Introduction
Gastric cancer is the third leading cause of
cancer-associated mortality, with an estimated 723,027 mortalities
worldwide in 2012 (1). Compared with
the best supportive care, first-line chemotherapy for advanced
gastric cancer (AGC) improves survival time and quality of life
(2–4);
however, the median overall survival (OS) time is short (9–13
months) (5–7). Several previous studies have
demonstrated that second-line chemotherapy confers a significant
survival benefit over best supportive care in AGC, with median OS
time ranging from 4 to 9.5 months (8–11).
Among patients with AGC, the rate of human epidermal
growth factor receptor 2 (HER2) positivity reportedly ranges from
8.2 to 23.0% as assessed by immunohistochemistry (IHC), and from
7.1 to 27.1% when assessed by fluorescence in situ
hybridization (FISH) (12–15). On the basis of the Trastuzumab for
Gastric Cancer (ToGA) trial results, addition of trastuzumab to
platinum-based chemotherapy has become the standard first-line
therapy for HER2-positive AGC (16).
However, in the second-line setting, the Tykerb with Taxol in Asian
ErbB2+ Gastric Cancer (TyTAN) trial did not demonstrate a survival
benefit from the addition of lapatinib, a small-molecule tyrosine
kinase inhibitor targeting HER2, to paclitaxel for the treatment of
HER2-positive AGC (17). Preliminary
evidence from a small phase II study demonstrated that a
trastuzumab plus docetaxel-containing regimen was well tolerated
and effective for HER2-positive AGC previously treated with
fluorouracil-based chemotherapy; however, in this study, the
patients had received no previous therapy with trastuzumab
(18). On the other hand, several
studies have reported that continued therapy with trastuzumab
beyond progression (TBP) improves the survival outcome of patients
with metastatic HER2-positive breast cancer (19,20), and
represents a practical therapeutic option. However, there is
currently no clinical data regarding the efficacy of trastuzumab
for HER2-positive AGC progressing during trastuzumab-based
chemotherapy. The aim of the present, retrospective study was to
assess the clinical benefit of the continuation of trastuzumab for
HER2-positive AGC patients who progressed during trastuzumab-based
chemotherapy.
Patients and methods
Patients
The study population comprised patients with
HER2-positive AGC who were treated at three Japanese institutions
(Aichi Cancer Center Hospital, Nagoya, Japan; Tsuchiura Kyodo
General Hospital, Tsuchiura, Japan; and Saitama Cancer Center
Hospital, Saitama, Japan) between January 2006 and March 2014. The
principal inclusion criteria were as follows: Histologically proven
inoperable gastric cancer; an Eastern Cooperative Oncology Group
performance status of 0–2 (21);
sufficient bone marrow, liver and renal function; progressive
disease diagnosed by computed tomography scans during first-line
chemotherapy of trastuzumab combined with fluoropyrimidine plus
platinum; and treatment with cytotoxic agents (docetaxel, 60
mg/m2 every 3 weeks; paclitaxel 80 mg/m2 on
days 1, 8 and 15, every 4 weeks; irinotecan 150 mg/m2
every 2 weeks; or irinotecan 60 mg/m2 plus cisplatin 30
mg/m2 every 2 weeks) with or without trastuzumab (8
mg/kg on day 1 of the first cycle, followed by 6 mg/kg every 3
weeks) as second-line chemotherapy. The second-line treatment
regimens were selected by the treating physicians at each
institution. HER2-positivity was defined as an IHC score of 3+, or
an IHC score of 2+ and an in situ hybridization
(ISH)-positive result determined by FISH or dual-color ISH
(16,22), which are considered to be indications
for using trastuzumab on the basis of subset analysis of the ToGA
trial results (16,22). The HER2 status examination was
performed prior to the start of the first-line chemotherapy.
Written informed consent for treatment was obtained from each
patient prior to treatment initiation. The Institutional Review
Board of each participating center approved the study.
Data analysis
The main purpose of this study was to compare the
efficacy and safety of trastuzumab treatment for patients who
subsequently continued trastuzumab (TBP group) vs. those who
discontinued trastuzumab beyond progression (non-TBP group).
