Introduction
The telomere is a region of repetitive nucleotide
sequences at each end of a chromosome. The telomere protects the
ends of chromosomes from deterioration or from fusion with adjacent
chromosomes. Therefore, telomeres are necessary for maintaining
genomic integrity and stability (1).
Furthermore, telomerase reverse transcriptase (TERT) is a
ribonucleoprotein polymerase that maintains telomere ends (2). Activation mutations in the TERT promoter
lead to increased expression of telomerase, which sustains telomere
length (TL) and genomic stability, thereby allowing cancer cells to
continuously divide, and preventing senescence or apoptosis
(2). TERT promoter mutations were
primarily established in melanoma; however, were subsequently
discovered in a number of other common types of cancer, including
hepatocellular cancer, bladder cancer, glioblastoma, and thyroid
cancer (3–7). In addition, a previous study reported
that TERT promoter mutations with ultraviolet signatures are
frequent in skin cancer, suggesting that the overexpression of
telomerase serves an important role in the pathogenesis of these
tumors (2).
Lung cancer is a primary public health problem and a
principal cause of cancer-associated mortality worldwide (8). There are two primary types of lung
cancer: Small cell lung cancer (SCLC) and non-small cell lung
cancer (NSCLC). Between 10 and 15% of lung cancer cases are SCLC,
named for the size of the cancer cells when observed under a
microscope (9). By contrast, between
85 and 90% of lung cancer cases are NSCLC (9). As NSCLC typically grows and spreads more
slowly compared with SCLC, it does not frequently respond to
chemotherapy, in contrast to SCLC (10). Therefore, there are numerous efforts
to develop NSCLC targeted chemotherapy based on oncogenic
mutations, including those in epidermal growth factor receptor
(EGFR) (10).
However, whether patients with lung cancer exhibit
TERT promoter mutations and their prevalence remains unclear due to
the low frequency of TERT promoter mutations in lung cancer. In the
present study, the presence of TERT promoter mutations in NSCLC was
investigated, and the potential association between
clinicopathological features and TERT promoter mutations evaluated.
To elucidate the role of TERT mutations, TL was also investigated
based on previous reports of its prognostic value (11,12).
Patients and methods
Patients and tissue samples
Tumor specimens and corresponding non-malignant lung
tissue specimens (n=188) were provided by the National
Biobank of Korea, Kyungpook National University Hospital (KNUH;
Daegu, South Korea), supported by the Ministry of Health, Welfare,
and Family Affairs (Sejong, South Korea). A total of 89 patients
were <60 years old and 99 patients were ≥60 years old. All
materials derived from the National Biobank of Korea, KNUH, were
obtained under institutional review board-approved protocols
(approval no. KNUMCBIO_14-1010). None of the patients received
chemotherapy or radiotherapy prior to surgery.
TERT promoter mutation
Genomic DNA (gDNA) was isolated from tumor samples
using QIAamp DNA mini kits (Qiagen, Inc., Valencia, CA, USA). The
TERT promoter region was amplified from isolated gDNA using the
polymerase chain reaction (PCR) as described previously (13). PCR was performed using AmpliTaq Gold
DNA polymerase (Applied Biosystems; thermo Fisher Scientific, Inc.,
Waltham, MA, USA). The sequences of the forward and reverse primers
were 5′-CACCCGTCCTGCCCCTTCACCTT-3′ and
5′-GGCTTCCCACGTGCGCAGCAGGA-3′, respectively. Thermocycling
conditions were 40 cycles of at 94°C for 30 sec, 55°C for 30 sec
and 72°C for 60 sec. The PCR products were separated on a 1.5%
agarose gel using electrophoresis and stained with ethidium bromide
for 20 mins to confirm the size of the bands. Direct DNA sequencing
of the TERT promoter region was subsequently performed using an ABI
3730 DNA sequencer (Bionics Inc., Seoul, South Korea).
Relative telomere length
determination
Telomere length was analyzed using quantitative PCR
(qPCR) as aforementioned. For the quantitative determination of
telomere length relative to nuclear DNA (nDNA), specific primers
for telomere (T) and nDNA-encoded β-globin (S) were selected,
according to a previous study (13).
