Introduction
Bladder cancer is one of the most common malignant
tumors of the urinary system worldwide, affecting 16.6 per 100,000
males and 3.6 per 100,000 females (1). Although surgery is the usual and
effective way to treat bladder cancer, the 5-year recurrence rate
is ~50% following surgical treatment (2,3). At
present, intravesical chemotherapy is the most frequently used
method to prevent the recurrence of bladder cancer, subsequent to
surgery (4). However, chemotherapy is
often associated with multidrug resistance and toxic side effects,
with little specificity to cancer cells, which elucidates the
challenges of applying chemotherapy to bladder cancers. Natural
compounds have previously emerged as a potential treatment for
cancer therapy; therefore, natural compounds may be a promising
target for bladder cancer therapy.
Matrine, the active compound that may be extracted
from the Chinese herb Sophora flavecens, has previously been
used to treat chronic hepatitis B, allergic dermatitis and
hypoleukocytosis, in China (5,6). Matrine
may transform into oxymatrine, which may also be extracted from
Sophora flavecens, and the nitrogen-oxygen bond may split
under certain conditions transforming oxymatrine back to matrine
(7). Several studies have
demonstrated the multiple beneficial effects of matrine, including
anti-arrhythmia (8),
anti-inflammation (9,10) and anti-fibrosis (11,12)
effects, with minimal side effects reported. In addition, previous
studies have shown that matrine induces apoptosis in several cancer
cell lines, including breast cancer MCF-7 cells (13) and pancreatic cancer PANC1 cells
(14), using various mechanisms,
which potentiate the role of matrine in cancer therapy. However, it
remains unclear whether matrine is able to inhibit the growth of
bladder cancer cells. Therefore, the present study was designed to
investigate whether matrine exerts an antitumor effect on the
bladder cancer T24 cell line. The results revealed that matrine
exhibits an anti-proliferative effect on the T24 cells, by inducing
apoptosis and cell cycle arrest.
Materials and methods
Cell culture
The T24 cell line was obtained from the Department
of Laboratory Diagnosis, Chongqing Medical University (Chongqing,
China). The cells were incubated in Roswell Park Memorial Institute
(RPMI)-1640 medium containing 10% fetal bovine serum (Sijiqing,
Hangzhou, China), at 37°C in a humidified atmosphere with 5%
CO2.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) assay. T24 cells in the exponential growth phase
(24–96 h after plating) were seeded into 96-well plates at
~4×104 cells/well, and then treated with oxymatrine
(0.625, 1.25, 2.5, 5.0 and 10 mg/ml; Ningxia Qiyuan Pharmaceutical
Co., Ltd., Yinchuan, Ningxia, China) for 24, 48 and 72 h.
Subsequent to incubation for various times, 20 µl MTT reagent (5
mg/ml; Sigma-Aldrich, St. Louis, MO, USA) was added to each well
and incubated for an additional 4 h. Dimethyl sulfoxide was used to
dissolve the MTT formazan. Mitomycin C (MMC) was used as a positive
control. The absorbance values at 600 nm were obtained using a
spectrometer (Infinite® 200; Tecan Group Ltd.,
Männedorf, Switzerland).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The total RNA of the treated cells was isolated
using the Invitrogen TRIzol reagent (Thermo Fisher Scientific,
Waltham, MA, USA), according to the manufacturer's protocols. The
ReverTra Ace® qPCR RT kit (Toyobo Co., Ltd., Shanghai,
China) was used to synthesize cDNA from the total RNA, producing a
0.5 µg sample per assay. A SYBR® Premix Ex Taq II kit
(Takara Biotechnology (Dalian) Co., Ltd., Dalian, China) was used
to perform the qPCR on the CFX96 Touch™ Real-Time PCR Detection
System (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The primers
were synthesized by Sangon Biotech Co., Ltd. (Shanghai, China) and
the sequences were as follows: i) Survivin forward,
5′-GCCCAGTGTTTCTTCTGCTT-3′ and reverse, 5′-CCGGACGAATGCTTTTTATG-3′;
ii) caspase-3 forward, 5′-GAGTGCTCGCAGCTCATACCT-3′ and reverse,
5-'CCTCACGGCCTGGGATTT-3′; iii) glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) forward, 5′-AGCCACATCGCTCAGACAC-3′ and
reverse, 5′-GCCCAATACGACCAAATCC-3′. The PCR protocol was as
follows: Survivin: 94°C for 2 min, 94°C for 30 sec 54°C for 30 sec
(30 cycles) and 72°C for 5 min; and caspase-3: 94°C for 5 min, 95°C
for 30 sec, 52°C for 30 sec (35 cycles) and 72°C for 5 min. The
gene expression was normalized to GAPDH, using the
2−ΔΔCq method (15) and
the experiments were performed in triplicate.
