Introduction
The present review will discuss the cystic lesions
of the retroperitoneum, an uncommon and heterogeneous group of
lesions that originate behind the retroperitoneum. Lesions that
occur in the retroperitoneal space are divided into solid masses
and cystic lesions, as previously described, and classified
(1,2).
The precise incidence of these lesions is difficult to define, as
the neoplastic lesions are of rare occurrence and the
non-neoplastic lesions, such as postsurgical lymphoceles, are more
frequent (1,2). Even if rarely identified in the
retroperitoneal space (1,2), cystic lesions are highly challenging
regarding the clinical presentation, the diagnostic and the choice
of therapeutic approaches (3).
Similar masses may be discovered during routinely performed
examinations, including abdominal sonography, or may present with
symptoms associated with compression of other bodily structures.
Computed tomography (CT) scan and Nuclear Magnetic Resonance (NMR)
represent the standard imaging approaches to evaluate these lesions
(1). Due to anatomical complexity of
the retroperitoneal space, the diagnostic procedures may include
ultrasound-guided drainage or surgical removal. To introduce the
clinical diagnostic approach and management, the present study will
discuss a representative patient who presented with a cystic
retroperitoneal mass.
Case report
A 68-year-old man was admitted to the Emergency
Department of San Luigi Hospital (Orbassano, Italy) in December
2014 with abdominal discomfort, nausea, vomiting and oedema of the
left inferior leg. The patient had a past medical history of
hypertension, diabetes mellitus, hepatic steatosis and
colelithiasis, with a recent (~three months prior to admission)
diagnosis and treatment for a deep venous thrombosis of the left
leg. No previous history of trauma or pancreatitis was reported. No
significant abnormalities were observed in blood tests (normal
complete blood count; normal renal and hepatic functions; normal
pancreatic enzyme and lactate dehydrogenase levels). As part of the
original examination, an abdominal ultrasound was performed
revealing a voluminous cystic lesion (maximum diameter, 14 cm) in
the abdomen. The patient was transferred to the Division of
Internal Medicine (San Luigi Hospital, Orbassano, Turin, Italy) for
appropriate diagnosis and treatment. A computed tomography (CT)
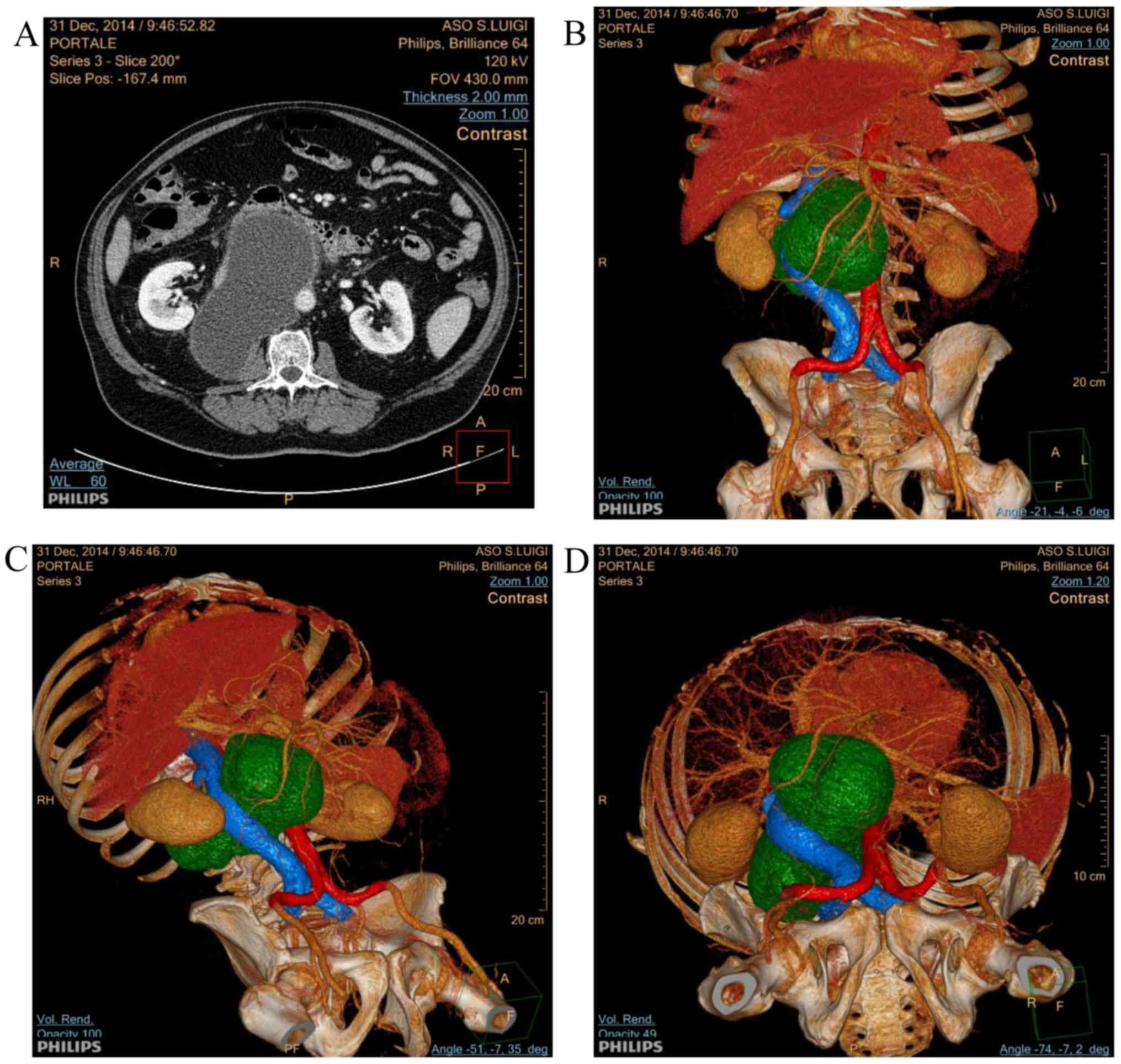
scan was performed, which confirmed the presence of a voluminous
retroperitoneal cystic lesion (diameter, 15×7.5 cm) in the
perivascular retroperitoneum, occupying the space between the aorta
and inferior vena cava, causing its compression and lateral
deviation (Fig. 1A).
