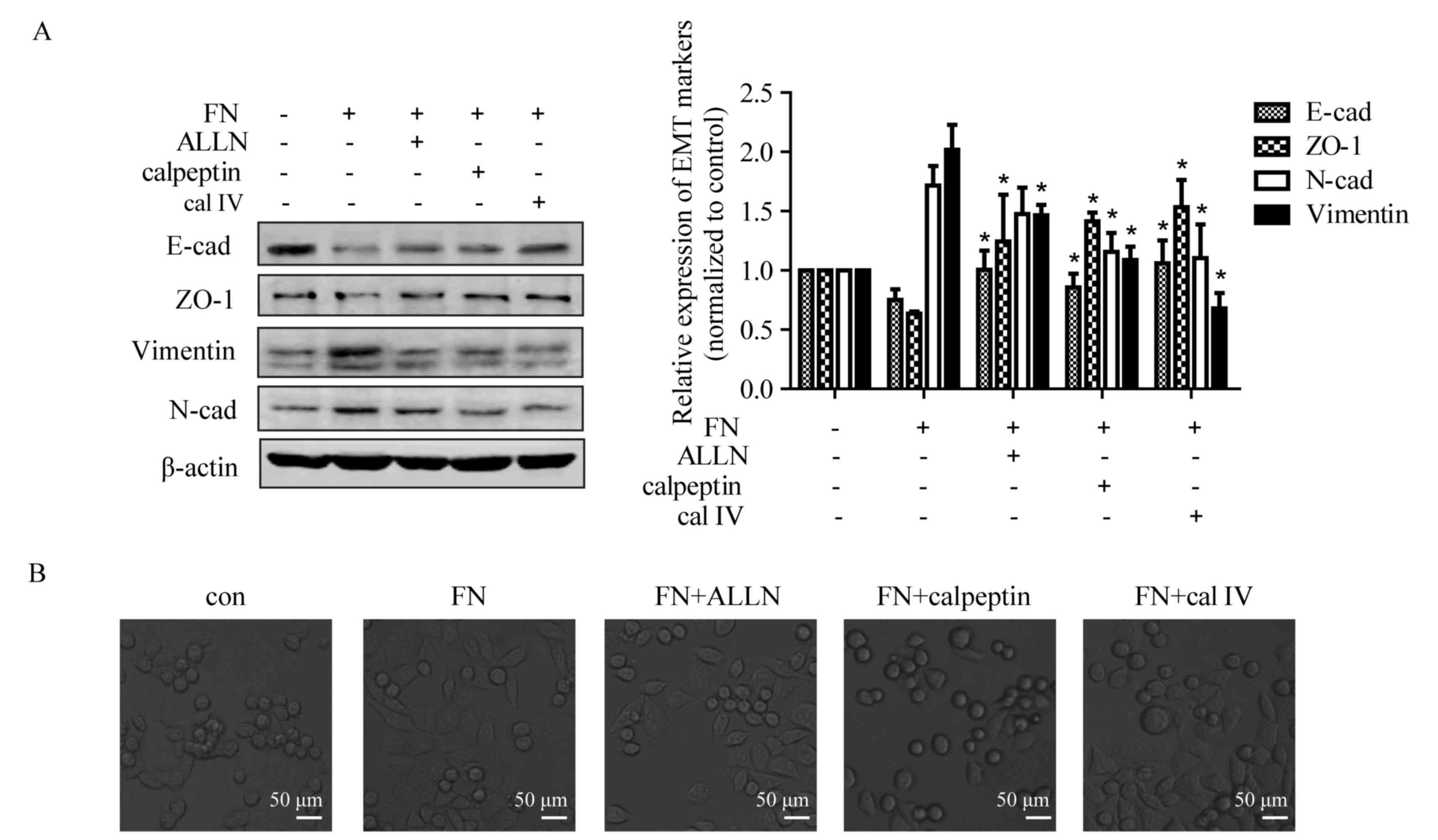 | Figure 3.Calpain mediates FN-induced EMT of
MCF-7 cells. Cells were pretreated with and without ALLN (10 µM),
calpeptin (50 µM) and calpain inhibitor IV (25 µM) for 1 h prior to
treatment with FN for 48 h. (A) Inhibition of calpain activation
blocked FN-induced change in EMT marker expression. The expression
of EMT markers was detected by western blotting, with β-actin as
loading control. Values are expressed the mean ± standard error.