Treatment responses were evaluated in accordance with the Response
Evaluation Criteria in Solid Tumors (version 1.1) (23) and the best overall response was
recorded as the antitumor effect for each patient. The objective
response rate (ORR) was defined as the proportion of patients with
a complete response (CR) or a partial response (PR), and the
disease control rate (DCR) was defined as the proportion of
patients with a CR, a PR or stable disease (SD).
Progression-free survival (PFS) time was measured
from the date of second-line therapy initiation to the date of
progressive disease or mortality due to any cause. OS time was
calculated from the date of second-line therapy initiation to the
date of mortality or the last follow-up visit. The PFS and OS
curves were constructed by using the Kaplan-Meier method. Survival
status was updated in October 2014.
The P-values for the comparison of differences
between the baseline patient characteristics of each group were
calculated using χ2 tests for homogeneity or trend, or
by using Fisher's exact test. Univariate Cox proportional hazards
models were used to assess the effects of TBP in terms of PFS and
OS, which were indicated by hazard ratios (HR) and 95% confidence
intervals (CI). The incidence and severity of adverse events were
graded using the National Cancer Institute Common Toxicity Criteria
version 4.0 (24). Statistical
analyses were performed with R software version 2.13.2 (R Project
for Statistical Computing, Vienna, Austria). All tests were
two-tailed and P<0.05 was considered to indicate statistical
significance.
Results
Patient characteristics
The characteristics of the patients are summarized
in Table I. During the study period,
108 consecutive patients with HER2-positive AGC received
trastuzumab combined with fluoropyrimidine plus platinum as
first-line treatment, of which 34 patients enrolled in clinical
trials, 28 patients did not receive second-line chemotherapy, and
46 patients met the inclusion criteria. A total of 26 patients
(57%) continued and 20 (43%) did not continue trastuzumab. For all
patients, HER2 status could not be tested at the time of disease
progression during trastuzumab treatment. Between the TBP and
non-TBP groups, no statistically significant differences were
identified in histological subtypes (intestinal-type, 61.5 vs.
65.0%, respectively; P=0.81), IHC score (3+, 80.8 vs. 60.0%,
respectively; P=0.19), presence of one or more measurable lesions
(84.6 vs. 95.0%, respectively; P=0.34), time-to-first disease
progression (first PFS time, 6.4 vs. 6.2 months respectively;
log-rank P=0.59), or >6-month first PFS rate (57.7 vs. 60.0%,
