Introduction
Retinoblastoma (RB) is the most common childhood
malignant ocular tumor, which is characterized by an abnormal
appearance of the pupil and leukocoria (1–3). A total
of >9,000 new cases of RB are reported annually (4), and the incidence may be increased in
developing countries; in certain Central and South American
countries RB is one of the most common tumor malignancies in youth
(5). Generally, onset of RB is
initiated by mutation of the RB1 gene, which was the first
tumor-suppressor gene to be described (6–8). The
majority of patients with RB are born with loss of one RB1
allele and subsequently lose the other one during the development
of retinal cells, eventually leading to the carcinogenesis of RB
within several years following birth (9).
Currently, treatment modalities for RB include
enucleation, systemic chemotherapy, external beam radiation
therapy, focal treatments, intra-arterial or subconjunctival
chemotherapy (10). However,
prognosis of these therapies depends on the stages of RB. To
improve the effectiveness of therapy, comprehensive understanding
of the mechanism involved in the oncogenesis and development of RB
is important. In RB cells, the expression status of numerous genes
is substantially altered. These differentially expressed genes
include components involved in the immune system, signaling
pathways, angiogenesis, cell structure and proliferation (11). Among the potential signaling
transductions involved in the genesis of RB, angiogenesis has been
demonstrated to be associated with local invasive growth and
metastasis of this cancer type (12).
In addition, a previous study by Marback et al (13) employed tumor angiogenesis as a
prognostic factor for disease dissemination of RB, which confirmed
the crucial role of angiogenesis in RB carcinogenesis. Therefore,
concatenated application of anti-angiogenic agents and traditional
therapies may be synergistic for a greater alleviation of the
impairments of RB (14).
Quercetin (Que; 3,30,40,5,7-pentahydroxylflavone) is
a typical flavonoid that is widespread in nature and found in
fruits, vegetables and plants (15,16).
Numerous studies on the effects of Que and associated flavonols on
signal transduction associated with carcinogenic processes have
been reported, including effects on cell cycle distribution,
apoptosis, pro-inflammatory protein induction and angiogenesis
(17). Que induced apoptosis in human
hepatoma HepG2 cells via activation of the mitochondrial signaling
pathway and blockade of Akt and extracellular signal-regulated
kinase (18). Duraj et al
(19) also demonstrated that Que
triggered DNA fragmentation, cleavage of poly-polymerase,
modification of B-cell lymphoma (Bcl) and upregulation of
Bcl-associated X protein in human leukemia cells. In addition, the
effect of Que on tumor angiogenesis was validated. Que inhibits
human prostate tumor growth in an angiogenesis inhibition-dependent
manner by targeting vascular endothelial growth factor receptor
(VEGFR)-2 (20). However, to the best
of our knowledge, few studies have focused on the treatment
potential of Que against RB. Therefore, it the present study
comprehensively investigated the effect of Que on the process of
angiogenesis in RB tumors and evaluated its potential as an anti-RB
agent.
In the present study, the human RB Y79 cell line was
employed as an in vitro model of RB. The cells were
subjected to various doses of Que. Cell viability, cell invasion
and migration ability, as well as cell apoptosis were subsequently
measured to demonstrate the effect of Que on RB cells. To verify
the possibility that Que exerted its function in an angiogenesis
inhibition-dependent manner, the expression of VEGFR, which is the
major mediator of the specific action of VEGF on endothelial cells
(20), was subsequently quantified
using reverse transcription-quantitative polymerase chain reaction
(RT-qPCR), western blotting and immunofluorescence assay. The
present study may provide a preliminary illustration of the effect
of Que on RB cells and promote the treatment of this malignant
cancer.
Materials and methods
Chemicals and cell culture
Que (>99% pure) was purchased from Sigma-Aldrich
(Merck KGaA, Darmstadt, Germany), dissolved in dimethyl sulfoxide
(DMSO), aliquoted and stored at −22°C. Antibodies against VEGFR
(cat. no. ab36844) and GAPDH (cat. no. ab8245) were purchased from
Abcam (Cambridge, UK). The human RB Y79 cell line (Shanghai Bioleaf
Biotech Co., Ltd., Shanghai, China), preserved at the Second
Hospital of Shandong University (Jinan, China), was cultured in
HyClone™ RPMI-1640 medium (GE Healthcare, Logan, UT, USA)
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and 1% (v/v) antibiotics
mixture (100 U/ml penicillin and 100 U/ml streptomycin) in an
atmosphere of 95% air and 5% CO2 at 37°C.