*P<0.05, FN vs. FN+ALLN, FN+calpeptin and FN+calpain inhibitor
IV. (B) Repression of calpain activation inhibited FN-induced
morphological changes. Morphological observation of MCF-7 cells was
performed following FN treatment with or without calpain inhibitors
(magnification, ×400). ALLN, calpain inhibitor I; con, control;
EMT, epithelial-mesenchymal transition; FN, fibronectin; cal IV,
calpain IV; E-cad, E-cadherin; N-cad, N-cadherin; ZO-1, tight
junction protein ZO-1. |
Calpain is required for FN-induced
cell migration, invasion and proteolytic cleavage of FAK
The results of the present study demonstrated that
calpain inhibitors reversed FN-induced EMT, which prompted an
investigation into whether calpain inhibition affects FN-induced
cell motility. MCF-7 cells were pre-incubated with or without
calpain inhibitors for 1 h prior to being placed on FN. As shown in
Fig. 4A, ALLN, calpeptin and calpain
inhibitor IV caused a significant reduction in FN-induced
migration. Furthermore, treatment with ALLN, calpeptin and calpain
inhibitor IV inhibited FN-induced cell invasion (Fig. 4B). Whether FN-induced FAK cleavage was
mediated via calpain activation was investigated. The results
demonstrated that all three calpain inhibitors markedly blocked
FN-induced FAK proteolytic cleavage, with significant
downregulation of the 90 kDa form (Fig.
4C). These data indicated that calpain has an important role in
FN-induced cell motility.
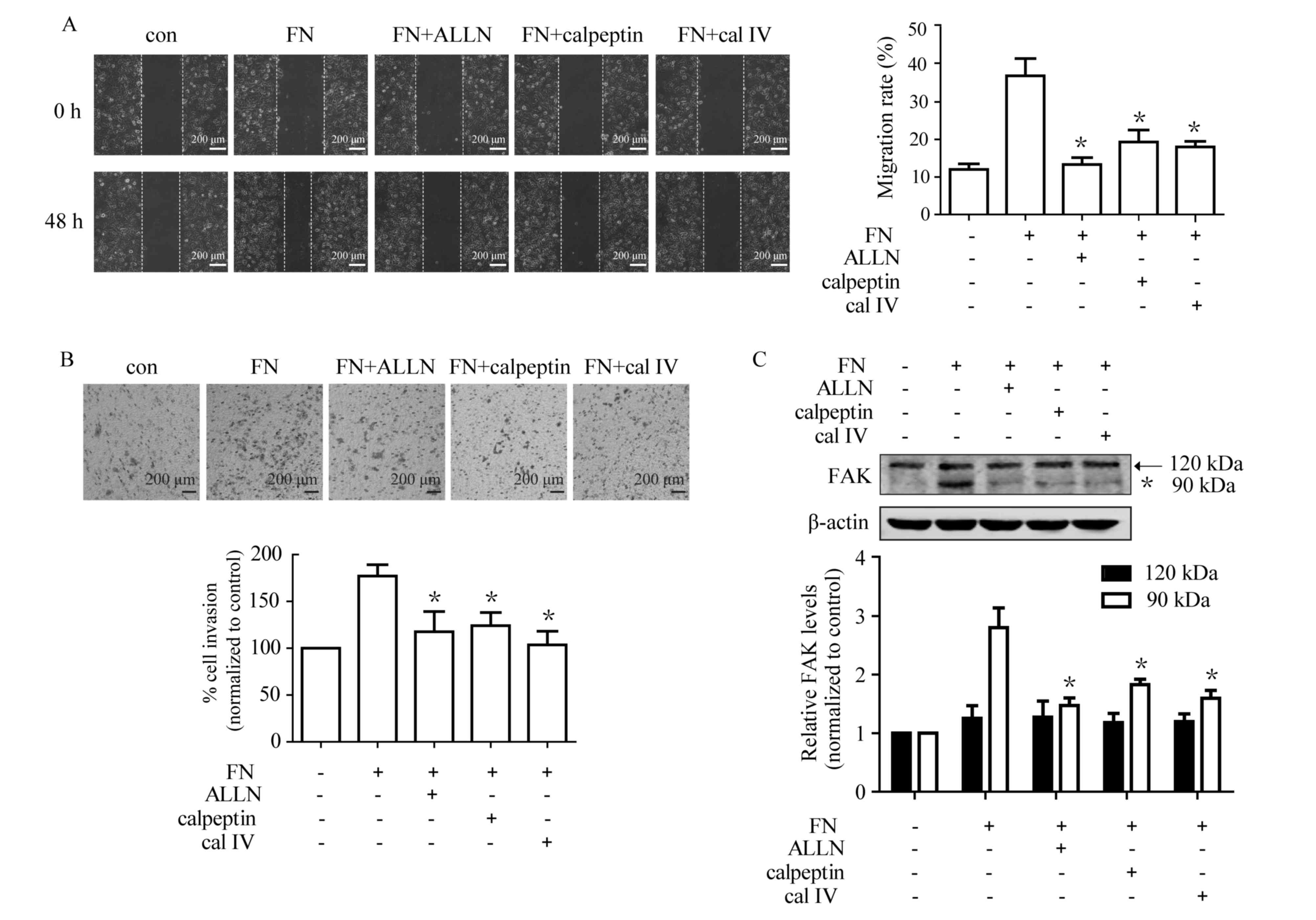 | Figure 4.Calpain is involved in cell
migration, invasion and FAK cleavage during FN-induced EMT in MCF-7
cells. Cells were pretreated with and without ALLN (10 µM),
calpeptin (50 µM) and calpain inhibitor IV (cal IV, 25 µM) for 1 h