respectively; P=0.87). In the TBP group, for second-line
chemotherapy, 22 patients (84.6%) received trastuzumab plus
taxanes, 3 patients (11.6%) received trastuzumab plus irinotecan,
and 1 (3.8%) received cisplatin plus irinotecan combined with
trastuzumab. For third-line chemotherapy, 7 patients (26.9%)
received taxanes/irinotecan and 8 (30.8%) received
taxanes/irinotecan combined with trastuzumab. In the non-TBP group,
13 patients (65.0%) received taxanes, and the remaining patients
(35.0%) received irinotecan as second-line therapy. For third-line
chemotherapy, 12 patients (60.0%) received taxanes/irinotecan and 3
(15.0%) received taxanes/irinotecan combined with trastuzumab.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristic | Non-TBP group
(n=20) | TBP group (n=26) | P-value |
|---|
| Age, years [median
(range)] | 64 (33–74) | 62 (29–86) | 0.97b |
| Gender, n (%) |
|
| 0.40c |
| Male | 14 (70.0) | 21 (80.8) |
|
|
Female | 6 (30.0) | 5 (19.2) |
|
| ECOG PS, n (%) |
|
| 0.26c |
| 0 | 9 (45.0) | 6 (23.1) |
|
| 1 | 9 (45.0) | 18 (69.2) |
|
| 2 | 2 (10.0) | 2 (7.7) |
|
| Disease status, n
(%) |
|
| 0.71c |
|
Unresectable | 17 (85.0) | 20 (76.9) |
|
|
Recurrent | 3 (15.0) | 6 (23.1) |
|
| Number of
metastatic sites, n (%) |
|
| 0.73c |
| 1 | 4 (20.0) | 7 (26.9) |
|
| ≥2 | 16 (80.0) | 19 (73.1) |
|
| Metastatic sites, n
(%) |
|
Liver | 12 (60.0) | 11 (42.3) | 0.23b |
|
Lung | 3 (15.0) | 9 (34.6) | 0.18c |
| Lymph
node | 15 (75.0) | 22 (84.6) | 0.47c |
|
Peritoneum | 12 (60.0) | 10 (38.5) | 0.14b |
| Prior gastrectomy,
n (%) |
|
| 0.34c |
|
Yes | 4 (20.0) | 9 (34.6) |
|
| Histological
subtypes, n (%) |
|
| 0.81b |
|
Diffuse | 7 (35.0) | 10 (38.5) |
|
|
Intestinal | 13 (65.0) | 16 (61.5) |
|
| HER2 status, n
(%) |
|
| 0.19c |
| IHC
2+/FISH positive | 8 (40.0) | 5 (19.2) |
|
| IHC
3+ | 12 (60.0) | 21 (80.8) |
|
| ≥1 measureable
target lesion, n (%) |
|
| 0.34c |
|
Yes | 19 (95.0) | 22 (84.6) |
|
| Adjuvant
chemotherapy, n (%) |
|
| 1.00c |
|
Yes | 2 (10.0) | 3 (11.5) |
|
| Prior chemotherapy,
n (%) |
|
| 0.01c |
| XP with
trastuzumab | 19 (95.0) | 19 (73.1) |
|
| SP with
trastuzumab | 0 (0) | 7 (26.9) |
|
| FP with
trastuzumab | 1 (5.0) | 0 (0) |
|
| Second-line
chemotherapy, n (%) |
|
| 0.16d,c |
|
Taxanesa | 13 (65.0) | 22 (84.6) |
|
|
Irinotecan | 7 (35.0) | 4 (15.4) |
|
|
Cisplatin | 0 (0) | 1 (3.8) |
|
|
Trastuzumab | 0 (0) | 26 (100) |
|
| PFS of first-line
chemotherapy, months [median (range)] | 6.2 (2.118.1) | 6.4 (1.621.6) | 0.59e |
Treatment and efficacy
At a median follow-up time of 8.7 months, 43 (87.8%)
patients had experienced disease progression and 32 (70%) patients