Administration of Que and VEGF
To assess the effect of Que on RB cells, Y79 cells
were divided into four groups: The control group, Y79 cells; the
LQUE group, Y79 cells incubated with 25 µM Que; the MQUE group, Y79
cells incubated with 50 µM Que; and the HQUE group, Y79 cells
incubated with 100 µM Que.
Cell Counting Kit-8 (CCK-8) assay
The cell viabilities of Y79 cells in different
groups were estimated by CCK-8 test. Briefly, 50 µl exponentially
growing cells (3×103 cells/ml) were seeded onto a
96-well plate and cultured for 72 h at room temperature. Every 24 h
subsequent to the start of the incubation, 5 mg/ml of CCK-8 was
added to five randomly selected wells in each group. Subsequent to
incubation for an additional 4 h at 37°C, 200 µl of DMSO was added
to each well, and the cell viability in the various treatments was
assessed by measuring the optical density values at 450 nm with a
microplate reader.
Transwell experiment
The Transwell experiment, which evaluated the
migration ability of Y79 cells in various groups, was performed.
Incubation medium (with 1 mM MgCl2; 200 µl) containing
1×104 cells was seeded into the upper Transwell chambers
(Costar; Corning Incorporated, Corning, NY, USA). The cells were
subsequently incubated at 37°C for 48 h to allow cell migration
through the porous membrane (pore size, 8 µm). Upon completion of
the culture, cells remaining on the upper surface of the chamber
were completely removed using a cotton swab. The lower surfaces of
the membranes were fixed with 4% paraformaldehyde for 20 min and
stained in a solution containing 0.5% (w/v) crystal violet for 5–10
min at room temperature. Subsequent to being washed with
ddH2O, results of different groups were observed using
the Olympus CX41 microscope (Olympus Corporation, Tokyo, Japan) at
×200 magnification and the numbers of cells were determined using
Image-Pro Plus 6.0 software (Nikon Corporation, Tokyo, Japan). The
invasion ability of Y79 cells was then measured as aforementioned,
with polycarbonate membranes pre-coated with 100 µl Matrigel (in
0.8 µg/µl Dulbecco's modified Eagle's medium; BD Biosciences, San
Jose, CA, USA) at 37°C for 2 h to form a reconstituted basement
membrane.
Flow cytometry assay
To assess the effect of Que administration on the
apoptotic process in Y79 cells in various groups, an Annexin
V-fluorescein isothiocyanate (FITC) Apoptosis Detection kit
(Jingmei Biotech Co., Ltd., Beijing, China) was employed according
to the manufacturer's protocol. The apoptotic rates were analyzed
using a FACScan flow cytometer (Accuri™ C6; BD Biosciences). The
apoptotic cell rate was equal to the sum of the late apoptotic rate
(upper right quadrant-advanced stage apoptosis cell percentage) and
the early apoptotic rate (lower right quadrant-prophase apoptosis
cell percentage).
RT-qPCR
For RT-qPCR detection, whole RNA in cells from
various groups was extracted using TRIzol reagent according to the
manufacturer's protocol (Takara Bio, Inc., Otsu, Japan). GAPDH was
selected as the reference gene. cDNA templates were achieved by
reverse transcribing the RNA using a RT-PCR kit (DBI Bioscience,
Shanghai, China), and the final RT-qPCR reaction mixture of volume
20 µl contained 10 µl of Bestar® SYBR-Green qPCR master
mix (DBI Bioscience, Shanghai, China), 0.5 µl of each primer (VEGFR
forward, 5′-CTCTCTCTGCCTACCTCACCTG-3′ and reverse,
5′-CGGCTCTTTCGCTTACTGTTC-3′; and GAPDH forward,
5′-TGTTCGTCATGGGTGTGAA-3′ and reverse, 5′-ATGGCATGGACTGTGGTCAT-3′;
Sangon Biotech, Shanghai, China), 2 µl of the cDNA template and 7
µl of RNase-free H2O. Thermocycling parameters for the
amplification were as follows: A denaturation step at 95°C for 2
min; followed by 40 cycles at 94°C for 20 sec; 58°C for 20 sec; and
72°C for 30 sec. Relative expression levels of targeted genes were
calculated with Data Assist Software version 3.0 (Applied
Biosystems; Thermo Fisher Scientific, Inc.) according to the
expression of 2−∆∆Cq (21).