prior to treatment with FN for 48 h. Cell migration and invasion,
as well as processing of FAK, were subsequently analyzed. (A) The
FN-induced cell migration was markedly suppressed by calpain
inhibitors. (magnification, ×100) (B) FN-induced cell invasion was
significantly inhibited by ALLN, calpeptin and calpain inhibitor
IV. (magnification, ×100) (C) Pretreatment with calpain inhibitors
suppressed FN-induced FAK processing. Values are expressed as the
mean ± standard error. *P<0.05, FN vs. FN+ALLN, FN+calpeptin and
FN+calpain inhibitor IV. ALLN, calpain inhibitor I; cal IV, calpain
IV; EMT, epithelial-mesenchymal transition; FAK, focal adhesion
kinase 1; FN, fibronectin; con, control. |
Discussion
According to Cancer Statistics 2014 (29), breast cancer is the most common type
of cancer diagnosed and the second leading cause of cancer
mortality among women. Metastasis is considered as one of the most
important stages of tumor progression and remains responsible for
~90% of patient mortalities, despite advances in the diagnosis and
treatment of breast cancer (30). The
process of tumor invasion and metastasis requires complex changes
in cell-cell and cell-matrix interactions, thus apart from the
accumulation of genetic and epigenetic changes in tumor cells, ECM
components in the tumor microenvironment also have roles in tumor
spreading, progression and therapeutic response (31). In recent years, a number of matrix
components have been identified as important constituents of
metastatic niches in breast cancer. Thus, performing an analysis of
these ECM proteins and the associated signaling pathways is of
enormous interest in the effort to identify therapeutic targets
against advanced stages of breast cancer (1).