had succumbed to the disease at the time of analysis. In the TBP
group, among the 22 assessable patients, no patients achieved CR, 3
achieved PR, and 9 achieved SD, with an ORR of 13.6% and a DCR of
54.5%. In the non-TBP group, among the 19 assessable patients, 3
achieved PR, and 4 achieved SD, with an ORR of 15.8% and a DCR of
36.8%. The differences between the two groups were not significant,
with P-values of 1.00 and 0.21 for ORR and DCR, respectively. No
significant differences in response rates for any subgroups (age,
gender, ECOG PS, disease status, number of metastatic sites,
metastatic sites, prior gastrectomy, histological subtypes, HER2
status, adjuvant chemotherapy) between the TBP group and non-TBP
group were identified.
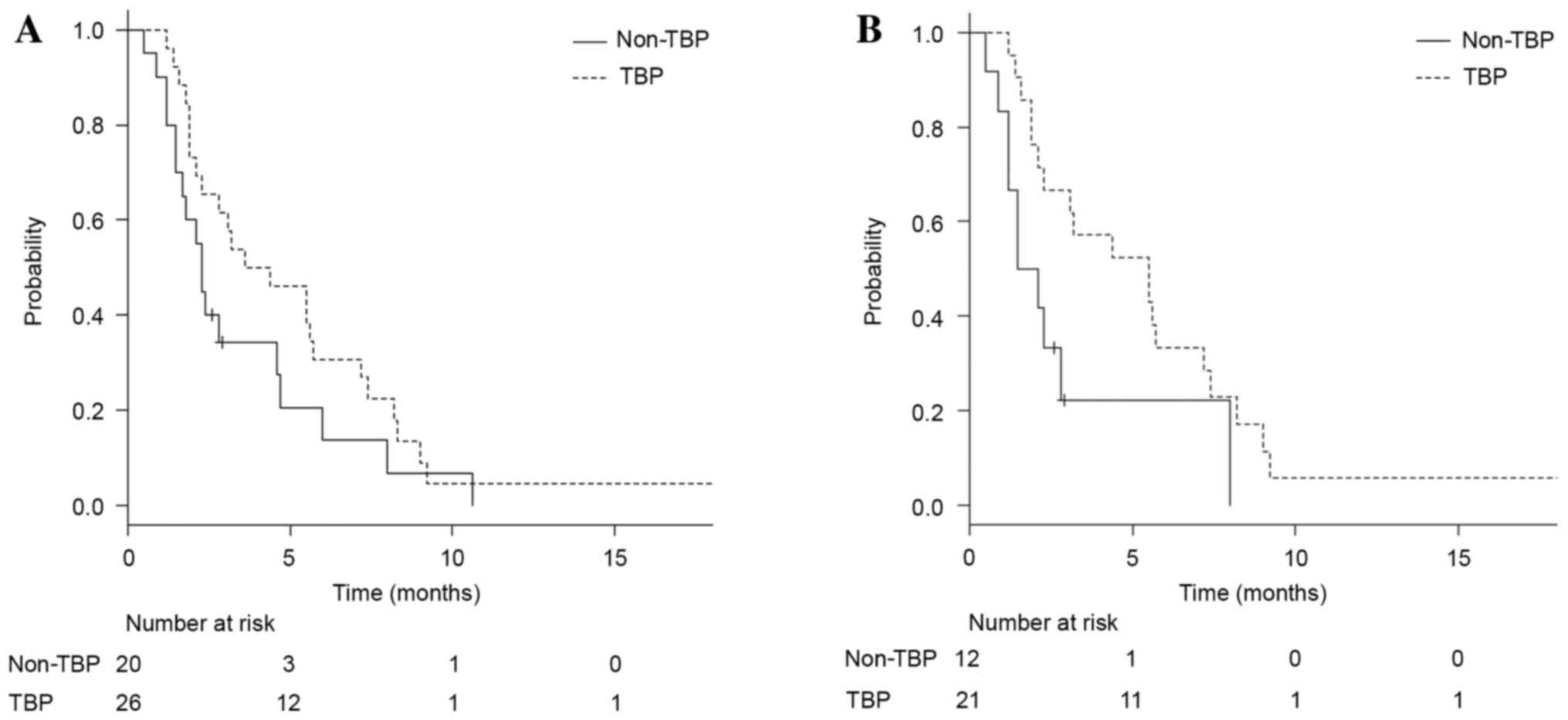
Only 3 patients had not experienced disease
progression at the time of analysis. Addition of trastuzumab (vs.
no addition) numerically improved PFS time, with no significance
(median, 4.0 vs. 2.3 months, respectively; HR, 0.63; 95% CI,
0.34–1.17; P=0.14; Fig. 1A). In the
exploratory subset analysis of PFS time, three subgroups that could
potentially benefit from continuing TBP vs. not continuing TBP were
identified: Patients with a HER2 IHC score of 3+ (median, 5.5 vs.
1.8 months, respectively; HR, 0.41; 95% CI, 0.18–0.94; P=0.04;
Fig. 1B), patients with histology of
intestinal type (median, 5.7 vs. 2.3 months, respectively; HR,
0.32; 95% CI, 0.14–0.75; P<0.01), and patients with a first PFS
of >6 months (median, 5.7 vs. 2.2 months respectively; HR, 0.44;
95% CI, 0.20–0.96; P=0.04).
The analysis of OS times revealed that the TBP group
had a survival time similar to that of the non-TBP group (median,
10.8 vs. 9.5 months, respectively; HR, 1.06; 95% CI, 0.52–2.16;
P=0.88). The OS time of the TBP group was also equivalent to that
of the non-TBP group in the subgroups with HER2 IHC 3+ (median,
11.3 vs. 8.8 months respectively; HR, 0.97; 95% CI, 0.39–2.43;
P=0.94), histology of intestinal type (median, 16.4 vs. 8.8 months,
respectively; HR, 0.53; 95% CI, 0.21–1.36, P=0.19), and first PFS
of >6 months (median, 16.3 vs. 11.5 months respectively; HR,
0.62; 95% CI, 0.25–1.57; P=0.31).
No statistically significant differences in the
percentages of patients who received third-line chemotherapy (69.2
vs. 80.0%, respectively; P=0.51), and subsequent
trastuzumab-containing therapy (30.7 vs. 15.0%, respectively;
P=0.15), were identified between the TBP and non-TBP groups.
Fourth-line chemotherapy or beyond was administered to 7 (26.9%)
patients in the TBP group and 8 (40.0%) in the non-TBP group.