Western blot analysis
The protein in various samples was extracted for
western blot analysis. GAPDH was used as reference protein.
Concentrations of protein samples were determined using the
bicinchoninic acid method, and 40 µg of protein was subject to a
10% SDS-PAGE. Following transfer of targeted proteins to
polyvinylidene difluoride membranes, the membranes were washed with
TBS-Tween-20 (TTBS) for 5 min and subsequently incubated with skim
milk powder solution for 1 h at room temperature. Primary
antibodies against VEGFR (dilution, 1:1,500) or GAPDH (dilution,
1:1,000) were incubated with membranes at 4°C overnight. Following
an additional four washes using TTBS, secondary horseradish
peroxidase-conjugated IgG antibodies (cat. no. A0216; dilution,
1:20,000; Beyotime Institute of Biotechnology, Haimen, China) were
added and incubated with the membranes for 45 min at 37°C.
Following another six washes using TTBS, the blots were developed
using Beyo ECL Plus reagent (Beyotime Institute of Biotechnology)
and the results were recorded in the Gel Documentation System
(Liuyi, Inc., Beijing, China). The relative expression levels of
gremlin 1 and GLI family zinc finger 3 in various samples were
calculated with Gel-Pro-Analyzer (Media Cybernetics, Inc.,
Rockville, MD, USA).
Immunofluorescence assay
The expression of VEGFR in various groups was
detected using immunofluorescence microscopy. Briefly, cells
(2×106) were transferred into a 1.5 ml Eppendorf tube,
and supernatant was then discarded following centrifugation for 5
min at 447 × g at 37°C. Subsequent to being washed three times with
PBS, cells were centrifuged for 5 min at 447 × g at 37°C.
Subsequently, cells were transferred to 24-well plates and fixed
with 4% paraformaldehyde for 15 min. The cells were then
permeabilized with 0.5% Triton X-100 for 30 min at room
temperature. Subsequent to being washed with PBS for three cycles,
5 min for each, the cells were blocked in 10% goat serum (Thermo
Fisher Scientific, Inc.) for 15 min. Primary rabbit polyclonal
antibodies (dilution, 1:200) to VEGFR were subsequently added and
the cells were incubated overnight at 4°C in 1% goat serum.
Staining was performed by incubating the cells with FITC-conjugated
secondary antibody (cat. no. A0521; Beyotime Biotechnology,
Shanghai, China) at dilution of 1:1,000 for 1 h. Following
incubation with the secondary antibody, cells were washed and
stained with FITC for 5 min at room temperature. Following three
cycles of 5-min washes with PBS buffer, the cells were fixed in
slides and imaged with fluorescent microscopy at ×400
magnification.