The primer sequences were: telomere forward,
5′-GGTTTTTGAGGGTGAGGGTGAGGGTGAGGGTGAGGGT-3′ and reverse,
5′-TCCCGACTATCCCTATCCCTATCCCTATCCCTATCC-CTA-3′; β-globin forward,
5′-CAGCAAGTGGGAAGGTGTAATCC-3′ and reverse,
5′-CCCATTCTATCATCAACGGGTACAA-3′. qPCR was carried out using a
LightCycler 480 II system (Roche Diagnostics, Basel, Switzerland)
with SYBR PCR master mix (Toyobo, Osaka, Japan). Relative telomere
length was determined by calculating T/S values using the following
formula: T/S=2−ΔCq, where ΔCq=mean CqT-mean
CqS (14). Each
measurement was repeated in triplicate and five serially diluted
control samples were included in each experiment.
Statistical analysis
χ2, Fisher's exact test, the Mann-Whitney
U test and Spearman's rank correlation analysis were used to
analyze the association between variables using SPSS version 20.0
(IBM SPSS, Armonk, NY, USA). Survival curves, constructed using the
univariate Kaplan-Meier estimators, were compared using the
log-rank test. Overall survival (OS) was defined as the time
between diagnosis and mortality. Disease-free survival (DFS) was
defined as the time between diagnosis, and disease recurrence or
the development of distant metastasis. Hazard ratios (HRs) and 95%
confidence intervals (CIs) were estimated using a multivariate Cox
proportional hazards model, with adjustment for age, gender, EGFR
mutation and histological grade. P<0.05 was considered to