Toxicity
The majority of adverse events associated with
chemotherapy (Table II) were grade 1
or 2. The frequency of any hematological toxicity of grades 3–4 was
62% in the TBP group and 50% in the non-TBP group, with no
significant difference observed (P=0.43). The most common grade 3–4
hematological toxicity was neutropenia in the two groups: 42% in
the TBP group and 40% in the non-TBP group. The frequencies of any
non-hematological toxicity of grade 3–4 were also not different
between the TBP and non-TBP groups (23 vs. 25%, respectively;
P=0.88). No cardiac dysfunction occurred in either group.
 | Table II.Adverse events of all grades and
grades 3–4 occurring in the non-TBP and TBP groups. |
Table II.
Adverse events of all grades and
grades 3–4 occurring in the non-TBP and TBP groups.
|
| Non-TBP (n=20) | TBP (n=26) |
|
|---|
|
|
|
|
|
|---|
| Adverse events | All grades, n
(%) | Grades 3–4, n
(%) | All grades, n
(%) | Grades 3–4, n
(%) |
P-valuea |
|---|
| Hematological
toxicities |
|
Any | 16 (80) | 10 (50) | 23 (88) | 16 (62) | 0.43b |
|
Leukopenia | 12 (60) | 5
(25) | 18 (69) | 7
(27) | 0.88c |
|
Neutropenia | 11 (55) | 8
(40) | 19 (73) | 11 (42) | 0.87b |
|
Anemia | 14 (70) | 6
(30) | 18 (69) | 7
(27) | 0.82b |
|
Thrombocytopenia | 4
(20) | 1 (5) | 1 (4) | 0 (0) | 0.43c |
| Non-hematological
toxicities |
|
Any | 18 (90) | 5
(25) | 21 (81) | 6
(23) | 0.88c |
|
Nausea | 11 (55) | 2
(10) | 7
(27) | 2 (8) | 1.00c |
|
Anorexia | 7
(35) | 1 (5) | 5
(19) | 1 (4) | 1.00c |
|
Fatigue | 8
(40) | 2
(10) | 12 (46) | 1 (4) | 0.57c |
|
Diarrhea | 6
(30) | 2
(10) | 3
(12) | 0 (0) | 0.18c |
|
Stomatitis | 2
(10) | 0 (0) | 3
(12) | 0 (0) | 1.00d |
|
Peripheral neuropathy | 7
(35) | 0 (0) | 11 (42) | 1 (4) | 1.00c |
|
Infusion reaction | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1.00d |
| Cardiac
dysfunction | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1.00d |
| Febrile
neutropenia | 1 (5) | 1 (5) | 2 (8) | 2 (8) | 1.00c |
Discussion
To the best of our knowledge, the present study is
the first to evaluate a TBP strategy in second-line chemotherapy
for HER2-positive AGC. There were two major findings. Firstly, the
trastuzumab-combined therapy was numerically superior to
chemotherapy alone for PFS, particularly in the subgroups with HER2
IHC 3+ tumors, histology of intestinal type, or a longer first PFS
time (>6 months). Secondly, there was no significant difference
in toxicity identified between the two groups, including cardiac
dysfunction. These results suggest that the TBP strategy may be a
second-line treatment option for patients with HER2-positive
AGC.
Compared with the results from a prospective large
observational study and a randomized phase III study involving
metastatic breast cancer, which showed that the continued therapy
with TBP improves the survival outcome of patients with metastatic
HER2-positive breast cancer (19,20), the
results of the present study did not demonstrate a clear survival
time benefit associated with trastuzumab therapy following first
disease progression. However, the magnitude of unadjusted HR (0.63)
for PFS in the present study was of the same order as that (0.69)
in a German phase III trial comparing trastuzumab plus capecitabine
vs. capecitabine alone in HER2-positive breast cancer patients
whose disease had progressed during trastuzumab therapy (20), a finding which justified further
investigation of HER2-positive AGC. The phenomenon of clinically
relevant improvement of PFS time in the subgroup of patients with
HER2 IHC 3+ tumors is congruent with the exploratory findings from
the ToGA and TyTAN trials (16,17). The
preplanned analyses of the ToGA trial revealed the greatest
survival time gain in the subgroup of patients with HER2 IHC 3+
tumors, and in the TyTAN trial, the subgroup analysis based on HER2
IHC status identified significant survival time improvement only in
the IHC 3+ subgroup.