Three-dimensional rendering of the CT scan image allowed the
extension of the lesion and its interaction with vessels to be
described more clearly (Fig. 1B-D).
The lesion appeared isolated from the pancreas and the posterior
pararenal space, where it almost reached the psoas muscle. No other
pathological repertoires were found at the CT scan. Based on the CT
scan findings, the patient underwent ultrasound-guided drainage of
the lesion. A total of 800 ml of clear liquid was removed, causing
a marked improvement in the patient's level of discomfort. The
liquid was examined to assess the levels of amylase and lipase, to
perform microbiological analysis and to exclude the presence of
neoplastic cells. All tests were found to be negative. In the
absence of any CT scan elements suggestive of the neoplastic origin
of the cystic lesion, and due to the lack of any significant
abnormalities in the liquid examinations, the patient was
discharged and a close follow-up of the retroperitoneal space was
performed by abdominal ultrasound and CT. After a 3-month follow-up
period, the patient did not present with any signs of relapse (the
abdominal CT confirmed the absence/disappearance of any lesions),
supporting the non-neoplastic origin of the cystic lesion.
Discussion
The present case prompted a review of the diagnostic
approaches and clinical management of retroperitoneal cystic
lesions. In order to describe these lesions, we here summarize the
most comprehensive classification and review published by Dr Yang
and colleagues (1). These masses
arise within the retroperitoneal space, which is a complex region
located behind the peritoneum (1,4,5). This region houses few organs (such as
the adrenal glands and kidneys, portions of the duodenum, pancreas
and colon, and the esophagus), major vessels (the aorta and
inferior cava vein), deep lymphatic vessels and structures,
ligaments and fatty tissues (6).
Among those diseases that originate in the retroperitoneal space,
neoplasms are uncommon (2). Although
they are rare, retroperitoneal masses are a challenging dilemma to
clinicians, either due to the lack of specific symptoms or the
difficult diagnostic approach. Almost 80% of retroperitoneal masses
display malignant features, but no clear diagnostic flow charts
have been proposed thus far. As reported in the present clinical
case, neither radiological imaging or drainage are able to clearly
rule out a malignant nature, imposing the challenging decision to
propose surgical diagnostic investigations of the lesion vs. an
appropriate radiological follow-up. Following on from the present
case report, the current study reviews the biological, clinical and
radiological features of retroperitoneal cystic lesions.
As reviewed and classified elsewhere (1), benign neoplastic lesions include
neoplasms with biologically benign behavior, including cystic
lymphangioma, cystic teratoma, mucinous cystadenoma, pseudomyxoma
retroperitonei, cystic mesothelioma, tailgut cysts, epidermoid
cysts and bronchogenic cysts. Cystic lymphangiomas are generally
benign congenital malformations characterized by abnormal lymphatic
tissues unable to develop normal lymphatic vessel communications
(7). Cystic lymphangiomas are more
common in men and can occur at any age, even though ~90% are found
in children <2 years of age (7–10).
Frequently, these lesions involve the head and the neck (11), while they are unusual in
retroperitoneal locations (1,12–15). When
they develop during the first two trimesters of pregnancy, cystic
lymphangiomas are associated with genetic disorders such as Noonan
syndrome (16) and chromosome 21
trisomy (17). By contrast, acquired
forms may result from trauma, inflammation or lymphatic
obstruction. Clinically, most cystic lymphangiomas result only in a
soft, slow-growing mass, but they can eventually compress their
surrounding structures, causing significant clinical consequences
(1,18). Generally, cystic lymphangiomas are
unilocular or multilocular cysts that contain a clear or milky
fluid, with a single flattened endothelial layer lining them. Upon
NMR, cystic lymphangioma typically demonstrates low T1 and high T2
signal intensity, with no significant enhancement on post-contrast
images (1,19). Wall calcifications are rare.
A cystic teratoma is a neoplasm composed of
different tissues that are not native to the region where it is
generated. The neoplasm is composed of tissues that are derived
from three germinal layers: |The endoderm, the mesoderm and the
ectoderm (20–22). These lesions can be classified as
mature (benign) or immature (malignant), with cystic lesions being
more likely to be benign and solid lesions generally associated
with an immature and malignant behavior. Cystic teratomas generally
arise within the gonadal and sacrococcygeal regions of adults and
children, while they are much more infrequent at the
retroperitoneum (23). The majority
of cases are asymptomatic or associated with non-specific
manifestations. The definitive diagnosis requires surgical excision
of these lesions, with a curative intent in the case of mature
(benign) teratoma (24). At the
histopathological analysis, teratomas are easily recognized due to
the mixture of mature components, including bone, squamous
epithelium, glandular epithelium, stroma and muscle cells. At CT
scan, a mature teratoma of the retroperitoneum manifests as a well
circumscribed mass containing a fluid component, adipose tissue and
calcifications (25). The presence of
hypo-attenuating fat tissues within the cyst is considered highly
suggestive of a cystic teratoma.