Western blot analysis
The treated T24 cells were digested using 1% trypsin
for 3–10 min at 37°C. The cells were then treated with 100 µl lysis
buffer for each sample. The cells were subsequently placed on ice
for 30 min. Each sample was centrifuged at 14,000 × g for 10
min and the supernatants were collected. The protein concentrations
of the supernatants were determined using a bicinchoninic acid kit
(Beyotime Institute of Biotechnology, Haimen, China). For the
western blot analysis, 30 µg denatured total protein for each
sample was separated on a sodium dodecyl sulfate polyacrylamide gel
electrophoresis gel and transferred onto a polyvinylidene fluoride
membrane. The membranes were blocked in 5% skimmed milk for 2 h and
were incubated with primary antibodies [rabbit anti-human survivin
monoclonal antibody (catalog no., BA14055; dilution, 1:1,000); and
rabbit anti-human caspase-3 monoclonal antibody (catalog no.,
BA3592; dilution, 1:1,000) purchased from Wuhan Boster Biological
Technology, Ltd., Wuhan, China] overnight at 4°C. The horseradish
peroxidase-conjugated secondary antibodies [goat anti-rabbit IgG
(catalog no., BA1055; dilution, 1:5,000) purchased from Wuhan
Boster Biological Technology, Ltd.] were used to detect the primary
antibodies on the membrane and the bands were visualized using
3,3-diaminobenzidine (DAB). Each experiment was performed in
triplicate.
Immunohistochemistry (IHC)
The cells were seeded onto coverslips and examined
using IHC staining. The HRP Conjugated anti-Mouse/Rabbit IgG SABC
kit (catalog no., SA1020) was purchased from Wuhan Boster
Biological Technology, Ltd., and the staining procedure was
performed according to the manufacturer's protocols. DAB was used
to develop the color, and the cells were counterstained with
hematoxylin and eosin (H&E). The rate of expression was
calculated manually.
H&E and Wright's staining
The T24 cells were cultured in RPMI-1640 medium
containing 10% bovine serum (Gibco; Thermo Fisher Scientific) at
37°C with 5% CO2 and placed on coverslips where they
were treated with 1.25 mg/ml oxymatrine. The staining was performed
using the Hematoxylin-Eosin Staining kit (Wuhan Boster Biological
Technology, Ltd.), according to the manufacturer's protocols.
Electron microscopy
The treated cells were fixed in 1% osmic acid and
subsequently subjected to gradient dehydration in ethanol. The
cells were then embedded in epoxy resin, sectioned (100 µm thick)
and stained with lead citrate. Electron microscopic images were
captured using the JEM-100CXII transmission electron microscope
(JEOL Ltd., Tokyo, Japan).