indicate a statistically significant difference.
Results
Frequency and clinicopathological
characteristics, of TERT promoter mutation and TL in patients with
NSCLC
The TERT promoter regions of 188 patients with NSCLC
were sequenced and mutations were observed in 2.2% (4/188) of
patients. Previous studies reported that C250T (−146C>T) and
C228T (−124C>T) were points of frequent TERT promoter mutation
in the majority of cancer types, and that lung cancer exhibited
only the C228T mutation. In the present study, all cases with a
TERT promoter mutation exhibited the C228T mutation and one case
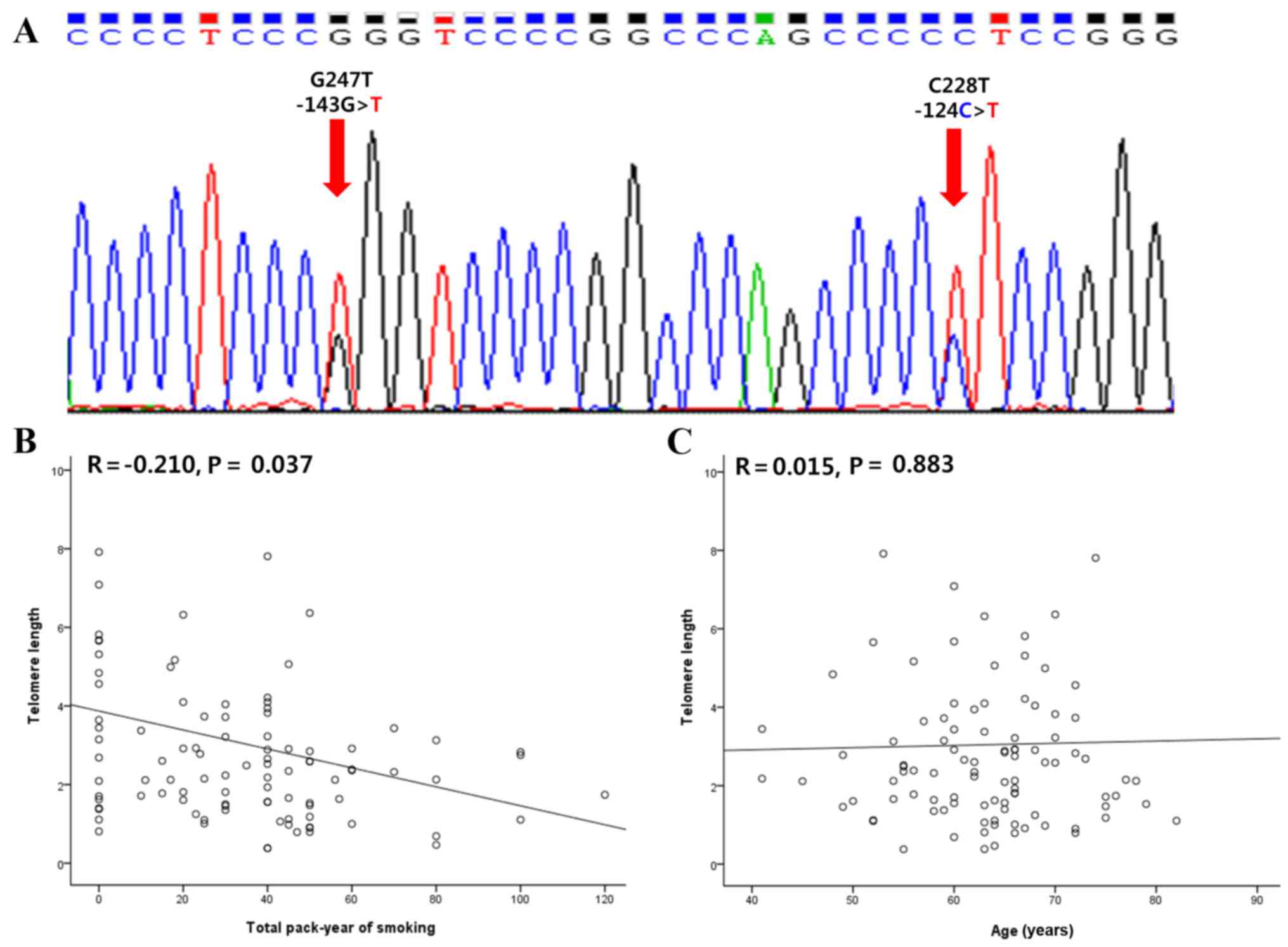
also exhibited a novel C247 (−143G>T) mutation (Fig. 1A). The clinicopathological
characteristics of patients with NSCLC and TERT promoter mutations
are presented in Table I. TERT
promoter mutation was statistically associated with lymph node
invasion (P<0.001). TERT promoter mutation was observed more in
patients with poor differentiation compared with patients with
well-differentiated NSCLC (0.7% vs. 7.7%); however, the difference
was not statistically significant (P=0.060; Table I).
 | Table I.Association between
clinicopathological characteristics and TERT promoter mutation
status in patients with non-small cell lung cancer. |
Table I.
Association between
clinicopathological characteristics and TERT promoter mutation
status in patients with non-small cell lung cancer.