High levels of FN in the primary tumor have been
linked to poor overall survival in breast cancer patients. In
addition, increased expression of FN was also observed in lymph
node metastases and was associated with an increased probability of
metastasis (3,32). Balanis et al (33) demonstrated that breast cancer cells at
an early stage utilize Src-dependent epidermal growth factor (EGF)
receptor signaling to promote the activation of signal transducer
and activator of transcription 3 (STAT3). However, in metastatic
breast cancer cells there is a switch to utilize FN-induced
FAK/FAK2:JAK2:STAT3 signaling after cancer cells have acquired EMT,
which indicates that a loss of responsiveness to growth factor is
associated with an increase in the ability of FN to stimulate an
alternative oncogenic pathway during metastatic progression. In
addition, FN is able to increase the migratory ability and
secretion of active matrix metalloproteinase-2 (MMP-2) in
non-invasive MCF-7 breast cancer cells, and induce MMP-2 expression
by decreasing its promoter methylation (34). In the present study, the ability of FN
to promote cell migration and invasion as well as to induce the EMT
progress in MCF-7 cells was also demonstrated. These findings
indicate the important functional role of FN in breast cancer
development, and its signaling pathway may be a potential target
for novel therapeutic agents. This is consistent with a recent
study that observed immunization against the alternatively spliced
extra domain-A (ED-A) of FN by anti-ED-A antibody vaccination
attenuates the progression of metastatic breast cancer (35).
Previously, studies have demonstrated that
proteolytic activity of the calpain family regulates numerous
intracellular proteins and is implicated in a variety of cellular
processes, including cytoskeletal remodeling, cell adhesion,
migration, proliferation and apoptosis (36). Additionally, calpain has been
associated with metastatic potential of breast cancer both in
vitro and in vivo (15,16). The
expression and activity of calpain is subject to complex
regulation. Epidermal growth factor, v-Src, the Ras signaling
pathway, ERK 1/2 and estrogen can all stimulate the activity and
expression of calpain (37). The
present study demonstrated that calpain-2, which is involved in
breast cancer cell migration and invasion (19,38), was
significantly upregulated following treatment with FN. It was
further demonstrated that calpain inhibitors inhibited FN-induced
migration and invasion of MCF-7 cells. Notably, treatment with FN
caused no apparent changes in calpain-1 expression, indicating that
FN may promote cell motility via calpain-2 activation. However, the
underlying molecular mechanisms remain to be elucidated. FAK, a
central component of focal adhesions, regulates cell-substrate
attachment and cell motility. More notably, it is a specific
substrate of calpain, which can be truncated into low molecular
weight forms via calpain (39). The
30 kDa C-terminal fragment of FAK contains the focal adhesion
targeting sequence, which is required for association with other
focal adhesion proteins, including breast cancer anti-estrogen
resistance protein 1, paxillin and talin (40). The 90 kDa amino-terminal fragment is
essential for kinase function and integrin-binding. The cleavage of
FAK reduces its activity at cell adhesions, which leads to
disassembling of focal adhesion complexes, loss of cell adhesion
and increased cell motility (41).
Previous studies have demonstrated that FAK is able to transduce
FN-induced survival signals (42).
Additionally, attachment of serum-starved MCF-10A cells to FN
stimulates the activation of FAK-Src signaling (43). In the present study, in response to FN
stimulation, the cleavage of FAK from 120 kDa to 90 kDa was
increased. However the generation of a 90 kDa FAK was significantly
repressed by calpain inhibitors, which implied that modulation of
FAK function through calpain-dependent cleavage is likely to play a
significant role in the process of FN-induced motility.
EMT is a physiological phenomenon during embryonic
development and tissue remodeling. Notably, EMT is essential for
the development of cancer metastasis (44). The impaired expression of epithelial
markers (E-cadherin and ZO-1), leads to the dissolution of cell
adherence and tight junctions and an increase in the expression of
mesenchymal markers (N-cadherin and vimentin), which are usually
correlated with increased tumor migration and invasion (45). A wide range of factors from the
micro-environment regulate this process, including TGF-β, tumor
necrosis factor α, and EGF. The results of the present study
indicated that FN also induces an EMT response in MCF-7 breast
cancer cells with a change in cell phenotype, including an
increased expression of N-cadherin and vimentin as well as a
decreased expression of E-cadherin and ZO-1 (Fig. 1). Notably, inhibition of calpain by
calpain inhibitors suppressed FN-induced EMT in MCF-7 cells
(Fig. 3), which suggests calpain is a
requisite for FN-induced EMT response. However, the detailed
mechanisms of how FN induces calpain remain to be elucidated.