Statistical analysis
All the data were expressed as the mean ± standard
deviation. One-way analysis of variance and post hoc multiple
comparisons using a least significant difference method were
conducted using SPSS version 19.0 (IBM SPSS, Armonk, NY, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Administration of Que inhibits
proliferation, migration and invasion in Y79 cells
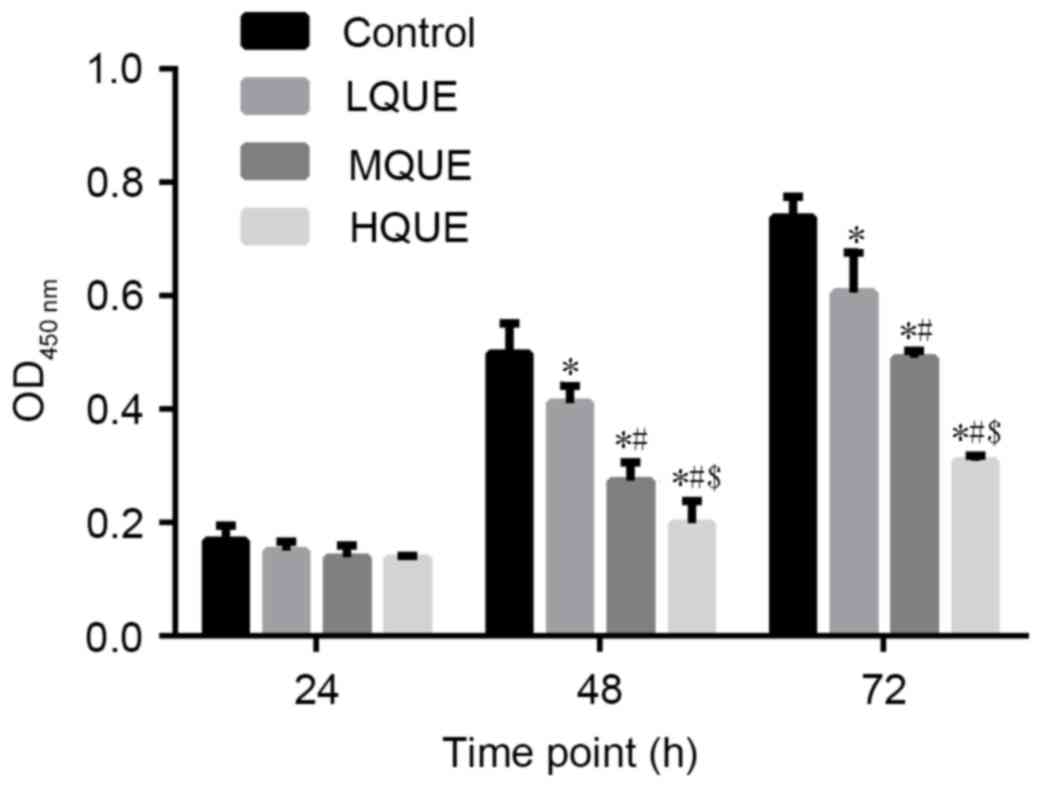
The viability of Y79 cells subsequent to being
subjected to Que was examined by CCK-8 assay. A decrease in cell
viability due to Que administration was observed for cells sampled
from 48 and 72 h (Fig. 1). The
difference between the control group and the MQUE group or the
control group and the LQUE group was statistically significant for
the last two time points (P<0.05). In addition, Que affected Y79
cells in a dose-dependent manner, with 100 Μm Que causing the
strongest inhibitory effect on the proliferation of Y79 cells. The