|
|
| TERT promoter
status |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
characteristic | n | Wild-type (%) | Mutated (%) | P-value |
|---|
| Total | 188 | 184 (97.8) | 4 (2.2) |
|
| Age, years |
|
|
| 1.00 |
|
<60 | 89 | 87 (97.8) | 2 (2.2) |
|
| ≥60 | 99 | 97 (98.0) | 2 (2.0) |
|
| Gender |
|
|
| 0.575 |
| Male | 136 | 132 (97.1) | 4 (2.9) |
|
|
Female | 50 | 50 (100) | 0 (0) |
|
| pT |
|
|
| 1.00 |
| 1/2 | 157 | 153 (97.5) | 4 (2.5) |
|
| 3/4 | 27 | 27 (100) | 0 (0) |
|
| pN |
|
|
| <0.001 |
| 0 | 155 | 153 (98.7) | 2 (1.3) |
|
| 1 | 20 | 20 (100) | 0 (0) |
|
| 2 | 9 | 7 (77.8) | 2 (22.2) |
|
| Histological
type |
|
|
| 0.637 |
| Squamous
cell carcinoma | 97 | 94 (96.9) | 3 (3.1) |
|
|
Adenocarcinoma | 90 | 89 (98.9) | 1 (1.1) |
|
| Differentiation
status |
|
|
| 0.060 |
|
Well/moderate | 146 | 145 (99.3) | 1 (0.7) |
|
|
Poor/undifferentiated | 26 | 24 (92.3) | 2 (7.7) |
|
| Smoking |
|
|
| 0.574 |
|
Yes/ever | 138 | 134 (97.1) | 4 (2.9) |
|
|
Never | 49 | 49 (100) | 0 (0) |
|
| EGFR mutation
status |
|
|
| 1.00 |
|
Mutated | 37 | 37 (100) | 0 (0) |
|
|
Wild-type | 110 | 109 (99.1) | 1 (0.9) |
|
TL was analyzed in only 99/188 patients with NSCLC
due to a lack of DNA samples. The mean (± standard deviation) TL in
NSCLC was 3.04±0.30, calculated as the ratio of TL in tumors to
that of paired wild-type tissues. The mean TL of patients with
NSCLC with and without TERT promoter mutations, was 1.82±0.49 and
3.09±1.05, respectively, exhibiting no statistically significant
difference (P=0.282). To further investigate the association
between TL and the clinicopathological features of patients with
NSCLC, patients were categorized into two subgroups according to
the mean value of the tumor/wild-type ratios (3.04). The
clinicopathological characteristics of patients stratified to TL
are summarized in Table II. TL was
observed to be markedly decreased in males (72.2%) compared with
females (50.0%) (P=0.058). However, TL exhibited a significant
association with smokers compared with non-smokers (73.8 vs. 42.1%;
P=0.008; Table II). To confirm the
association between TL and smoking status, quantitative analysis
was performed using Spearman's rank correlation coefficient (R)
(Fig. 1B and C). The total pack-year
of smoking was negatively associated with TL (R=−0.210; P=0.037).
However, age did not demonstrate any significant association with
TL (R=0.015; P=0.883). Other variables exhibited no significant
association with TERT promoter mutation or TL.
 | Table II.Association between
clinicopathological characteristics and telomere length in patients
with non-small cell lung cancer. |
Table II.
Association between
clinicopathological characteristics and telomere length in patients