In conclusion, the results of the present study
demonstrated that FN enhances cell migration, invasion and the EMT
process, which may facilitate metastatic progression of breast
cancer. In addition, it has also been demonstrated that calpain has
an essential role in the FN-induced EMT response. Targeting calpain
may be a potential strategy to reduce breast cancer metastasis, and
it would also be of great value to evaluate the effects of
pharmacological calpain inhibitors on the treatment of breast
cancer.
Acknowledgements
The present study was supported by the following
funding bodies and programs: The Chinese National Natural Science
Foundation (grant nos. 81560598, 81402969 and 81302804), the
Jiangsu Natural Science Foundation (grant no. BK20130220), the
Chinese Postdoctoral Science Foundation (grant nos. 2013M541733 and
2015M582749XB), the Jiangsu Planned Projects for Postdoctoral
Research Funds (grant no. 1301015B), the Science Foundation of
Guiyang Science and Technology Bureau [grant no. (20141001) 06],
the Science and Technology Innovation Advanced Individual of
Guizhou Department of Education [grant no. QJHKY (2015) 492], the
Guizhou Natural Science Foundation [grant no. QKHJ (2014) 2007],
the Foundation for Training Programs of Innovation and
Entrepreneurship for Undergraduates at the Guiyang Medical
University (grant nos. 201610660042 and 201410660038), the Guizhou
Innovated Team of the Education Department (grant no. 2014-31), the
Guizhou Innovation team [grant no. (2015) 4025], the Program for
New Century Excellent Talents in University (grant no.
NCET-13-0747), the High level Innovation Talents (grant no.
2015-4029), the Fund of Innovation Team of Guizhou Province (grant
no. 2015-4025), the Fund of Innovated Team of the Education
Department of Guizhou Province (grant no. 2014-31) and the 2011
Modern Drug of Cooperation Innovation [grant no. (2013) 04].
References
|
1
|
Oskarsson T: Extracellular matrix
components in breast cancer progression and metastasis. Breast.
22:(Suppl 2). S66–S72. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lu P, Weaver VM and Werb Z: The
extracellular matrix: A dynamic niche in cancer progression. J Cell
Biol. 196:395–406. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ioachim E, Charchanti A, Briasoulis E,
Karavasilis V, Tsanou H, Arvanitis DL, Agnantis NJ and Pavlidis N:
Immunohistochemical expression of extracellular matrix components
tenascin, fibronectin, collagen type IV and laminin in breast
cancer: Their prognostic value and role in tumour invasion and
progression. Eur J Cancer. 38:2362–2370. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bae YK, Kim A, Kim MK, Choi JE, Kang SH
and Lee SJ: Fibronectin expression in carcinoma cells correlates
with tumor aggressiveness and poor clinical outcome in patients
with invasive breast cancer. Hum Pathol. 44:2028–2037. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou Z, Qutaish M, Han Z, Schur RM, Liu Y,
Wilson DL and Lu ZR: MRI detection of breast cancer micrometastases
with a fibronectin-targeting contrast agent. Nat Commun.
6:79842015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sceneay J, Smyth MJ and Möller A: The
pre-metastatic niche: Finding common ground. Cancer Metastasis Rev.
32:449–464. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Barkan D, Green JE and Chambers AF:
Extracellular matrix: A gatekeeper in the transition from dormancy
to metastatic growth. Eur J Cancer. 46:1181–1188. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kalluri R: EMT: When epithelial cells
decide to become mesenchymal-like cells. J Clin Invest.