Furthermore, the present study identified that
patients with histology of intestinal-type demonstrated increased
PFS time with trastuzumab treatment, which supported the subgroup
result from the ToGA study in which patients with intestinal-type
tumors had increased survival rates on trastuzumab-based
chemotherapy (HR, 0.69; 95% CI, 0.54–0.88). The differences in
efficacy according to HER2 IHC status and histological type may be
partly explained by HER2 heterogeneity, which is more frequent in
gastric cancer than in breast cancer (25). Previously, Lee et al (25) reported that only 8 (32.0%) of the
patients studied exhibited heterogeneous HER2 overexpression in the
IHC 3+ subgroup whereas, in the IHC 2+ subgroup, it was identified
in 46 (95.8%) of the 48 cases. Furthermore, only 3 (14.3%) of 21
patients with diffuse or mixed types exhibited homogeneous HER2
overexpression vs. 16 (30.8%) of the 52 cases with histology of
intestinal type (25). Presumably,
selection of subclones lacking HER2 overexpression is likely to
confer resistance to anti-HER2 therapy in tumors with HER2
heterogeneity, and verification of this hypothesis is required in
the future. In the present study, patients who had a longer first
PFS (>6 months) benefited from continuing TBP, and this result
is in accord with that of a previous study on metastatic breast
cancer, in which patients with a longer first time-to-progression
(≥8.6 months) had significantly improved survival times following
first progression compared with those who had a shorter first
time-to-progression (median, 24.3 vs. 15.4 months, respectively;
P=0.024) (26). The dose of
trastuzumab was 8 mg/kg on day 1 of the first cycle, followed by 6
mg/kg every 3 weeks, and this dose was representative of doses used
by other studies (26). In the
current study, it is unclear why OS time was not statistically
different between the two groups, but it may be due to a high
percentage of third-line or later treatment and trastuzumab use
following second progression in the non-TBP group.
The limitations of this study are its retrospective
nature and small sample size. In addition, the TBP strategy
involves the additional cost of trastuzumab, and the current
standard care in second-line treatment for gastric cancer is
paclitaxel plus ramucirumab regardless of HER2 status (27); consequently, a certain degree of
prudence is required when adapting this strategy to patients.
Therefore, these findings require confirmation in a well-designed
prospective study.
There was no significant difference in toxicity
identified between the two groups, a finding that is consistent
with the results of the ToGA trial, in which the addition of
trastuzumab did not increase the toxic effects associated with
standard chemotherapy (16). In
addition, no cardiac dysfunction was observed in either group, in
contrast to the findings from a previous study of breast cancer
(28) and the ToGA trial. In the
study of breast cancer, the incidence rates of cardiotoxicity were
27% for patients who received trastuzumab combined with
anthracyclines and 13% for those who received paclitaxel (28). In the ToGA trial, the incidence rate
of cardiac events was as low as 6% for AGC patients (16). The lack of previous treatment with
anthracyclines, the short duration of exposure to trastuzumab, and
underestimation of cardiac dysfunction due to less-frequent
evaluation may lead to a lack of detection of cardiac events.
To summarize, although the retrospective nature of
this study and the small number of patients analyzed are major
limitations, these findings indicate that trastuzumab-combined
therapy provided a possible benefit of improved PFS time for
subgroups of AGC patients with HER2 IHC 3+ tumors, histology of
intestinal type, or a longer first PFS time (>6 months). An
ongoing randomized phase II study (UMIN000009297; target sample
size, n=90) comparing trastuzumab plus paclitaxel vs.
paclitaxel-only following disease progression in patients treated
with trastuzumab as first-line chemotherapy for HER2-positive AGC
is likely to provide further insights into this strategy.
Acknowledgements
The authors thank Enago (www.enago.jp) for the English language review.