differences between the LQUE and HQUE groups, the LQUE and MQUE
groups, and the MQUE and HQUE groups were all statistically
significant for the last two time points (P<0.05).
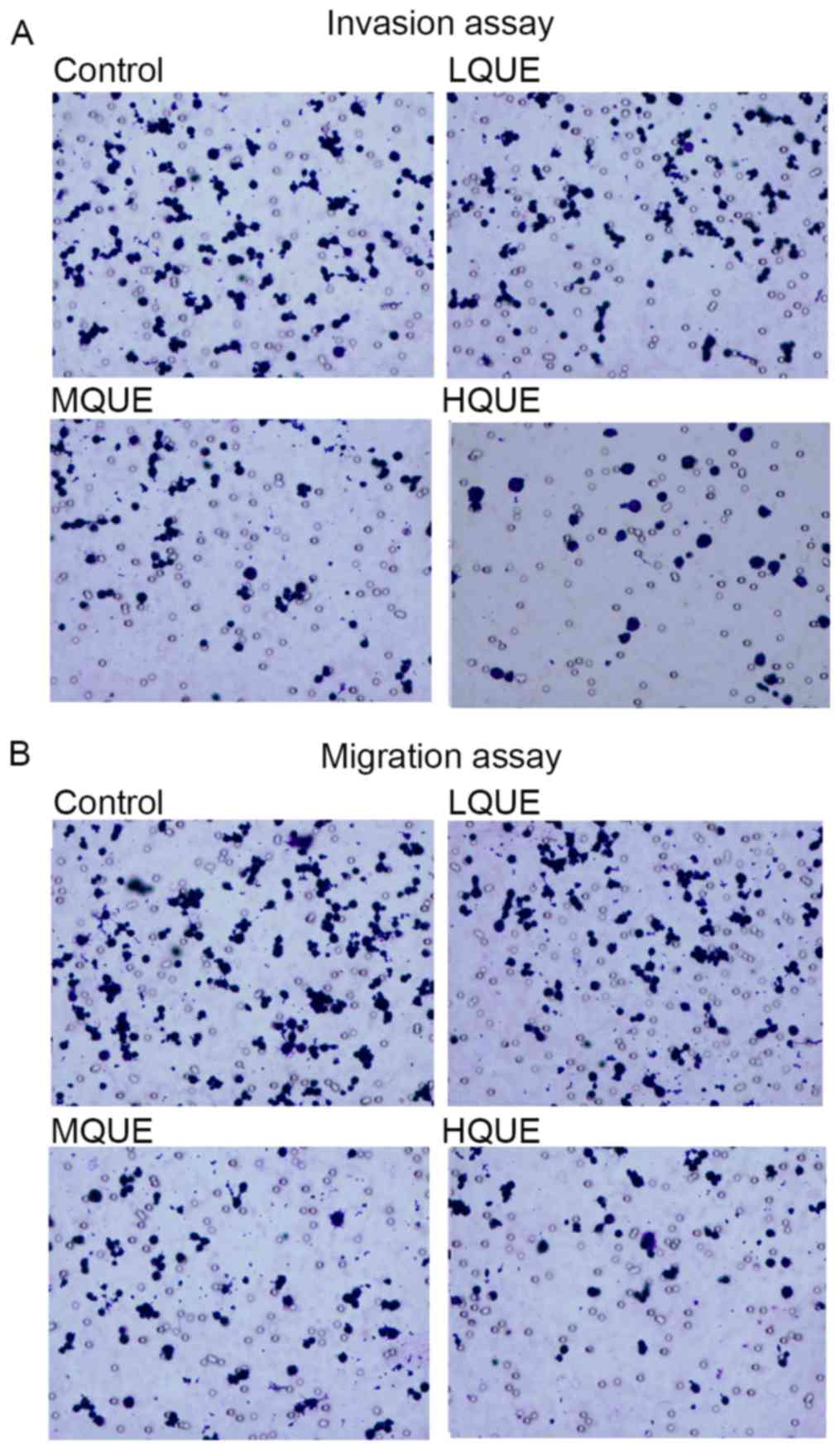
Migration and invasion of Y79 cells was detected
using Transwell experiments. Exposure to Que markedly reduced the
cell numbers moving through the porous membranes (Fig. 2A and B), representing the potential of
Y79 to inhibit the metastasis of RB.
Administration of Que induces
apoptosis in Y79 cells
The apoptotic process in Y79 cells was also induced
by Que administration (Fig. 3).
Apoptotic rate of the control group (15.4±1.0%) was significantly
different from the other three groups (28.1±0.9% for the LQUE
group, 29.3±0.7% for the MQUE group and 42.8±0.8% for the HQUE
group) (P<0.05). The effect of Que on the apoptosis of Y79 cells
was also dose-dependent, but only the data of the HQUE group was
significantly increased compared with the other two groups
(P<0.05).