119:1417–1419. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park J and Schwarzbauer JE: Mammary
epithelial cell interactions with fibronectin stimulate
epithelial-mesenchymal transition. Oncogene. 33:1649–1657. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hood JL, Brooks WH and Roszman TL:
Subcellular mobility of the calpain/calpastatin network: An
organelle transient. Bioessays. 28:850–859. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Storr SJ, Carragher NO, Frame MC, Parr T
and Martin SG: The calpain system and cancer. Nat Rev Cancer.
11:364–374. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Storr SJ, Thompson N, Pu X, Zhang Y and
Martin SG: Calpain in breast cancer: Role in disease progression
and treatment response. Pathobiology. 82:133–141. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shiba E, Kambayashi JI, Sakon M, Kawasaki
T, Kobayashi T, Koyama H, Yayoi E, Takatsuka Y and Takai SI:
Ca²+;-dependent neutral protease (Calpain) activity in
breast cancer tissue and estrogen receptor status. Breast Cancer.
3:13–17. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Libertini SJ, Robinson BS, Dhillon NK,
Glick D, George M, Dandekar S, Gregg JP, Sawai E and Mudryj M:
Cyclin E both regulates and is regulated by calpain 2, a protease
associated with metastatic breast cancer phenotype. Cancer Res.
65:10700–10708. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Storr SJ, Lee KW, Woolston CM, Safuan S,
Green AR, Macmillan RD, Benhasouna A, Parr T, Ellis IO and Martin
SG: Calpain system protein expression in basal-like and
triple-negative invasive breast cancer. Ann Oncol. 23:2289–2296.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Franco SJ, Rodgers MA, Perrin BJ, Han J,
Bennin DA, Critchley DR and Huttenlocher A: Calpain-mediated
proteolysis of talin regulates adhesion dynamics. Nat Cell Biol.
6:977–983. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chan KT, Bennin DA and Huttenlocher A:
Regulation of adhesion dynamics by calpain-mediated proteolysis of
focal adhesion kinase (FAK). J Biol Chem. 285:11418–11426. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cortesio CL, Chan KT, Perrin BJ, Burton
NO, Zhang S, Zhang ZY and Huttenlocher A: Calpain 2 and PTP1B
function in a novel pathway with Src to regulate invadopodia
dynamics and breast cancer cell invasion. J Cell Biol. 180:957–971.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hoskin V, Szeto A, Ghaffari A, Greer PA,
Côté GP and Elliott BE: Ezrin regulates focal adhesion and
invadopodia dynamics by altering calpain activity to promote breast
cancer cell invasion. Mol Biol Cell. 26:3464–3479. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wells A, Kassis J, Solava J, Turner T and
Lauffenburger DA: Growth factor-induced cell motility in tumor
invasion. Acta Oncol. 41:124–130. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Keshamouni VG, Jagtap P, Michailidis G,
Strahler JR, Kuick R, Reka AK, Papoulias P, Krishnapuram R,
Srirangam A, Standiford TJ, et al: Temporal quantitative proteomics
by iTRAQ 2D-LC-MS/MS and corresponding mRNA expression analysis
identify post-transcriptional modulation of actin-cytoskeleton
regulators during TGF-beta-induced epithelial-mesenchymal
transition. J Proteome Res. 8:35–47. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Meng XN, Jin Y, Yu Y, Bai J, Liu GY, Zhu
J, Zhao YZ, Wang Z, Chen F, Lee KY and Fu SB: Characterisation of
fibronectin-mediated FAK signalling pathways in lung cancer cell
migration and invasion. Br J Cancer. 101:327–334. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li C, Zhao Y, Yang D, Yu Y, Guo H, Zhao Z,
Zhang B and Yin X: Inhibitory effects of kaempferol on the invasion
of human breast carcinoma cells by downregulating the expression
and activity of matrix metalloproteinase-9. Biochem Cell Biol.
93:16–27. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Drasin DJ, Robin TP and Ford HL: Breast
cancer epithelial-to-mesenchymal transition: Examining the
functional consequences of plasticity. Breast Cancer Res.