Mucinous cystadenomas are homogenous and
well-defined unilocular cysts found in women with normal ovaries
(26–29). The pathogenesis is unclear, although
it has been suggested that these lesions arise from invagination of
the peritoneum, with consequent mucinous metaplasia and cyst
formation. The mucinous cystadenoma cysts are lined by a single
layer of tall columnar epithelial cells, with pale cytoplasm and
basal nuclei (30–32). Notably, even if benign per se, these
lesions could transform into malignant cystadenocarcinoma (33–36). Upon
CT scan, mucinous cystadenoma does not display specific
characteristics over the other cystic retroperitoneal masses
(37). Due to the potential
differential diagnosis with other ovarian lesions, to rule out
malignant lesions, exploratory laparotomy with complete excision of
the cysts should always be proposed (38).
Pseudomyxoma retroperitonei is a rare clinical
condition that is often associated with the development of mucinous
ascites as the consequence of a rupture of mucinous lesions
(39,40). These lesions generally originate from
the appendix and ovary, and, more rarely, from other primary sites,
including the colon, stomach, pancreas and urachus (41). Pseudomyxoma of the retroperitoneum,
also known as pseudomyxoma retroperitonei and pseudomyxoma
extraperitonei, is extremely rare (42,43).
Clinically, pseudomyxoma retroperitonei usually presents with
abdominal or lumbar pain and the presence of a palpable mass.
Occasionally, the formation of abscess, with a high fever, or
discharge is possible and weight loss can also be observed. At CT,
pseudomyxoma retroperitonei appears as masses that are usually
multicystic and are characterized by the presence of septa or thick
walls. Calcifications, either single spot or curvilinear, may be
discovered (1).
Although the name evokes an image of malignant
cancer, cystic mesotheliomas are mostly benign neoplasms that
originate in the serous lining of the pleural, pericardial or
peritoneal spaces (44). Peritoneal
mesotheliomas affect mainly young women, and are characterized by
cysts of variable size and number, with a single layer of benign
mesothelial cells. With regard to causative factors, this lesion
appears to be unrelated to asbestos exposure. The life expectancy
of affected patients is normal due to the low occurrence of
malignant transformation (45–47). The
radiological appearance may be indistinguishable from lymphangiomas
and other retroperitoneal cysts, with the lesions generally
appearing as thin-walled multilocular cysts (48,49).
Tailgut cysts are rare congenital malformations that
may present in the presacral space. The tailgut normally regresses
by the 6th week of gestation, but if the mucous-secreting remnants
fail to regress, a tailgut cyst is formed. These lesions are more
frequently identified in middle-aged women (50), are generally asymptomatic and are only
occasionally associated with symptoms due to compression (51,52). The
major complication of tailgut cysts is the infection of the cysts
themselves, leading to a condition that mimics perianal or pelvic
abscesses (53). During pathological
examination, tailgut cysts are typically lined by different types
of epithelium, including columnar, transitional and squamous
epithelium. The identification of glandular or transitional
epithelium aids in the differential diagnosis with epidermoid and
dermoid cysts. These lesions are mostly benign, but certain cases
of malignant transformation have been reported (54). CT examination shows a well-defined
multicystic mass, with values of density varying from that of water
to that of soft tissues (55). On
NMR, a tailgut cyst typically demonstrates low signal intensity on
T1-weighted images and high signal intensity on T2-weighted images
(56).
Epidermoid cysts are frequently occurring benign
cutaneous tumors, however, they also rarely present as
retroperitoneal cysts (57). One case
has even been reported in the round ligament (58). At the histological analysis, the
hallmark of epidermoid cysts is the presence of a squamous
epithelium, with a mixture of desquamated debris, cholesterol,
keratin and water. Upon CT scan, these lesions appear as unilocular
cysts. The location in the presacral space can facilitate the
differentiation from other pelvic masses (59). Notably, these lesions can be better
identified by MRI as hypo-intense on T1-weighted imaging and
hyper-intense on T2-weighted imaging.
The bronchogenic cyst is a spherical cyst that
originates as an embryonic out-pouching of the foregut or trachea
during the fifth week of gestation. The cyst is generally found in
the mediastinum or lungs and is usually asymptomatic. The major
risk associated with these lesions is infection. However, it should
be noted that these lesions may migrate to an atypical location.
The occurrence of such cysts inside the retroperitoneal space is
extremely rare, with a consequent highly challenging differential
diagnosis (60–62). Upon histopathological analysis,
bronchogenic cysts are mainly unilocular or oligolocular, with a
pseudostratified ciliated columnar epithelial lining, and mucoid
material, bronchial glands, smooth muscle and cartilage. Upon CT
scan, bronchogenic cysts usually present as spherical lesions, with
well-circumscribed smooth or lobulated borders. These lesions lack
enhancement following intravenous contrast administration, while
the occurrence of hyperattenuation should suggest concomitant
hemorrhage or proteinaceous secretions inside the lesions (63). On MRI, bronchogenic cysts usually
display an intermediate to high signal intensity on T1-weighted
images and an high signal intensity on T2-weighted images (64,65). Even
if generally asymptomatic, these lesions could eventually transform
into malignant lesions (66), and
should therefore always be removed (60).