Flow cytometric analysis of DNA
content
The T24 cells were synchronized for 24 h and were
treated with 1.25 and 2.50 mg/ml oxymatrine for 72 h. Following
treatment, the cells were collected using trypsin and resuspended
in pre-cooled ethanol. The cell suspensions were mixed with an
equal volume of propidium iodide (PI) staining buffer for 30 min,
and then passed through a 40-µm strainer. The PI stain was excited
at a wavelength of 488 nm. The results were analyzed using ModFit
LT 2.0 software (Verity Software House, Inc., Topsham, ME,
USA).
Statistical analysis
The data are presented as the mean ± standard error.
Student's t-test was used to compare the differences between two
groups and the differences among three or more groups were compared
using a one-way analysis of variance, followed by the Bonferroni
post hoc test. A two-tailed P-value of <0.05 was considered to
indicate a statistically significant difference.
Results
Oxymatrine inhibits the proliferation
of T24 cells
Previous studies have shown that matrine exerts a
growth inhibition effect on breast cancer cells (13); however, whether matrine has a similar
effect on bladder cancer cells has not been elucidated. In order to
address this issue, an MTT assay was performed to examine the role
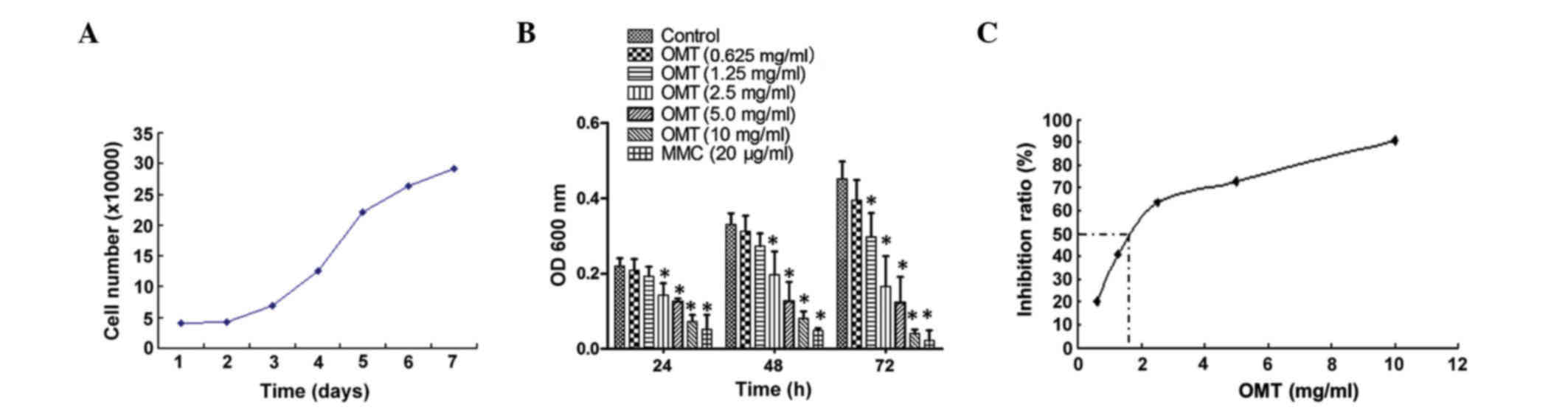
of oxymatrine in cell growth. As shown in Fig. 1A, the exponential growth phase of the
T24 cells was between 24–96 h after plating. The cells were treated
with various doses of oxymatrine for 24, 48 and 72 h. Low doses
(0.625 mg/ml) of oxymatrine showed no significant effect on the
cell inhibition ratio, whereas a medium to high dose (1.25–10.00
mg/ml) of oxymatrine significantly inhibited the growth of the T24
cells in a time- and dose-dependent manner (Fig. 1B). In order to better understand the
effect of oxymatrine on T24 cells, the data was analyzed using a
regression equation, which indicated that the 50% inhibition
concentration of oxymatrine on the T24 cells was 1.82 mg/ml
(Fig. 1C).
Oxymatrine induces apoptosis in T24
cells
Multiple causes may account for the growth
inhibition effect of oxymatrine, including apoptosis and necrosis.
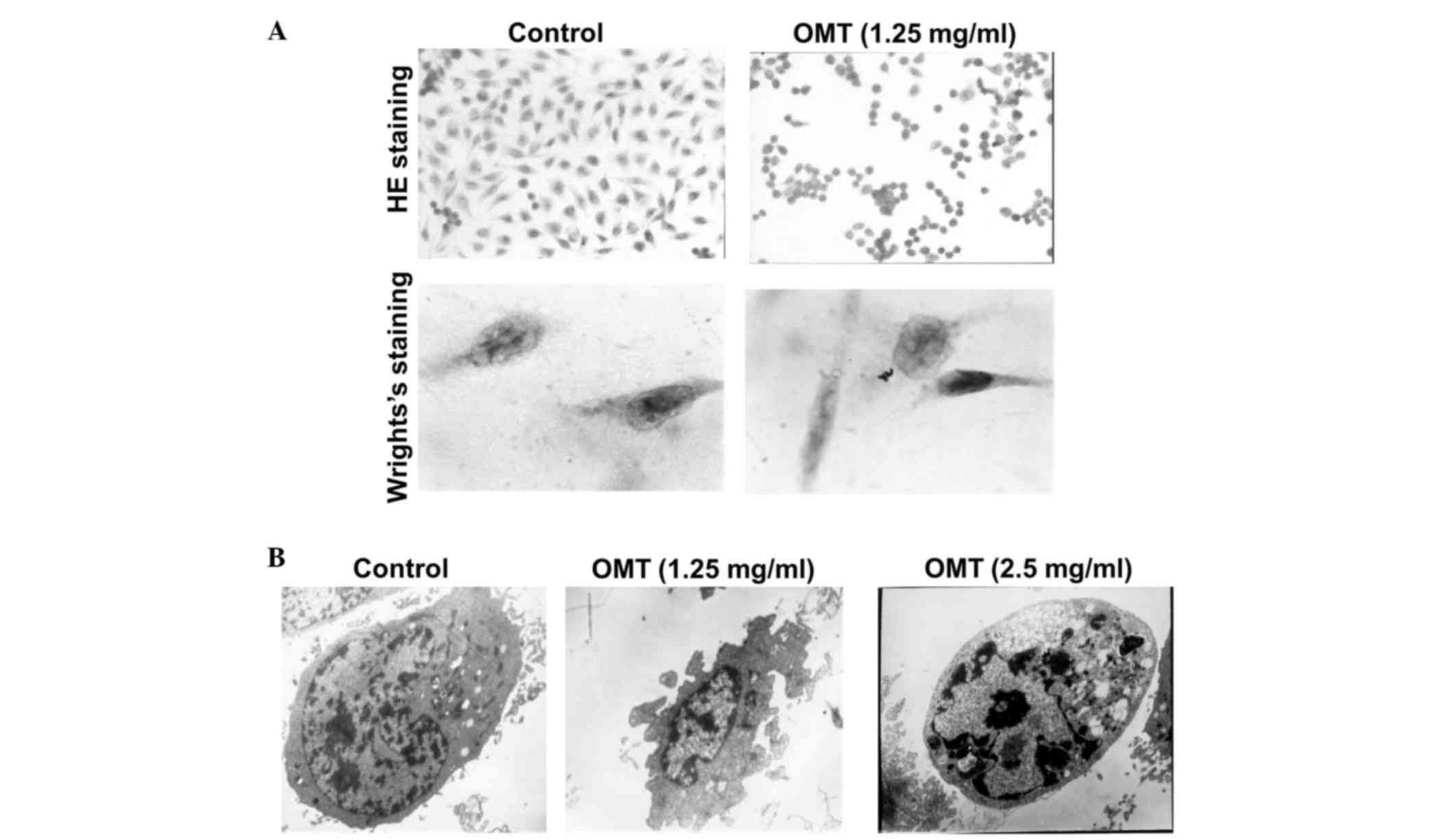
In order to examine whether the growth inhibition was caused by
apoptosis, the T24 cells were subjected to 1.25 mg/ml oxymatrine
for 72 h. H&E staining revealed that the cells in the control
group were spindle-shaped, the nuclei were hyperchromatic and the
nucleoli were presented clearly. By contrast, the
oxymatrine-treated cells exhibited an irregular cell shape and
shrinkage of the cytoplasm (Fig. 2A).