with non-small cell lung cancer.
|
|
| Telomere length |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
characteristic | n | Short (%) | Long (%) | P-value |
|---|
| Total | 99 | 67 (67.7) | 32 (32.3) | 1.00 |
| Age, years |
|
|
| 0.71 |
|
<60 | 53 | 35 (66.0) | 18 (34.0) |
|
|
≥60 | 46 | 32 (69.6) | 14 (30.4) |
|
| Gender |
|
|
| 0.058 |
|
Male | 79 | 57 (72.2) | 22 (27.8) |
|
|
Female | 20 | 10 (50.0) | 10 (50.0) |
|
| pT |
|
|
| 0.83 |
|
1/2 | 78 | 53 (67.9) | 25 (32.1) |
|
|
3/4 | 17 | 12 (70.6) | 5 (29.4) |
|
| pN |
|
|
| 0.78 |
| 0 | 68 | 45 (66.2) | 23 (33.8) |
|
| 1 | 19 | 13 (68.4) | 6 (31.6) |
|
| 2 | 9 | 7 (77.8) | 2 (22.2) |
|
| Histological
type |
|
|
| 0.100 |
|
Squamous cell carcinoma | 61 | 45 (73.8) | 16 (26.2) |
|
|
Adenocarcinoma | 38 | 22 (57.9) | 16 (42.1) |
|
|
Differentiation |
|
|
| 0.85 |
|
Well/moderate | 67 | 45 (67.2) | 22 (32.8) |
|
|
Poor/undifferentiated | 17 | 11 (64.7) | 6 (35.3) |
|
| Smoking |
|
|
| 0.008 |
|
Yes/ever | 80 | 59 (73.8) | 21 (26.3) |
|
|
Never | 19 | 8 (42.1) | 11 (57.9) |
|
| EGFR mutation
status |
|
|
| 1.0 |
|
Mutated | 6 | 4 (66.7) | 2 (33.3) |
|
|
Wild-type | 53 | 38 (71.7) | 15 (28.3) |
|
Prognostic value of TERT promoter
mutation and TL in NSCLC
We then assessed survival analysis to clarify any
prognostic significance of TERT mutations and TL in NSCLC. The
median follow-up of patients for survival analysis was 78.8 months
(range, 1–123 months). Univariate survival analysis was performed
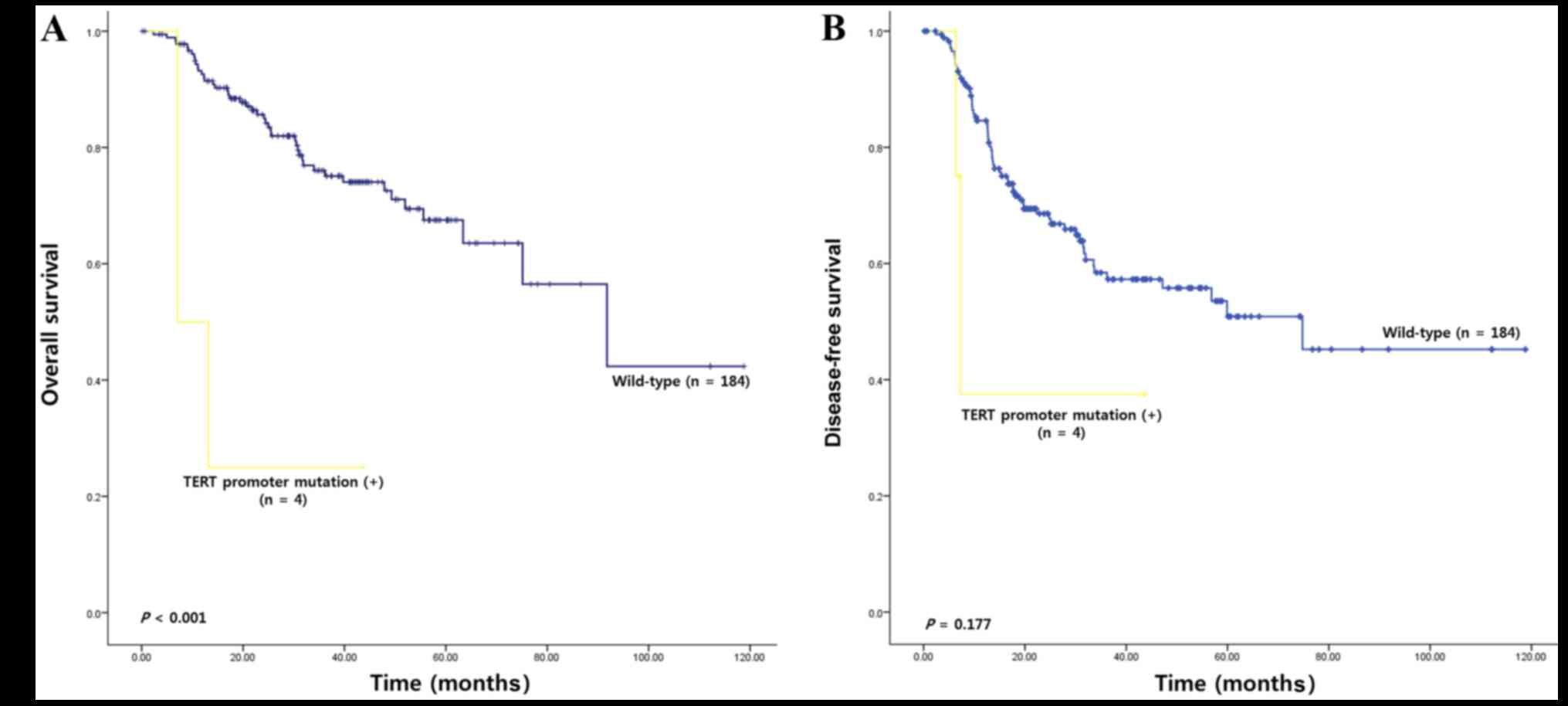
using Kaplan-Meier estimators and demonstrated a decreased OS in
patients with NSCLC with TERT promoter mutations compared with
wild-type TERT (17.74 vs. 79.90 months; χ2=13.25;
P<0.001; Fig. 2A). However, TERT
promoter mutation was not significantly associated with DFS
(Fig. 2B). Survival analysis was also
performed using TL and exhibited no significant prognostic value
(OS, P=0.64; DFS, P=0.68; data not shown). To evaluate whether TERT
promoter mutation is an independent prognostic marker in patients
with NSCLC, the data was further analyzed using the Cox
proportional hazards model, following adjustment for possible
confounders of survival (Table
III). Following multivariate analysis, TERT promoter mutation
(OS, HR=13.16; 95% CI; 1.06–163.59; P=0.045; and DFS, HR=9.99; 95%
CI, 0.87–114.18; P=0.064) exhibited potential value as an
independent prognostic factor of OS. Histological type and EGFR
mutation status also exhibited prognostic value, whereas TL did not
demonstrate any statistical significance as an independent
prognostic marker (OS, HR=2.08; 95% CI, 0.79–5.92; P=0.170; DFS,
HR=1.17; 95% CI, 0.41–3.40; P=0.768).
 | Table III.Multivariate analyses of the