Malignant neoplastic cysts include neurilemmoma,
paraganglioma (only rarely cystic) and perianal mucinous carcinoma.
Neurilemmoma is mostly a benign encapsulated tumor of the nerve
sheath. This tumor is rarely found in the retroperitoneum, where it
represents 0.3–3% of all Schwannomas. This disease presentation
peaks at the fifth to sixth decades and can occur as part of
neurofibromatosis type 2. While it is commonly a solid tumor,
neurilemmoma can also undergo cystic degeneration, therefore posing
as a cystic lesion (67–69). The cellular origin of this tumor is
the Schwann cells, which originate from the neural crest. The
lesions are generally well encapsulated, eccentric from their
parent nerve, with a unique histological presentation that is
characterized by Antoni A and B cells, and positivity for the S-100
antigen. Up to 60% of retroperitoneal Schwannomas display cystic
degeneration, while 1% of retroperitoneal Schwannomas can behave
with malignant features and be associated with cystic degeneration.
Hemorrhage can occur in 5% of the cases, while calcification is
rare. Upon CT scan, the lesions can be isodense to hypodense, with
contrast enhancement (70,71).
Paragangliomas are rare neuroendocrine tumors
originating from the autonomic ganglia, with pathological features
indistinguishable from pheochromocytomas (72,73).
Generally, these lesions are associated with hormone secretion
(catecholamines), with a high rate of malignant behavior (up to
25%) (74,75). The occurrence of paraganglioma peaks
at the third to fifth decades, with a higher female incidence.
Clinically, these lesions may be totally asymptomatic, particularly
if non-functional, or occur with abdominal pain, nausea, emesis,
abdominal distension and weight loss. By contrast, secreting forms
may occur with paroxysmal hypertension, palpitations and profuse
sweating. Paragangliomas are mostly benign tumors with an overall
good prognosis, but almost one-third of cases can be locally
invasive and metastatic. Upon CT scan, paragangliomas are
well-circumscribed masses, with contrast enhancement due to
increased vascularization. Upon MRI, paragangliomas are hypointense
or isointense to the liver parenchyma on T1-weighted images and
markedly hyperintense on T2-weighted images (76,77). In
certain cases, scintigraphy with iodine-123-labeled
metaiodobenzylguanidine can facilitate the diagnosis due to its
superior specificity compared with CT and MRI imaging (78).
Perianal mucinous carcinoma is a rare carcinoma
characterized by abundant mucin production (79,80).
Perianal involvement constitutes ~2% of colorectal cancer and is
more prevalent in middle-aged men. Notably, mucinous adenocarcinoma
appears to either cause fistulation or be a potential consequence
of a chronic fistula (81,82). At the pathological examination, these
lesions are characterized by well-differentiated neoplastic glands
associated with large lakes of mucin. On CT scan, perianal mucinous
adenocarcinoma typically presents as a multiloculated cystic mass,
with the possible presence of septal calcifications. Upon MRI,
these lesions appears as masses filled with a markedly hyperintense
content on T2-weighted images (83,84).
Non-neoplastic lesions are a group of
retroperitoneal lesions composed of pancreatic pseudocysts,
non-pancreatic pseudocysts, lymphoceles, urinoma and hematoma.
Pancreatic pseudocysts are well described and well known among
clinicians (85,86). These lesions collect pancreatic
secretions, including pancreatic amylases and lipases. The
identification of these enzymes inside the cysts allows an easy
diagnosis (87). The lesions commonly
originate as extensions from the diseased pancreas, but could also
migrate to different sites of the retroperitoneal space (88). Generally, these lesions are identified
simultaneously with the diagnosis of pancreatic inflammation.
Non-pancreatic pseudocysts originate from the
mesentery and/or the omentum, and are characterized by a fibrous
wall (5,89,90). As an
important diagnostic element to differentiate them from pancreatic
pseudocysts, these lesions do not contain amylase.
Lymphoceles are a common consequence of a
lymphoadenectomy and/or urological surgery (91,92). These
lesions are generated due to the disruption of normal lymph flow
channels, causing the accumulation of the liquid in anatomical
spaces without epithelial delimitation.
Urinomas are characterized by encapsulated
extravasated urine (93). As a
consequence of surgery, trauma or invasive procedures, urine can
extravasate and accumulate in anatomical spaces.
Blood extravasation in the retroperitoneal space in
the form of a hematoma is generally a consequence of trauma
(94), the rupture of aneurisms
and/or anticoagulation.
Overall, the identification of retroperitoneal
cystic lesions is always a challenging diagnostic dilemma. As has
been reviewed in the present study, following a previously reported
classification and review (63),
cystic lesions of the retroperitoneal space include either
non-neoplastic lesions, or benign and malignant neoplasms. CT
and/or MRI are mandatory to achieve a putative diagnosis (95). However, a dilemma exists as to whether
clinicians should always further investigate the lesions with
biopsy and/or surgical removal. The present study also described
the management of a single patient who presented with a cystic mass
of the retroperitoneum and signs/symptoms due to compression of
vessels and abdominal structures. The original diagnostic approach
consisted of sonography-guided drainage of the lesion. This
approach allowed a reduction in the compression of the surrounding
structures and the analysis of the nature of the cystic liquid.