Wright's staining clearly showed that the nuclei were condensed and
that the nuclear membranes were crescent-shaped, an indication of
apoptotic bodies (Fig. 2A). In
addition, the cells displayed the typical features of apoptosis,
including a decreased nuclear-cytoplasmic ratio, a decreased
nucleolus size, condensed chromatin and the invagination of cell
membranes, following treatment with oxymatrine (Fig. 2B). These results suggest that
oxymatrine induces apoptosis in T24 cells.
Oxymatrine induces cell cycle arrest
in T24 cells
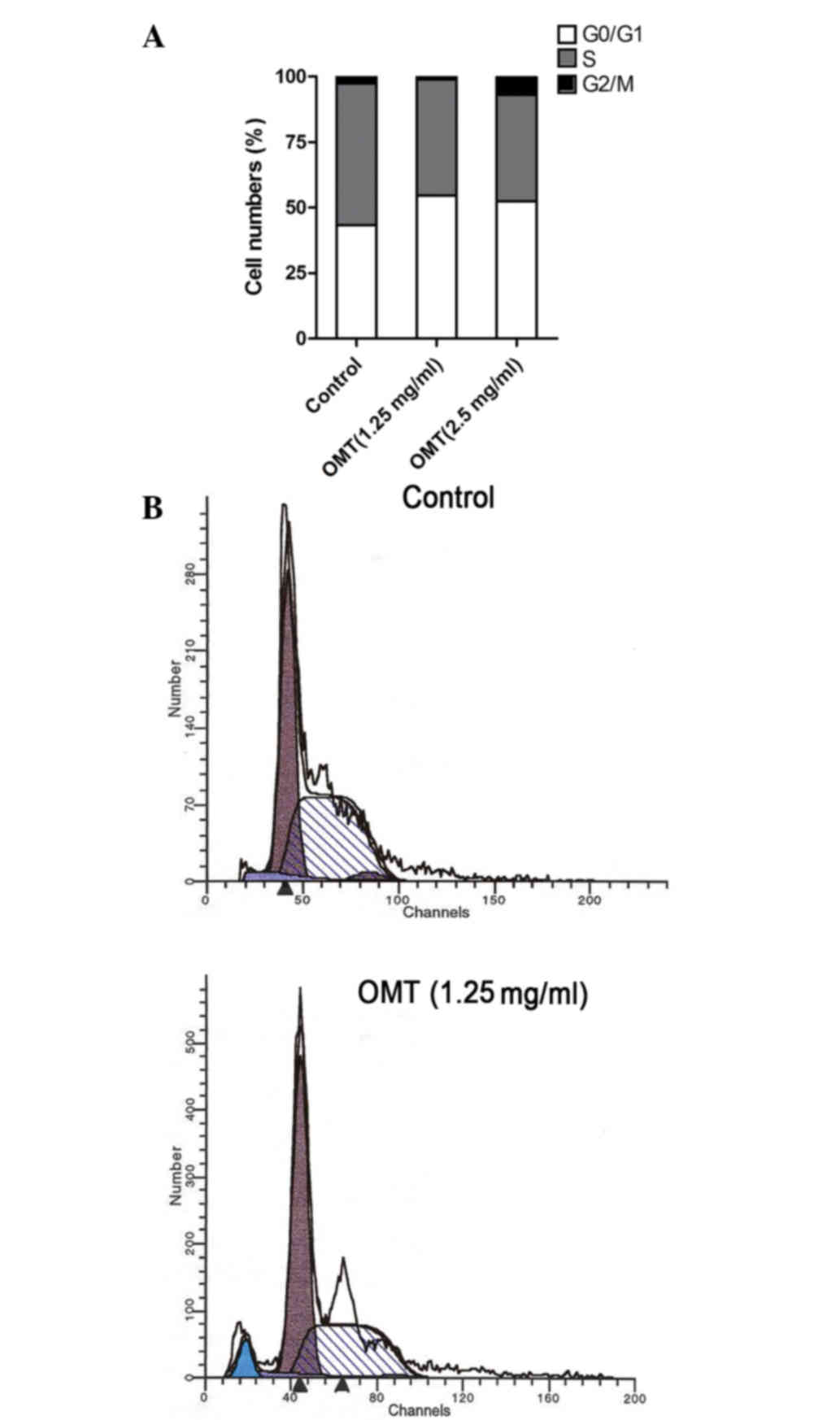
In order to evaluate the effect of oxymatrine on
cell cycle progression, the T24 cells were treated with oxymatrine
at the doses of 1.25 and 2.50 mg/ml. The DNA content was determined
using flow cytometry. As shown in Fig.