prognostic values of various factors. |
Table III.
Multivariate analyses of the
prognostic values of various factors.
|
| Overall
survival | Disease-free
survival |
|---|
|
|
|
|
|---|
| Factor | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (>60 vs. ≤60
years old) | 1.39
(0.58–3.33) | 0.455 | 1.64
(0.64–4.22) | 0.304 |
| Gender (female vs.
male) | 1.47
(0.43–5.05) | 0.534 | 1.63
(0.42–6.38) | 0.478 |
|
Differentiation | 1.78
(1.10–2.88) | 0.019 | 2.00
(1.18–3.39) | 0.01 |
| EGFR mutation
status | 3.59
(0.87–14.76) | 0.077 | 6.70
(1.37–32.87) | 0.019 |
| TERT promoter
mutation status | 13.16
(1.06–163.59) | 0.045 | 9.99
(0.87–114.18) | 0.064 |
| Telomere length
(short vs. long) | 2.08
(0.73–5.92) | 0.170 | 1.17
(0.41–3.40) | 0.768 |
Discussion
NSCLC, as a heterogeneous disease, has various
outcomes even in patients with identical clinicopathological
features. Identification of oncogenic driver mutations in NSCLC has
promoted clinical treatment options, including targeted drugs. For
example, EGFR-targeted therapy using gefitinib increases survival
results and prognosis of patients with NSCLC (15). To define molecular subtypes of NSCLC
for personalized therapy, it is necessary to understand the driver
genes and their molecular mechanisms, which currently remain
unclear. Therefore, in the present study the clinicopathological
characteristics and prognostic effects, of TERT promoter mutations
and TL, were evaluated in patients with surgically resected
NSCLC.
The results of the present study demonstrated that
TERT promoter mutations were infrequent in patients with NSCLC;
however, predicted poor OS following the construction of univariate
and multivariate survival models. Previous studies reported that
C250 (−146C>T) and C228 (−124C>T) were sites of frequent
mutation, and that lung cancer exhibits only the C228T mutation
(2/68 cell lines) (3). Recently, Li
et al (16) identified no TERT
promoter mutation in 174 NSCLC cases; however, Ma et al
(17) reported it in 2.57% (12/467)
of patients with NSCLC. Previous reports identified certain novel
TERT promoter mutations, which were associated with old age. In the
present study, TERT promoter mutations were detected in 2.2%
(4/188) of patients with NSCLC, which was consistent with previous
studies (17). All cases with TERT
promoter mutations exhibited a C228T mutation and one case
exhibited the novel mutation, G247T (−143G>T). Certain studies
have reported novel mutations of the TERT promoter region; however,
the G247T mutation has not been reported in any type of cancer. In
addition, TERT promoter mutations were associated with poor
differentiation and lymph node invasion, which may predict a poor
prognosis.