Glossary
Abbreviations
Abbreviations:
|
AGC
|
advanced gastric cancer
|
|
CI
|
confidence intervals
|
|
CR
|
complete response
|
|
DCR
|
disease control rate
|
|
FISH
|
fluorescent in situ hybridization
|
|
HER2
|
human epidermal growth factor receptor
2
|
|
HR
|
hazard ratio
|
|
IHC
|
immunohistochemistry
|
|
non-TBP
|
discontinued trastuzumab beyond
progression
|
|
ORR
|
objective response rate
|
|
OS
|
overall survival
|
|
PFS
|
progression-free survival
|
|
PR
|
partial response
|
|
SD
|
stable disease
|
|
TBP
|
trastuzumab beyond progression
|
|
ToGA trial
|
Trastuzumab for Gastric Cancer
trial
|
|
TyTAN trial
|
Tykerb with Taxol in Asian ErbB2+
Gastric Cancer trial
|
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Glimelius B, Ekström K, Hoffman K, Graf W,
Sjödén PO, Haglund U, Svensson C, Enander LK, Linné T, Sellström H
and Heuman R: Randomized comparison between chemotherapy plus best
supportive care with best supportive care in advanced gastric
cancer. Ann Oncol. 8:163–168. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Murad AM, Santiago FF, Petroianu A, Rocha
PR, Rodrigues MA and Rausch M: Modified therapy with
5-fluorouracil, doxorubicin, and methotrexate in advanced gastric
cancer. Cancer. 72:37–41. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pyrhonen S, Kuitunen T, Nyandoto P and
Kouri M: Randomised comparison of fluorouracil, epidoxorubicin and
methotrexate (FEMTX) plus supportive care with supportive care
alone in patients with non-resectable gastric cancer. Br J Cancer.
71:587–591. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ajani JA, Moiseyenko VM, Tjulandin S,
Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi
E, et al: Clinical benefit with docetaxel plus fluorouracil and
cisplatin compared with cisplatin and fluorouracil in a phase III
trial of advanced gastric or gastroesophageal cancer
adenocarcinoma: The V-325 Study Group. J Clin Oncol. 25:3205–3209.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cunningham D, Starling N, Rao S, Iveson T,
Nicolson M, Coxon F, Middleton G, Daniel F, Oates J and Norman AR;
Upper Gastrointestinal Clinical Studies Group of the National
Cancer Research Institute of the United Kingdom, : Capecitabine and
oxaliplatin for advanced esophagogastric cancer. N Engl J Med.
358:36–46. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Koizumi W, Narahara H, Hara T, Takagane A,
Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama
W, et al: S-1 plus cisplatin versus S-1 alone for first-line
treatment of advanced gastric cancer (SPIRITS trial): A phase III
trial. Lancet Oncol. 9:215–221. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ford HE, Marshall A, Bridgewater JA,
Janowitz T, Coxon FY, Wadsley J, Mansoor W, Fyfe D, Madhusudan S,
Middleton GW, et al: Docetaxel versus active symptom control for
refractory oesophagogastric adenocarcinoma (COUGAR-02): An
open-label, phase 3 randomised controlled trial. Lancet Oncol.
15:78–86. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hironaka S, Ueda S, Yasui H, Nishina T,
Tsuda M, Tsumura T, Sugimoto N, Shimodaira H, Tokunaga S, Moriwaki
T, et al: Randomized, open-label, phase III study comparing
irinotecan with paclitaxel in patients with advanced gastric cancer
without severe peritoneal metastasis after failure of prior
combination chemotherapy using fluoropyrimidine plus platinum: WJOG
4007 trial. J Clin Oncol. 31:4438–4444. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kang JH, Lee SI, Lim DH, Park KW, Oh SY,
Kwon HC, Hwang IG, Lee SC, Nam E, Shin DB, et al: Salvage
chemotherapy for pretreated gastric cancer: A randomized phase III
trial comparing chemotherapy plus best supportive care with best
supportive care alone. J Clin Oncol. 30:1513–1518. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim HS, Kim HJ, Kim SY, Kim TY, Lee KW,
Baek SK, Kim TY, Ryu MH, Nam BH and Zang DY: Second-line
chemotherapy versus supportive cancer treatment in advanced gastric
cancer: A meta-analysis. Ann Oncol. 24:2850–2854. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aizawa M, Nagatsuma AK, Kitada K, Kuwata
T, Fujii S, Kinoshita T and Ochiai A: Evaluation of HER2-based
biology in 1,006 cases of gastric cancer in a Japanese population.