3A and Table I, the cell number
in the S phase was significantly decreased for each dose (% cell
population in phase, 40.59 and 44.42% for 1.25 and 2.5 mg/ml,
respectively, vs. 54.10% for control; P<0.05) in the
oxymatrine-treated groups compared with the control group. Cell
cycle arrest at the G0/G1 phase was also
observed in the treated groups (% cell population in phase, 54.52
and 52.41% for 1.25 and 2.5 mg/ml, respectively, vs. 43.31% for
control; P<0.05). Consistent with the findings of the
morphological studies, the apoptotic sub-G1 peak was
detected, and the apoptotic ratio of the cells that were treated
with 1.25 mg/ml oxymatrine was 5.37% (Fig. 3A and B).
 | Table I.Cell cycle distribution and apoptotic
ratio following treatment with oxymatrine (%). |
Table I.
Cell cycle distribution and apoptotic
ratio following treatment with oxymatrine (%).
| Groups |
G0/G1 | S | G2/M | Apoptotic ratio |
|---|
| Control | 43.31 | 54.10 | 2.59 | 0.00 |
| 1.25 mg/ml
oxymatrine | 54.52 | 44.42 | 1.06 | 5.37 |
| 2.50 mg/ml
oxymatrine | 52.41 | 40.59 | 6.99 | –a |
Oxymatrine affects the expression of
apoptosis regulator proteins
In order to investigate the mechanism that mediates
the pro-apoptotic effect of oxymatrine on T24 cells, the cells were
firstly treated with oxymatrine. The levels of caspase-3 and
survivin were evaluated, and the results from the RT-qPCR and
western blot analysis revealed that the mRNA and protein expression
levels of the apoptosis effector, caspase-3, increased in a
dose-dependent manner (Fig. 4A and
B). By contrast, the mRNA and protein expression levels of the
anti-apoptotic gene, survivin, were decreased (Fig. 4A and B). In addition, the expression
of tumor protein p53 (p53), B-cell lymphoma 2 (Bcl-2) and
BCL2-associated X protein (Bax) was examined using IHC. As shown in
Fig. 4C, p53 expression was
significantly decreased (25.75 vs. 76.25%; P<0.05), Bax
expression was markedly increased (58.25 vs. 23.38%; P<0.05) and
Bcl-2 expression was notably increased (27.13 vs. 64.38%;
P<0.05) in the T24 cells of the oxymatrine-treated group
compared with the control group.
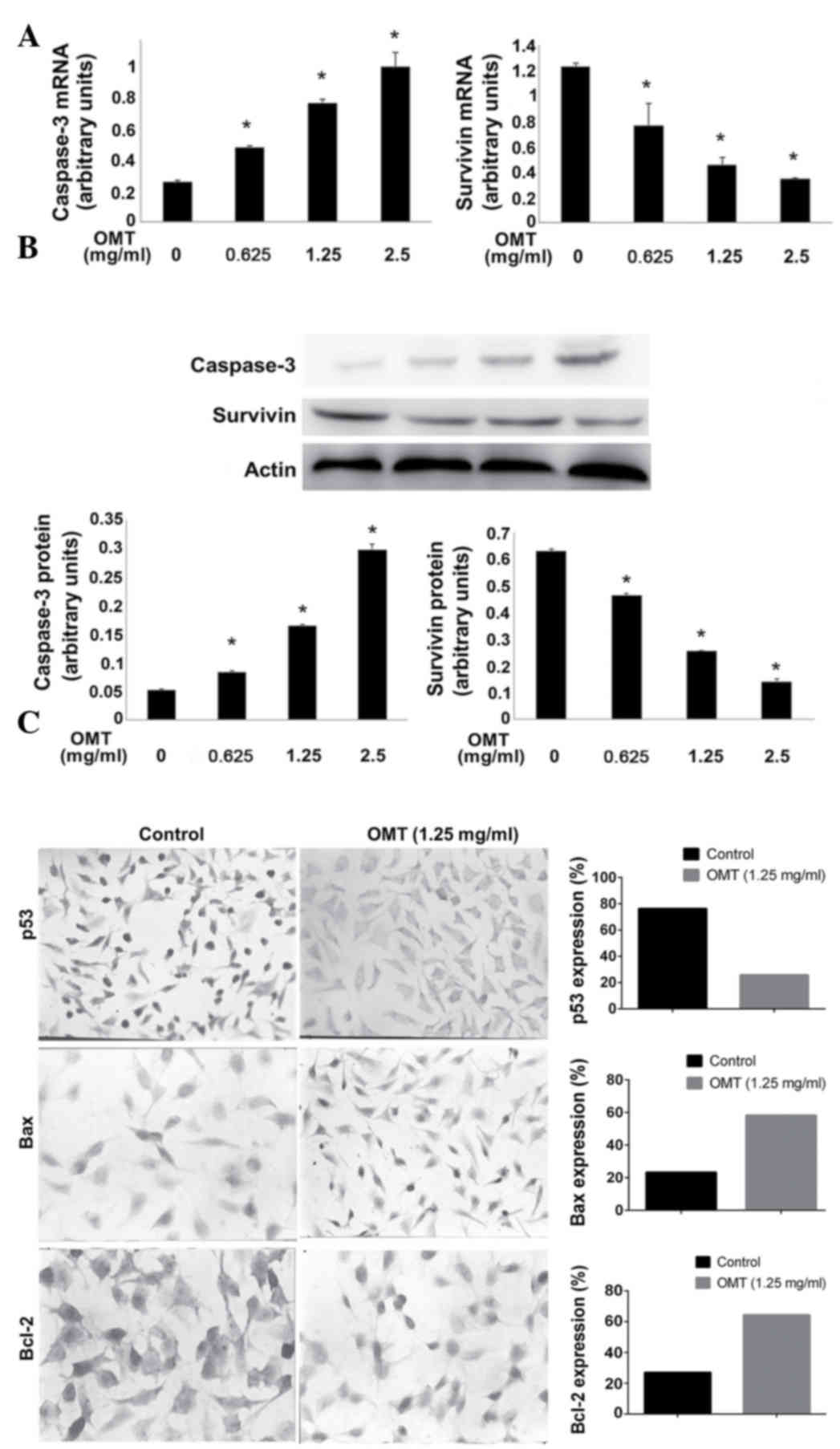 | Figure 4.Effect of OMT on the expression of
apoptosis-associated mRNAs and proteins. Effect of various doses of
OMT on: (A) Caspase-3 and survivin mRNA levels, determined using
reverse transcription-quantitative polymerase chain reaction; and
(B) caspase-3 and survivin protein levels, detected by western blot