Notably, no neoplastic cells, bacteria or pancreatic enzymes were
detectable. In the absence of these elements, the precise diagnosis
of this lesion was only speculative. Upon consideration of the
risks of surgical examination of this region, which was located
between the aorta and cava, it was decided that the evolution of
the residual lesion should be monitored only. Notably, after 3 and
then 6 months, no relapse of the cystic lesion was observed. As
time has passed, a lack of relapse has confirmed the benign nature
of the cystic lesion. The present study described this clinical
case and reviewed the literature to emphasize how retroperitoneal
cystic lesions must be managed case by case, based on surgical
removal risks, CT scan data and percutaneous interventional
drainage procedures. Not all lesions should undergo invasive
diagnostic procedures, and therefore, a challenging close follow-up
approach may be mandatory.
Acknowledgements
The authors would like to thank all the members of
the Division of Internal Medicine and the Department of Radiology
of San Luigi Hospital (Orbassano, Italy), for providing assistance
and useful discussions.
References
|
1
|
Yang DM, Jung DH, Kim H, Kang JH, Kim SH,
Kim JH and Hwang HY: Retroperitoneal cystic masses: CT, clinical,
and pathologic findings and literature review. Radiographics.
24:1353–1365. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Scali EP, Chandler TM, Heffernan EJ, Coyle
J, Harris AC and Chang SD: Primary retroperitoneal masses: What is
the differential diagnosis? Abdom Imaging. 40:1887–1903. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Renzulli P and Candinas D: Symptomatic
retroperitoneal cyst: A diagnostic challenge. Ann R Coll Surg Engl.
91:W9–W11. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Osman S, Lehnert BE, Elojeimy S, Cruite I,
Mannelli L, Bhargava P and Moshiri M: A comprehensive review of the
retroperitoneal anatomy, neoplasms and pattern of disease spread.
Curr Probl Diagn Radiol. 42:191–208. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tan JJ, Tan KK and Chew SP: Mesenteric
cysts: An institution experience over 14 years and review of
literature. World J Surg. 33:1961–1965. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tirkes T, Sandrasegaran K, Patel AA,
Hollar MA, Tejada JG, Tann M, Akisik FM and Lappas JC: Peritoneal
and retroperitoneal anatomy and its relevance for cross-sectional
imaging. Radiographics. 32:437–451. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ha J, Yu YC and Lannigan F: A review of
the management of lymphangiomas. Curr Pediatr Rev. 10:238–248.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gümüştaş OG, Sanal M, Güner O and Tümay V:
Retroperitoneal cystic lymphangioma: A diagnostic and surgical
challenge. Case Rep Pediatr. 2013:2920532013.PubMed/NCBI
|
|
9
|
Makni A, Chebbi F, Fetirich F, Ksantini R,
Bedioui H, Jouini M, Kacem M and Ben Safta Z: Surgical management
of intra-abdominal cystic lymphangioma. Report of 20 cases. World J
Surg. 36:1037–1043. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Waldhausen JH, Holterman MJ and Tapper D:
Identification and surgical management of cystic retroperitoneal
lymphangioma in children. Pediatr Surg Int. 11:283–285. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Adams MT, Saltzman B and Perkins JA: Head
and neck lymphatic malformation treatment: A systematic review.
Otolaryngol Head Neck Surg. 147:627–639. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nuzzo G, Lemmo G, Marrocco-Trischitta MM,
Boldrini G and Giovannini I: Retroperitoneal cystic lymphangioma. J
Surg Oncol. 61:234–237. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bhavsar T, Saeed-Vafa D, Harbison S and
Inniss S: Retroperitoneal cystic lymphangioma in an adult: A case
report and review of the literature. World J Gastrointest
Pathophysiol. 1:171–176. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Richmond B and Kister N: Adult
presentation of giant retroperitoneal cystic lymphangioma: Case
report. Int J Surg. 7:559–560. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hauser H, Mischinger HJ, Beham A, Berger
A, Cerwenka H, Razmara J, Fruhwirth H and Werkgartner G: Cystic
retroperitoneal lymphangiomas in adults. Eur J Surg Oncol.
23:322–326. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zarabi M, Mieckowski GC and Mazer J:
Cystic hygroma associated with Noonan's syndrome. J Clin
Ultrasound. 11:398–400. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gedikbasi A, Gul A, Sargin A and Ceylan Y:
Cystic hygroma and lymphangioma: Associated findings, perinatal
outcome and prognostic factors in live-born infants. Arch Gynecol
Obstet. 276:491–498. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Umap PS: Intra-abdominal cystic
lymphangioma. Indian J Cancer. 31:111–113. 1994.PubMed/NCBI
|
|
19
|
Hayami S, Adachi Y, Ishigooka M, Suzuki H,
Sasagawa I, Kubota Y and Nakada T: Retroperitoneal cystic
lymphangioma diagnosed by computerized tomography, magnetic
resonance imaging and thin needle aspiration. Int Urol Nephrol.
28:21–26. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ashley DJ: Origin of teratomas. Cancer.