Gastric Cancer. 17:34–42. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Barros-Silva JD, Leitão D, Afonso L,
Vieira J, Dinis-Ribeiro M, Fragoso M, Bento MJ, Santos L, Ferreira
P, Rêgo S, et al: Association of ERBB2 gene status with
histopathological parameters and disease-specific survival in
gastric carcinoma patients. Br J Cancer. 100:487–493. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Takehana T, Kunitomo K, Kono K, Kitahara
F, Iizuka H, Matsumoto Y, Fujino MA and Ooi A: Status of c-erbB-2
in gastric adenocarcinoma: A comparative study of
immunohistochemistry, fluorescence in situ hybridization and
enzyme-linked immuno-sorbent assay. Int J Cancer. 98:833–837. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yano T, Doi T, Ohtsu A, Boku N, Hashizume
K, Nakanishi M and Ochiai A: Comparison of HER2 gene amplification
assessed by fluorescence in situ hybridization and HER2 protein
expression assessed by immunohistochemistry in gastric cancer.
Oncol Rep. 15:65–71. 2006.PubMed/NCBI
|
|
16
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
et al: Trastuzumab in combination with chemotherapy versus
chemotherapy alone for treatment of HER2-positive advanced gastric
or gastro-oesophageal junction cancer (ToGA): A phase 3,
open-label, randomised controlled trial. Lancet. 376:687–697. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Satoh T, Xu RH, Chung HC, Sun GP, Doi T,
Xu JM, Tsuji A, Omuro Y, Li J, Wang JW, et al: Lapatinib plus
paclitaxel versus paclitaxel alone in the second-line treatment of
HER2-amplified advanced gastric cancer in asian populations:
TyTAN-a randomized, phase III study. J Clin Oncol. 32:2039–2049.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dai GH, Shi Y, Chen L, Lv YL and Zhong M:
Trastuzumab combined with docetaxel-based regimens in previously
treated metastatic gastric cancer patients with HER2
over-expression. Hepatogastroenterology. 59:2439–2444.
2012.PubMed/NCBI
|
|
19
|
Jackisch C, Welslau M, Schoenegg W,
Selbach J, Harich HD, Schröder J, Schmidt M, Göhler T, Eustermann
H, Ringel R and Hinke A: Impact of trastuzumab treatment beyond
disease progression for advanced/metastatic breast cancer on
survival - results from a prospective, observational study in
Germany. Breast. 23:603–608. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
von Minckwitz G, du Bois A, Schmidt M,
Maass N, Cufer T, de Jongh FE, Maartense E, Zielinski C, Kaufmann
M, Bauer W, et al: Trastuzumab beyond progression in human
epidermal growth factor receptor 2-positive advanced breast cancer:
A German breast group 26/breast international group 03–05 study. J
Clin Oncol. 27:1999–2006. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
NCCN Clinical Practice Guidelines in
Oncology GC, Ver3. 2016.http://www.nccn.org/professionals/physician_gls/f_guidelines.aspOctober
30–2016
|
|
23
|
Nishino M, Jagannathan JP, Ramaiya NH and
Van den Abbeele AD: Revised RECIST guideline version 1.1: What
oncologists want to know and what radiologists need to know. AJR Am
J Roentgenol. 195:281–289. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
National Cancer Institute Common
Terminology Criteria for Adverse Events (CTCAE) version 4.0.
https://evs.nci.nih.gov/ftp1/CTCAE/About.html
|
|
25
|
Lee HE, Park KU, Yoo SB, Nam SK, do J
Park, Kim HH and Lee HS: Clinical significance of intratumoral HER2
heterogeneity in gastric cancer. Eur J Cancer. 49:1448–1457. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hayashi M, Okumura Y, Osako T, Toyozumi Y,
Arima N, Iwase H and Nishimura R: Time to first tumor progression
as a predictor of efficacy of continued treatment with trastuzumab
beyond progression in human epidermal growth factor receptor
2-positive metastatic breast cancer. Int J Clin Oncol. 16:694–700.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wilke H, Muro K, Van Cutsem E, Oh SC,
Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, et
al: Ramucirumab plus paclitaxel versus placebo plus paclitaxel in
patients with previously treated advanced gastric or
gastro-oesophageal junction adenocarcinoma (RAINBOW): A
double-blind, randomised phase 3 trial. Lancet Oncol. 15:1224–1235.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Seidman A, Hudis C, Pierri MK, Shak S,
Paton V, Ashby M, Murphy M, Stewart SJ and Keefe D: Cardiac
dysfunction in the trastuzumab clinical trials experience. J Clin
Oncol. 20:1215–1221. 2002. View Article : Google Scholar : PubMed/NCBI
|