analysis. The bottom panel shows the statistical band density
analysis (n=3; *P<0.05 vs. control). (C) Representative images
of the immunohistochemistry of p53, Bax and Bcl-2. The cells were
treated with 1.25 mg/ml OMT for 72 h. Left, control group; right,
OMT (1.25 mg/ml)-treated group. p53 is present in the nucleus,
while Bax and Bcl-2 are present in the cytoplasm. OMT, oxymatrine;
p53, tumor protein p53; Bax, Bcl-2-associated X protein; Bcl-2,
B-cell lymphoma 2. |
Discussion
The present study demonstrates that oxymatrine
initiates an anti-proliferative effect on the bladder cancer T24
cell line, in addition to inducing apoptosis and cell cycle arrest,
which is consistent with previous studies on other types of cancer
cells.
Bladder cancer is the most common malignancy of the
urinary system (16,17). As recurrent bladder cancers are often
characterized by a high occurrence, poor differentiation and a high
invasive ability, searching for effective chemotherapy drugs is a
priority for improving the prognosis. The natural compound
oxymatrine, which is an alkaloid that is extracted from the
traditional Chinese herb Sophora flavecens, has emerged as
an important chemical compound in the treatment of cancer.
Consistent with several previous studies that have
reported the antitumor effects of matrine on cancer cells (18–20), the
present study reinforced the findings of the anti-proliferative
effects of oxymatrine in bladder cancer cells. The MTT assay
indicated that oxymatrine decreased the cell survival rate in a
dose- and time-dependent manner, which indicates the significant
inhibitory effect of oxymatrine on T24 cell proliferation. In
addition, the morphologies of the cells were evidently altered to
an apoptotic phenotype by oxymatrine. Oxymatrine induced the
shrinkage of T24 cell cytoplasm and the condensation of the
nucleus. In particular, crescent-shaped apoptotic bodies were also
present in the cells. Normal cell cycle progression is important
for the proliferation and division of cancer cells. In the present
study, the findings of the flow cytometry analysis showed that
oxymatrine induced T24 cell cycle arrest at the
G0/G1 phase and therefore decreased the cell
number in the S phase. The mechanisms underlying the
anti-proliferative effect of oxymatrine were investigated in order
to strengthen the current understanding of the therapeutic value of
oxymatrine in prostate cancer.