Consistent with these characteristics, survival of
patients with NSCLC with TERT mutations was significantly decreased
compared with patients without TERT promoter mutations, following
univariate and multivariate analyses. Horn et al (4) previously demonstrated that TERT promoter
mutations may create an E-twenty-six binding motif, resulting in
the upregulation of TERT expression at the mRNA level in melanoma.
Although alterations in TERT mRNA levels were not evaluated in the
specimens of patients and the prognostic value of
telomere-associated pathways remain unclear, the results of the
present study suggested that TERT promoter mutations are an
independent prognostic marker. Recent independent studies suggest
that NSCLC patients with the 1st quartile (shortest) TL exhibit a
poor outcome (18,19). An age-dependent association between
TERT promoter mutations and TL was also suggested in patients with
thyroid cancer (5). As TL is
hypothesized to be different depending on the presence or absence
of TERT promoter mutations (3–5), TL was
analyzed in the present study to elucidate the role of TERT
promoter mutations and TL in NSCLC. The TL levels were not observed
to be significantly associated with TERT promoter mutations.
Conversely, TL exhibited an association with male gender and
positive smoking status; however, these results may have originated
from the high rate of smoking in Korean males (20). However, TL exhibited no statistically
significant association with other clinicopathological
characteristics or prognosis in Korean patients with NSCLC.
Following stratification of variables, including TL, TERT promoter
mutation status exhibited no prognostic significance (data not
shown). Certain recent studies demonstrated no difference in TERT
expression compared with the presence of TERT promoter mutations
(21,22). These reports indicate that tumor cells
without TERT promoter mutations may have other mechanisms for
activating TERT expression and changing TL. TERT promoter mutations
may play a minor but independent role in lung carcinogenesis,
inducing poor progression. To establish the true prognostic value
of TERT promoter mutation, further study is required in larger
cases. As EGFR mutation status was identified in the present study
to be of prognostic significance in patients with NSCLC, analysis
of EGFR mutation status in all samples is also required to
elucidate the effect of TERT promoter mutation on lung
carcinogenesis.
In conclusion, although the present study is limited
by its retrospective design and small number of sample cases, the
results demonstrate that TERT promoter mutations are infrequent in
patients with NSCLC; however, are significantly associated with an
unfavorable prognosis. These results provide novel insights into
the consequences of TERT promoter mutations in patients with NSCLC
and suggest their value as a prognostic marker in surgically
resected NSCLC. The results of the present study warrant future
large-scale studies to elucidate the underlying molecular
mechanisms and to determine potential clinical utility.
Acknowledgements
This study was supported by grants of the Basic
Science Research Program through the National Research Foundation
of Korea (NRF) funded by the Ministry of Education
(NRF-2014R1A6A3A04058057) and by the Korean Government (MSIP; No.
2014R1A5A2010008).
References
|
1
|
Yoo SS, Do SK, Choi JE, Lee SY, Cha SI,
Kim CH and Park JY: TERT Polymorphism rs2853669 Influences on lung
cancer risk in the korean population. J Korean Med Sci.
30:1423–1428. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Griewank KG, Murali R, Schilling B,
Schimming T, Möller I, Moll I, Schwamborn M, Sucker A, Zimmer L,
Schadendorf D and Hillen U: TERT promoter mutations are frequent in
cutaneous basal cell carcinoma and squamouscell carcinoma. PLoS
One. 8:e803542013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huang FW, Hodis E, Xu MJ, Kryukov GV, Chin
L and Garraway LA: Highly recurrent TERT promoter mutations in
human melanoma. Science. 339:957–959. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Horn S, Figl A, Rachakonda PS, Fischer C,
Sucker A, Gast A, Kadel S, Moll I, Nagore E, Hemminki K, et al:
TERT promoter mutations in familial and sporadic melanoma. Science.