32:390–394. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gatcombe HG, Assikis V, Kooby D and
Johnstone PA: Primary retroperitoneal teratomas: A review of the
literature. J Surg Oncol. 86:107–113. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gschwend J, Burke TW, Woodward JE and
Heller PB: Retroperitoneal teratoma presenting as an
abdominal-pelvic mass. Obstet Gynecol. 70:500–502. 1987.PubMed/NCBI
|
|
23
|
Sasi W, Ricchetti GA, Parvanta L and
Carpenter R: Giant mature primary retroperitoneal teratoma in a
young adult: Report of a rare case and literature review. Case Rep
Surg. 2014:9305382014.PubMed/NCBI
|
|
24
|
Liu H, Li W, Yang W and Qi Y: Giant
retroperitoneal teratoma in an adult. Am J Surg. 193:736–737. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Davidson AJ, Hartman DS and Goldman SM:
Mature teratoma of the retroperitoneum: Radiologic, pathologic, and
clinical correlation. Radiology. 172:421–425. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yan SL, Lin H, Kuo CL, Wu HS, Huang MH and
Lee YT: Primary retroperitoneal mucinous cystadenoma: Report of a
case and review of the literature. World J Gastroenterol.
14:5769–5772. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Matsubara M, Shiozawa T, Tachibana R,
Hondo T, Osasda K, Kawaguchi K, Kimura K and Konishi I: Primary
retroperitoneal mucinous cystadenoma of borderline malignancy: A
case report and review of the literature. Int J Gynecol Pathol.
24:218–223. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tapper EB, Shrewsberry AB, Oprea G and
Majmudar B: A unique benign mucinous cystadenoma of the
retroperitoneum: A case report and review of the literature. Arch
Gynecol Obstet. 281:167–169. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bortolozzi G, Grasso A and Zasso B:
Mucinous cystadenoma of the retroperitoneum. A case report and
review. Eur J Gynaecol Oncol. 16:65–68. 1995.PubMed/NCBI
|
|
30
|
Demirel D, Gun I, Kucukodaci Z, Balta AZ
and Ramzy I: Primary retroperitoneal mucinous cystadenoma with a
sarcoma-like mural nodule: An immunohistochemical study with
histogenetic considerations and literature review. Int J Gynecol
Pathol. 32:15–25. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Papadogiannakis N, Gad A and Ehliar B:
Primary retroperitoneal mucinous tumor of low malignant potential:
Histogenetic aspects and review of the literature. APMIS.
105:483–486. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hart WR: Mucinous tumors of the ovary: A
review. Int J Gynecol Pathol. 24:4–25. 2005.PubMed/NCBI
|
|
33
|
Lee IW, Ching KC, Pang M and Ho TH: Two
cases of primary retroperitoneal mucinous cystadenocarcinoma.
Gynecol Oncol. 63:145–150. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tangjitgamol S, Manusirivithaya S,
Sheanakul C, Leelahakorn S, Thawaramara T and Kaewpila N:
Retroperitoneal mucinous cystadenocarcinoma: A case report and
review of literature. Int J Gynecol Cancer. 12:403–408. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pearl ML, Valea F, Chumas J and Chalas E:
Primary retroperitoneal mucinous cystadenocarcinoma of low
malignant potential: A case report and literature review. Gynecol
Oncol. 61:150–152. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nelson H, Benjamin B and Alberty R:
Primary retroperitoneal mucinous cystadenocarcinoma. Cancer.
61:2117–2121. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zaheer A, Pokharel SS, Wolfgang C, Fishman
EK and Horton KM: Incidentally detected cystic lesions of the
pancreas on CT: Review of literature and management suggestions.
Abdom Imaging. 38:331–341. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bakker RF, Stoot JH, Blok P and Merkus JW:
Primary retroperitoneal mucinous cystadenoma with sarcoma-like
mural nodule: A case report and review of the literature. Virchows
Arch. 451:853–857. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Harshen R, Jyothirmayi R and Mithal N:
Pseudomyxoma peritonei. Clin Oncol (R Coll Radiol). 15:73–77. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sherer DM, Abulafia O and Eliakim R:
Pseudomyxoma peritonei: A review of current literature. Gynecol
Obstet Invest. 51:73–80. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Niwa H, Hiramatsu T and Ishihara Y:
Clinical challenges and images in GI. Pseudomyxoma retroperitonei.
Gastroenterology. 133(14): 3722007.
|
|
42
|
Smeenk RM, Verwaal VJ and Zoetmulder FA:
Pseudomyxoma peritonei. Cancer Treat Rev. 33:138–145. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ioannidis O, Cheva A, Paraskevas G,
Papadimitriou N, Konstantara A, Chatzopoulos S, Kotronis A,
Makrantonakis A and Kakoutis E: Pseudomyxoma retroperitonei: Report
of 2 cases and review of the literature. Rev Esp Enferm Dig.
104:268–275. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang TB, Dai WG, Liu DW, Shi HP and Dong
WG: Diagnosis and treatment of benign multicystic peritoneal
mesothelioma. World J Gastroenterol. 19:6689–6692. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Murro D, Harbhajanka A, Mahon B and Deziel
D: Benign cystic mesothelioma associated with ipsilateral renal
agenesis: A case report and review of literature. Pediatr Dev
Pathol. 17:487–490. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Park JY, Kim KW, Kwon HJ, Park MS, Kwon
GY, Jun SY and Yu ES: Peritoneal mesotheliomas: Clinicopathologic
features, CT findings, and differential diagnosis. AJR Am J
Roentgenol. 191:814–825. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pickhardt PJ and Bhalla S: Primary
neoplasms of peritoneal and sub-peritoneal origin: CT findings.