One of the major features of oxymatrine in the
present study is the induction of apoptosis, a self-killing process
that relies on the stepwise activation of caspases and substrates.
Among the caspase family, caspase-3 acts as the rate-limiting
enzyme that determines the extent of apoptosis. Survivin is another
important regulator of cell survival that has been demonstrated to
inhibit apoptotic signals by inhibiting the activation of
endogenous caspase-9 (21). Survivin
has also demonstrated the ability to exert an anti-apoptotic effect
through competitively binding with the p21-cdk4 complex, thereby
promoting the release of p21. p21 and caspase-3 form a complex that
may inhibit caspase-3 activity (22).
The results of the present study showed the increased expression of
caspase-3 mRNA and the decreased expression of survivin mRNA caused
by oxymatrine. In addition, similar results were observed for the
protein expression of caspase-3 and survivin. The present study
indicates that the transcriptional regulation of apoptosis via
caspase-3 and survivin regulation may be one of the mechanisms
underlying the effect of oxymatrine on cell proliferation. Another
important molecule that regulates apoptosis is p53. p53 is
important for G1/S cell cycle regulation, and cells that
express wild-type p53 are arrested in the G1 phase,
initiating apoptosis as a response to DNA damage. However, cells
that express mutated forms of p53 may exhibit a cancerous
aneuploidy phenotype, and are unable to initiate apoptosis
(23). p53 mutations have been
identified in the majority of cancer cell types (24). As the half-life of wild-type p53 is
extremely short, IHC is commonly used to detect the expression of
mutant p53. The results of the present study indicated that
oxymatrine significantly suppressed the nuclear expression of
mutant p53. Proteins located in the mitochondrial membrane are also
important for the regulation of apoptosis. In the present study,
Bcl-2 and Bax demonstrated anti-apoptotic and pro-apoptotic
effects, respectively. Additionally, p53 has been previously
indicated to transcriptionally activate Bax expression (25). The abnormal expression of the Bcl-2
and Bax proteins in bladder cancer has been previously illustrated
(26,27). The results of the present study
indicated an increased Bax/Bcl-2 ratio due to oxymatrine, which
indicates that a p53-Bax dependent mechanism may be involved in
this process.
Certain limitations were evident in the present
study. For example, the results were all obtained from in
vitro studies; therefore, careful assessment of the effect of
oxymatrine on bladder cancer cell apoptosis in vivo is
recommended in future studies.
In summary, the present study elucidated the
anti-proliferative effect of the natural compound oxymatrine on
bladder cancer cells. Oxymatrine was indicated to induce apoptosis
and cell cycle arrest in T24 cells via the regulation of survivin
and p53-Bax signaling. The findings may have clinical implications
for future chemotherapy strategies.
References
|
1
|
Chou R, Gore JL, Buckley D, Fu R,
Gustafson K, Griffin JC, Grusing S and Selph S: Urinary Biomarkers
for diagnosis of bladder cancer: A systematic review and
meta-analysis. Ann Intern Med. 163:922–931. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee CY, Yang KL, Ko HL, Huang RY, Tsai PP,
Chen MT, Lin YC, Hwang TI, Juang GD and Chi KH: Trimodality
bladder-sparing approach without neoadjuvant chemotherapy for
node-negative localized muscle-invasive urinary bladder cancer
resulted in comparable cystectomy-free survival. Radiat Oncol.
9:2132014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ku JH, Kim M, Jeong CW, Kwak C and Kim HH:
Risk prediction models of locoregional failure after radical
cystectomy for urothelial carcinoma: external validation in a
cohort of Korean patients. Int J Radiat Oncol Biol Phys.
89:1032–1037. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yoshida T, Okuyama H, Nakayama M, Endo H,
Nonomura N, Nishimura K and Inoue M: High-dose chemotherapeutics of
intravesical chemotherapy rapidly induce mitochondrial dysfunction
in bladder cancer-derived spheroids. Cancer Sci. 106:69–77. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu YQ, Li Y, Qin J, Wang Q, She YL, Luo
YL, He JX, Li JY and Xie XD: Matrine reduces proliferation of human
lung cancer cells by inducing apoptosis and changing miRNA
expression profiles. Asian Pac J Cancer Prev. 15:2169–2177. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li W, Liang H, Yin T, Wang B and Zhao YY:
Main flavonoids from Sophora flavescenes. Yao Xue Xue Bao.