13:2262011. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Franco SJ and Huttenlocher A: Regulating
cell migration: Calpains make the cut. J Cell Sci. 118:3829–3838.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Frame MC, Fincham VJ, Carragher NO and
Wyke JA: v-Src's hold over actin and cell adhesions. Nat Rev Mol
Cell Biol. 3:233–245. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Carragher NO, Levkau B, Ross R and Raines
EW: Degraded collagen fragments promote rapid disassembly of smooth
muscle focal adhesions that correlates with cleavage of pp125(FAK),
paxillin and talin. J Cell Biol. 147:619–630. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chaffer CL and Weinberg RA: A perspective
on cancer cell metastasis. Science. 331:1559–1564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Joyce JA: Therapeutic targeting of the
tumor microenvironment. Cancer Cell. 7:513–520. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fernandez-Garcia B, Eiró N, Marín L,
González-Reyes S, González LO, Lamelas ML and Vizoso FJ: Expression
and prognostic significance of fibronectin and matrix
metalloproteases in breast cancer metastasis. Histopathology.
64:512–522. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Balanis N, Wendt MK, Schiemann BJ, Wang Z,
Schiemann WP and Carlin CR: Epithelial to mesenchymal transition
promotes breast cancer progression via a fibronectin-dependent
STAT3 signaling pathway. J Biol Chem. 288:17954–17967. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pereira IT, Ramos EA, Costa ET, Camargo
AA, Manica GC, Klassen LM, Chequin A, Braun-Prado K, Fde Pedrosa O,
Souza EM, et al: Fibronectin affects transient MMP2 gene expression
through DNA demethylation changes in non-invasive breast cancer
cell lines. PLoS One. 9:e1058062014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Femel J, Huijbers EJ, Saupe F, Cedervall
J, Zhang L, Roswall P, Larsson E, Olofsson H, Pietras K, Dimberg A,
et al: Therapeutic vaccination against fibronectin ED-A attenuates
progression of metastatic breast cancer. Oncotarget. 5:12418–12427.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gora J and Latajka R: Involvement of
cysteine proteases in cancer. Curr Med Chem. 22:944–957. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Leloup L and Wells A: Calpains as
potential anti-cancer targets. Expert Opin Ther Targets.
15:309–323. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ho WC, Pikor L, Gao Y, Elliott BE and
Greer PA: Calpain 2 regulates Akt-FoxO-p27(Kip1) protein signaling
pathway in mammary carcinoma. J Biol Chem. 287:15458–15465. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Goll DE, Thompson VF, Li H, Wei W and Cong
J: The calpain system. Physiol Rev. 83:731–801. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Legate KR, Wickström SA and Fässler R:
Genetic and cell biological analysis of integrin outside-in
signaling. Genes Dev. 23:397–418. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Carragher NO, Fincham VJ, Riley D and
Frame MC: Cleavage of focal adhesion kinase by different proteases
during SRC-regulated transformation and apoptosis. Distinct roles
for calpain and caspases. J Biol Chem. 276:4270–4275. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ilic D, Almeida EA, Schlaepfer DD, Dazin
P, Aizawa S and Damsky CH: Extracellular matrix survival signals
transduced by focal adhesion kinase suppress p53-mediated
apoptosis. J Cell Biol. 143:547–560. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kim NG and Gumbiner BM: Adhesion to
fibronectin regulates Hippo signaling via the FAK-Src-PI3K pathway.
J Cell Biol. 210:503–515. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Polyak K and Weinberg RA: Transitions
between epithelial and mesenchymal states: Acquisition of malignant
and stem cell traits. Nat Rev Cancer. 9:265–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bedi U, Mishra VK, Wasilewski D, Scheel C
and Johnsen SA: Epigenetic plasticity: A central regulator of
epithelial-to-mesenchymal transition in cancer. Oncotarget.
5:2016–2029. 2014. View Article : Google Scholar : PubMed/NCBI
|