Radiographics. 25:983–995. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
O'Neil JD, Ros PR, Storm BL, Buck JL and
Wilkinson EJ: Cystic mesothelioma of the peritoneum. Radiology.
170:333–337. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Li YP, Guico R, Parikh S and Chiu S:
Cystic mesothelioma of the retroperitoneum. J Clin Ultrasound.
20:65–68. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Haydar M and Griepentrog K: Tailgut cyst:
A case report and literature review. Int J Surg Case Rep.
10:166–168. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hansen NH and Qvist N: Tailgut cyst
prolapsing through the anus. Eur J Pediatr Surg. 23:e3–e4.
2013.PubMed/NCBI
|
|
52
|
Leo JM, O'Connor KM, Pezim M, Nagy A and
Schaeffer DF: Benign tailgut cyst masquerading as a hemorrhoid. Can
J Gastroenterol Hepatol. 28:1832014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Johnson KN, Young-Fadok TM, Carpentieri D,
Acosta JM and Notrica DM: Case report: Misdiagnosis of tailgut cyst
presenting as recurrent perianal fistula with pelvic abscess. J
Pediatr Surg. 48:e33–e36. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
van Roggen JF Graadt, Welvaart K, de Roos
A, Offerhaus GJ and Hogendoorn PC: Adenocarcinoma arising within a
tailgut cyst: Clinicopathological description and follow up of an
unusual case. J Clin Pathol. 52:310–312. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Johnson AR, Ros PR and Hjermstad BM:
Tailgut cyst: Diagnosis with CT and sonography. AJR Am J
Roentgenol. 147:1309–1311. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Aflalo-Hazan V, Rousset P, Mourra N, Lewin
M, Azizi L and Hoeffel C: Tailgut cysts: MRI findings. Eur Radiol.
18:2586–2593. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Alaoui FZ Fdili, Oussaden A, Bouguern H,
El Fatemi H, Melhouf MA, Amarti A and Ait Taleb K: Giant pelvic
retroperitoneal epidermoid cyst: A rare case report. Case Rep Med.
2012:9813872012.PubMed/NCBI
|
|
58
|
Kim T and Feranec JB: Epidermoid cyst of
round ligament: Case report and review of literature. J Minim
Invasive Gynecol. 18:126–127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yang DM, Yoon MH and Kim HS, Oh YH, Ha SY,
Oh JH, Chung HS and Kim HS: Presacral epidermoid cyst: Imaging
findings with histopathologic correlation. Abdom Imaging. 26:79–82.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Govaerts K, Van Eyken P, Verswijvel G and
Van der Speeten K: A bronchogenic cyst, presenting as a
retroperitoneal cystic mass. Rare Tumors. 4:e132012. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
O'Neal PB, Moore FD, Gawande A, Cho NL,
King EE, Moalem J and Ruan D: Bronchogenic cyst masquerading as an
adrenal tumor: A case of mistaken identity. Endocr Pract.
18:e102–e105. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Liang MK, Yee HT, Song JW and Marks JL:
Subdiaphragmatic bronchogenic cysts: A comprehensive review of the
literature. Am Surg. 71:1034–1041. 2005.PubMed/NCBI
|
|
63
|
Dong B, Zhou H, Zhang J, Wang Y and Fu Y:
Diagnosis and treatment of retroperitoneal bronchogenic cysts: A
case report. Oncol Lett. 7:2157–2159. 2014.PubMed/NCBI
|
|
64
|
McAdams HP, Kirejczyk WM,
Rosado-de-Christenson ML and Matsumoto S: Bronchogenic cyst:
Imaging features with clinical and histopathologic correlation.
Radiology. 217:441–446. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Murakami R, Machida M, Kobayashi Y, Ogura
J, Ichikawa T and Kumazaki T: Retroperitoneal bronchogenic cyst: CT
and MR imaging. Abdom Imaging. 25:444–447. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Sullivan SM, Okada S, Kudo M and Ebihara
Y: A retroperitoneal bronchogenic cyst with malignant change.
Pathol Int. 49:338–341. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Dede M, Yagci G, Yenen MC, Gorgulu S,
Deveci MS, Cetiner S and Dilek S: Retroperitoneal benign
schwannoma: Report of three cases and analysis of
clinico-radiologic findings. Tohoku J Exp Med. 200:93–97. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Goh BK, Tan YM, Chung YF, Chow PK, Ooi LL
and Wong WK: Retroperitoneal schwannoma. Am J Surg. 192:14–18.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Fass G, Hossey D, Nyst M, Smets D,
Saligheh EN, Duttmann R, Claes K and da Costa PM: Benign
retroperitoneal schwannoma presenting as colitis: A case report.
World J Gastroenterol. 13:5521–5524. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Li Q, Gao C, Juzi JT and Hao X: Analysis
of 82 cases of retroperitoneal schwannoma. ANZ J Surg. 77:237–240.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Hughes MJ, Thomas JM, Fisher C and
Moskovic EC: Imaging features of retroperitoneal and pelvic
schwannomas. Clin Radiol. 60:886–893. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Verma A, Pandey D, Akhtar A, Arsia A and
Singh N: Non-functional paraganglioma of retroperitoneum mimicking
pancreatic mass with concurrent urinary bladder paraganglioma: An
extremely rare entity. J Clin Diagn Res. 9:XD09–XD11.