43:833–837. 2008.(In Chinese). PubMed/NCBI
|
|
7
|
Pan X, Wang L, Grundemann D and Sweet DH:
Inhibition of human organic cation transporters by the alkaloids
matrine and oxymatrine. Fitoterapia. 92:206–210. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou Y, Xu W, Han R, Zhou J, Pan Z, Rong
H, Li J, Xu C, Qiao G and Lu Y: Matrine inhibits pacing induced
atrial fibrillation by modulating I(KM3) and I(Ca-L). Int J Biol
Sci. 8:150–158. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang B, Liu ZY, Li YY, Luo Y, Liu ML,
Dong HY, Wang YX, Liu Y, Zhao PT, Jin FG and Li ZC:
Antiinflammatory effects of matrine in LPS-induced acute lung
injury in mice. Eur J Pharm Sci. 44:573–579. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chuang CY, Xiao JG and Chiou GC: Ocular
anti-inflammatory actions of matrine. J Ocul Pharmacol. 3:129–134.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu JL, Li JH, Chengz RG, Ma YM, Wang XJ
and Liu JC: Effect of matrine on transforming growth factor β1 and
hepatocyte growth factor in rat liver fibrosis model. Asian Pac J
Trop Med. 7:390–393. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ma X, Chen R, Liu X, Xie J, Si K and Duan
L: Effects of matrine on JAK-STAT signaling transduction pathways
in bleomycin-induced pulmonary fibrosis. Afr J Tradit Complement
Altern Med. 10:442–448. 2013.PubMed/NCBI
|
|
13
|
Zhang Y, Piao B, Zhang Y, Hua B, Hou W, Xu
W, Qi X, Zhu X, Pei Y and Lin H: Oxymatrine diminishes the side
population and inhibits the expression of β-catenin in MCF-7 breast
cancer cells. Med Oncol. 28:(Suppl 1). S99–S107. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ling Q, Xu X, Wei X, Wang W, Zhou B, Wang
B and Zheng S: Oxymatrine induces human pancreatic cancer PANC-1
cells apoptosis via regulating expression of Bcl-2 and IAP
families, and releasing of cytochrome c. J Exp Clin Cancer Res.
30:662011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Weintraub MD, Li QQ and Agarwal PK:
Advances in intravesical therapy for the treatment of non-muscle
invasive bladder cancer (Review). Mol Clin Oncol. 2:656–660.
2014.PubMed/NCBI
|
|
17
|
Willis DL, Flaig TW, Hansel DE, Milowsky
MI, Grubb RL, Al-Ahmadie HA, Plimack ER, Koppie TM, McConkey DJ,
Dinney CP, et al: Micropapillary bladder cancer: current treatment
patterns and review of the literature. Urol Oncol. 32:826–832.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guo B, Zhang T, Su J, Wang K and Li X:
Oxymatrine targets EGFR(p-Tyr845) and inhibits EGFR-related
signaling pathways to suppress the proliferation and invasion of
gastric cancer cells. Cancer Chemother Pharmacol. 75:353–363. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ren H, Zhang S, Ma H, Wang Y, Liu D, Wang
X and Wang Z: Matrine reduces the proliferation and invasion of
colorectal cancer cells via reducing the activity of p38 signaling
pathway. Acta Biochim Biophys Sin (Shanghai). 46:1049–1055. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xie M, He G, Wang R, Shi S, Chen J, Ye Y,
Xie L, Yi X and Tang A: Matrine-induced apoptosis of human
nasopharyngeal carcinoma cells via in vitro vascular endothelial
growth factor-A/extracellular signal-regulated kinase1/2 pathway
inactivation. Horm Metab Res. 46:556–560. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Athanasoula KC, Gogas H, Polonifi K,
Vaiopoulos AG, Polyzos A and Mantzourani M: Survivin beyond
physiology: Orchestration of multistep carcinogenesis and
therapeutic potentials. Cancer Lett. 347:175–182. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Suzuki A, Ito T, Kawano H, Hayashida M,
Hayasaki Y, Tsutomi Y, Akahane K, Nakano T, Miura M and Shiraki K:
Survivin initiates procaspase 3/p21 complex formation as a result
of interaction with Cdk4 to resist Fas-mediated cell death.
Oncogene. 19:1346–1353. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schmitt CA, Fridman JS, Yang M, Baranov E,
Hoffman RM and Lowe SW: Dissecting p53 tumor suppressor functions
in vivo. Cancer Cell. 1:289–298. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hollstein M, Sidransky D, Vogelstein B and
Harris CC: p53 mutations in human cancers. Science. 253:49–53.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang W, Ho WC, Dicker DT, MacKinnon C,
Winkler JD, Marmorstein R and El-Deiry WS: Acridine derivatives
activate p53 and induce tumor cell death through Bax. Cancer Biol
Ther. 4:893–898. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kunze D, Kraemer K, Erdmann K, Froehner M,
Wirth MP and Fuessel S: Simultaneous siRNA-mediated knockdown of
antiapoptotic BCL2, Bcl-xL, XIAP and survivin in bladder cancer
cells. Int J Oncol. 41:1271–1277. 2012.PubMed/NCBI
|
|
27
|
Hussain SA, Ganesan R, Hiller L, Murray
PG, el-Magraby MM, Young L and James ND: Proapoptotic genes BAX and
CD40L are predictors of survival in transitional cell carcinoma of
the bladder. Br J Cancer. 88:586–592. 2003. View Article : Google Scholar : PubMed/NCBI
|