339:959–961. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu X, Bishop J, Shan Y, Pai S, Liu D,
Murugan AK, Sun H, El-Naggar AK and Xing M: Highly prevalent TERT
promoter mutations in aggressive thyroid cancers. Endocr Relat
Cancer. 20:603–610. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Killela PJ, Reitman ZJ, Jiao Y, Bettegowda
C, Agrawal N, Diaz LA Jr, Friedman AH, Friedman H, Gallia GL,
Giovanella BC, et al: TERT promoter mutations occur frequently in
gliomas and a subset of tumors derived from cells with low rates of
self-renewal. Proc Natl Acad Sci USA. 110:pp. 6021–6026. 2013;
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vinagre J, Almeida A, Pópulo H, Batista R,
Lyra J, Pinto V, Coelho R, Celestino R, Prazeres H, Lima L, et al:
Frequency of TERT promoter mutations in human cancers. Nat Commun.
4:21852013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Torre LA, Siegel RL and Jemal A: Lung
Cancer Statistics. Adv Exp Med Biol. 893:1–19. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Travis WD, Travis LB and Devesa SS: Lung
cancer. Cancer. 75:191–202. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oser MG, Niederst MJ, Sequist LV and
Engelman JA: Transformation from non-small-cell lung cancer to
small-cell-lung cancer: Molecular drivers and cells of origin.
Lancet Oncol. 16:e165–e172. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gertler R, Rosenberg R, Stricker D,
Friederichs J, Hoos A, Werner M, Ulm K, Holzmann B, Nekarda H and
Siewert JR: Telomere length and human telomerase reverse
transcriptase expression as markers for progression and prognosis
of colorectal carcinoma. J Clin Oncol. 22:1807–1814. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Svenson U, Nordfjäll K, Stegmayr B, Manjer
J, Nilsson P, Tavelin B, Henriksson R, Lenner P and Roos G: Breast
cancer survival is associated with telomere length in peripheral
blood cells. Cancer Res. 68:3618–3623. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu T, Wang N, Cao J, Sofiadis A, Dinets
A, Zedenius J, Larsson C and Xu D: The age- and shorter
telomere-dependent TERT promoter mutation in follicular thyroid
cell-derived carcinomas. Oncogene. 33:4978–4984. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Reck M: Gefitinib in the treatment of
advanced non-small-cell lung cancer. Expert Rev Anticancer Ther.
9:401–412. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li C, Hao L, Li Y, Wang S, Chen H, Zhang
L, Ke B, Yin Y, Suo H, Sun B, et al: Prognostic value analysis of
mutational and clinicopathological factors in non-small cell lung
cancer. PLoS One. 9:e1072762014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ma X, Gong R, Wang R, Pan Y, Cai D, Pan B,
Li Y, Xiang J, Li H, Zhang J, et al: Recurrent TERT promoter
mutations in non-small cell lung cancers. Lung Cancer. 86:369–373.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fernández-Marcelo T, Gómez A, Pascua I, De
Juan C, Head J, Hernando F, Jarabo JR, Calatayud J, Torres-García
AJ and Iniesta P: Telomere length and telomerase activity in
non-small cell lung cancer prognosis: Clinical usefulness of a
specific telomere status. J Exp Clin Cancer Res. 34:782015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun B, Wang Y, Kota K, Shi Y, Motlak S,
Makambi K, Loffredo CA, Shields PG, Yang Q, Harris CC and Zheng YL:
Telomere length variation: A potential new telomere biomarker for
lung cancer risk. Lung Cancer. 88:297–303. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Park JJ and Park HA: Prevalence of
cigarette smoking among adult cancer survivors in Korea. Yonsei Med
J. 56:556–562. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen YL, Jeng YM, Chang CN, Lee HJ, Hsu
HC, Lai PL and Yuan RH: TERT promoter mutation in resectable
hepatocellular carcinomas: A strong association with hepatitis C
infection and absence of hepatitis B infection. Int J Surg.
12:659–665. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chiba K, Johnson JZ, Vogan JM, Wagner T,
Boyle JM and Hockemeyer D: Cancer-associated TERT promoter
mutations abrogate telomerase silencing. Elife. 4:Jul 21–2015.doi:
10.7554/eLife.07918. View Article : Google Scholar
|