2015.PubMed/NCBI
|
|
73
|
Lee KY, Oh YW, Noh HJ, Lee YJ, Yong HS,
Kang EY, Kim KA and Lee NJ: Extraadrenal paragangliomas of the
body: Imaging features. AJR Am J Roentgenol. 187:492–504. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Kiernan CM and Solórzano CC:
Pheochromocytoma and Paraganglioma: Diagnosis, genetics, and
treatment. Surg Oncol Clin N Am. 25:119–138. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Burnichon N, Buffet A and Gimenez-Roqueplo
AP: Pheochromocytoma and paraganglioma: Molecular testing and
personalized medicine. Curr Opin Oncol. 28:5–10. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Nishino M, Hayakawa K, Minami M, Yamamoto
A, Ueda H and Takasu K: Primary retroperitoneal neoplasms: CT and
MR imaging findings with anatomic and pathologic diagnostic clues.
Radiographics. 23:45–57. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Rha SE, Byun JY, Jung SE, Chun HJ, Lee HG
and Lee JM: Neurogenic tumors in the abdomen: Tumor types and
imaging characteristics. Radiographics. 23:29–43. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Srirangalingam U, Khoo B, Walker L,
MacDonald F, Skelly RH, George E, Spooner D, Johnston LB, Monson
JP, Grossman AB, et al: Contrasting clinical manifestations of SDHB
and VHL associated chromaffin tumours. Endocr Relat Cancer.
16:515–525. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Deruyter L, Venken R and Maetens M:
Perianal mucinous adenocarcinoma arising from a chronic
fistula-in-ano. Acta Chir Belg. 114:410–413. 2014.PubMed/NCBI
|
|
80
|
Hongo K, Kazama S, Sunami E, Kitayama J
and Watanabe T: Perianal adenocarcinoma associated with anal
fistula: A report of 11 cases in a single institution focusing on
treatment and literature review. Hepatogastroenterology.
60:720–726. 2013.PubMed/NCBI
|
|
81
|
Yang BL, Shao WJ, Sun GD, Chen YQ and
Huang JC: Perianal mucinous adenocarcinoma arising from chronic
anorectal fistulae: A review from single institution. Int J
Colorectal Dis. 24:1001–1006. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Ho CM, Tan CH and Ho BC: Clinics in
diagnostic imaging (143). Perianal mucinous adenocarcinoma arising
from chronic fistula-in-ano. Singapore Med J. 53:843–849.
2012.PubMed/NCBI
|
|
83
|
Hama Y, Makita K, Yamana T and Dodanuki K:
Mucinous adenocarcinoma arising from fistula in ano: MRI findings.
AJR Am J Roentgenol. 187:517–521. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Santos MD, Nogueira C and Lopes C:
Mucinous adenocarcinoma arising in chronic perianal fistula: Good
results with neoadjuvant chemoradiotherapy followed by surgery.
Case Rep Surg. 2014:3861502014.PubMed/NCBI
|
|
85
|
Gumaste VV and Aron J: Pseudocyst
management: Endoscopic drainage and other emerging techniques. J
Clin Gastroenterol. 44:326–331. 2010.PubMed/NCBI
|
|
86
|
Brugge WR: Diagnosis and management of
cystic lesions of the pancreas. J Gastrointest Oncol. 6:375–388.
2015.PubMed/NCBI
|
|
87
|
Ngamruengphong S and Lennon AM: Analysis
of pancreatic cyst fluid. Surg Pathol Clin. 9:677–684. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Karoumpalis I and Christodoulou DK: Cystic
lesions of the pancreas. Ann Gastroenterol. 29:155–161. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Prudnick C, Turnbull J, Jarosz S, Hofeldt
M and Richmond B: Benign mesothelial mesenteric cyst: Case report
and literature review. W V Med J. 111:20–21. 2015.PubMed/NCBI
|
|
90
|
Pozzi G, Ferrarese A, Busso M, Borello A,
Catalano S, Surace A, Marola S, Gentile V, Martino V, Solej M and
Nano M: Percutaneous drainage and sclerosis of mesenteric cysts:
Literature overview and report of an innovative approach. Int J
Surg. 12 Suppl 2:S90–S93. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Karcaaltincaba M and Akhan O: Radiologic
imaging and percutaneous treatment of pelvic lymphocele. Eur J
Radiol. 55:340–354. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Glass LL and Cockett AT: Lymphoceles:
Diagnosis and management in urologic patients. Urology. 51 5A
Suppl:S135–S140. 1998. View Article : Google Scholar
|
|
93
|
Adorisio O, Silveri M, Colajacomo M,
Bassani F and Rivosecchi M: The impact of perinatal urinoma
formation on renal function: Our experience and review of the
literature. J Paediatr Child Health. 47:217–222. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Manzini N and Madiba TE: The management of
retroperitoneal haematoma discovered at laparotomy for trauma.
Injury. 45:1378–1383. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Ayyappan AP, Jhaveri KS and Haider MA:
Radiological assessment of mesenteric and retroperitoneal cysts in
adults: Is there a role for chemical shift MRI? Clin Imaging.
35:127–132. 2011. View Article : Google Scholar : PubMed/NCBI
|