Administration of Que downregulates
the level of VEGF at the mRNA and protein levels
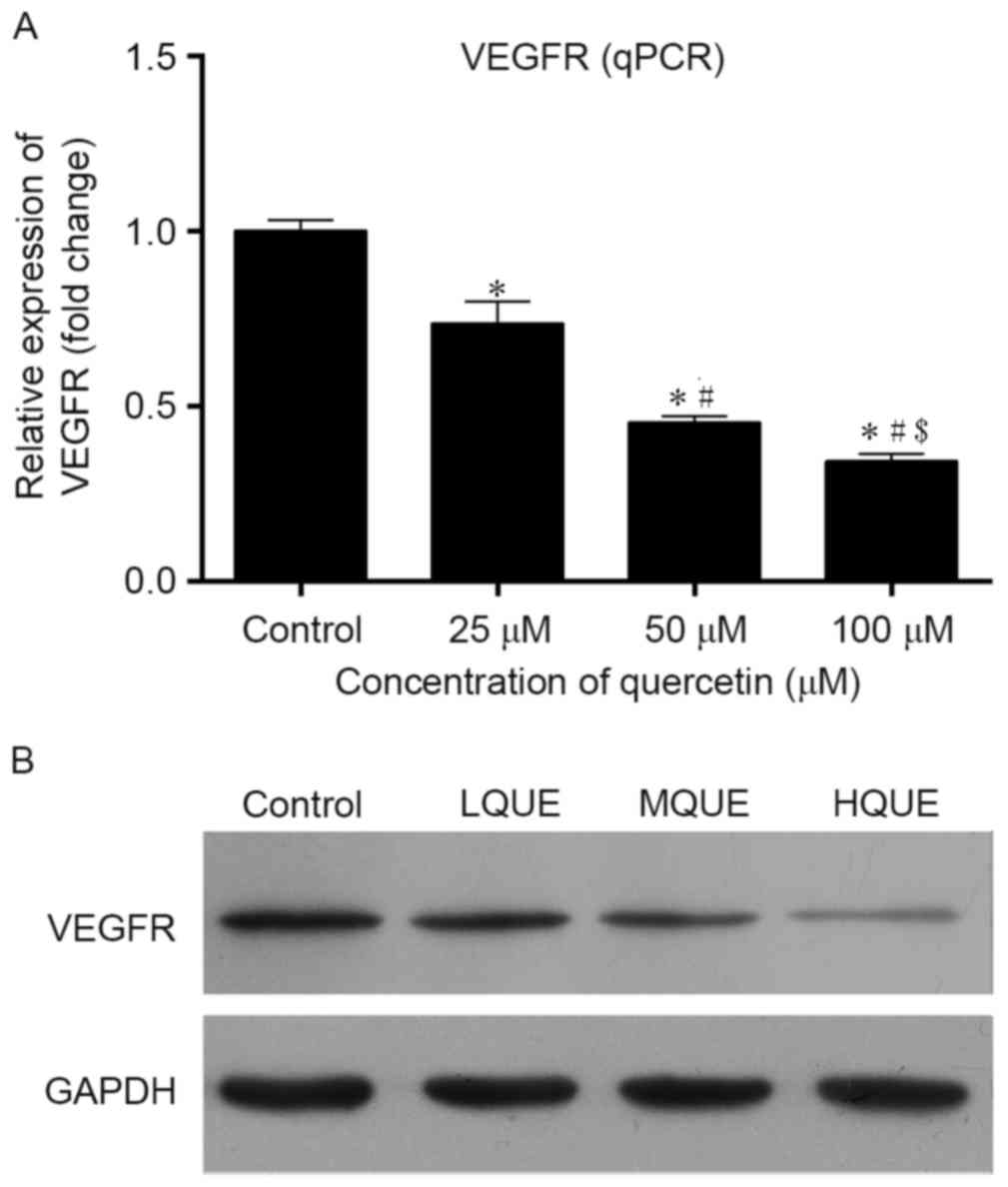
Expression of VEGF in Y79 cells post-Que
administration was detected using RT-qPCR, western blotting and
immunofluorescence assay. At the mRNA level, treatment of Que
reduced the transcription of VEGFR, and the differences between the
control group and the other three groups were all statistically
significant (P<0.05; Fig. 4A). In
addition, the effect of Que on the transcription of VEGFR was also
dose-dependent. Similar results were also detected for western blot
analysis; Que administration inhibited the expression of VEGFR in a
dose-dependent manner (Fig. 4B). For
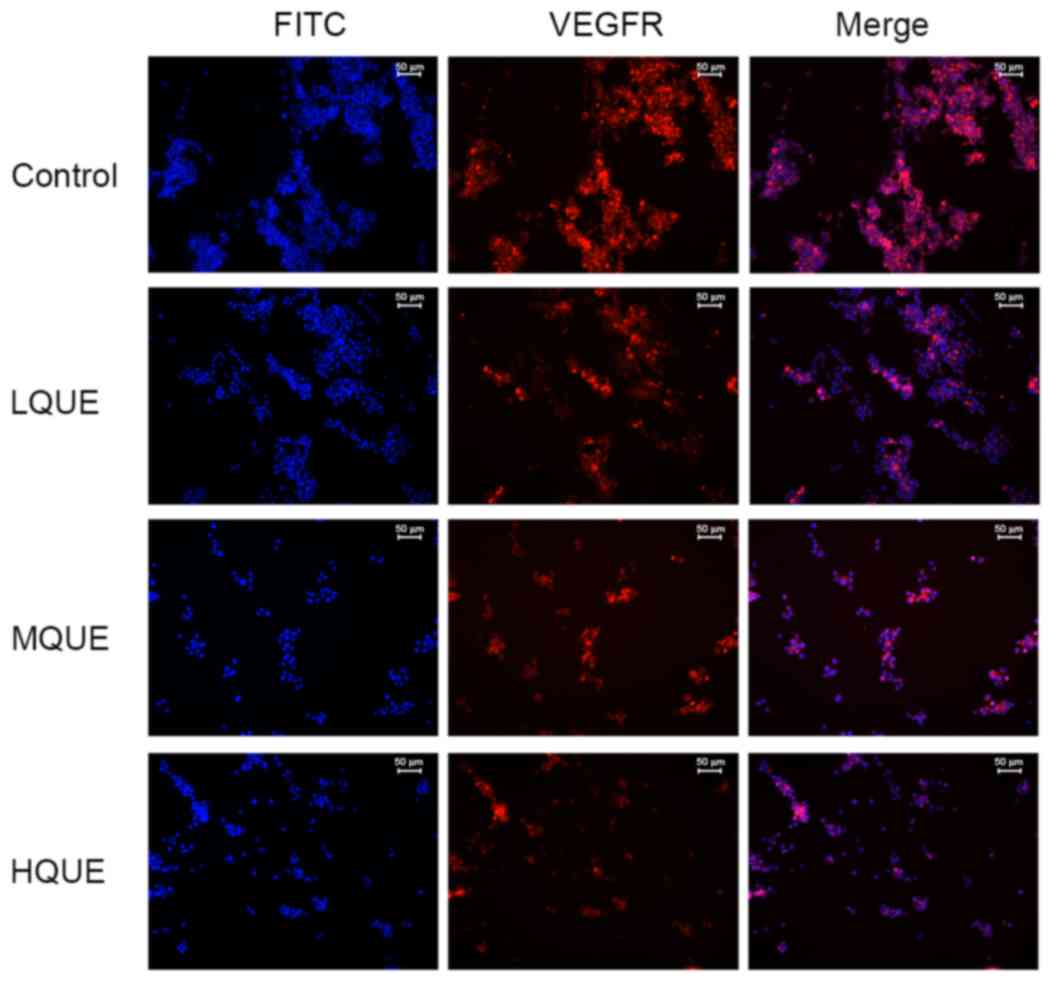
detection of the immunofluorescence assay, as shown in Fig. 5, VEGFR-positive cells were stained red
and FITC-positive cells were stained blue. It was demonstrated that
Que administration decreased the distribution and amount of VEGFR
in Y79 cells. These results confirmed the antagonizing effect of
Que on the process of angiogenesis in RB cells.
Discussion
Numerous attempts have been made to investigate risk
factors in patients with RB, for development of metastatic cases
and for extension of RB locally into the orbit (22–24). The
majority of these previous studies focused on the degree or extent
to which tumor cells invade the optic nerve or choroid in
enucleated eyes, and reported a positive association between tumor
cell invasion and an increased risk of disseminated disease
(22,23,25,26).
Currently, the most effective therapy against RB is chemotherapy
combined with immunotherapy, which may enhance cytotoxicity on RB
by increasing apoptosis (27).
Despite its high efficiency, the current treatment scheme remains
unsatisfactory considering the general side effects of
chemotherapy. Thus, development of mild therapeutic targets to
promote treatment of RB is imperative. In the present study, the
effect of Que on RB cells was evaluated. Que has previously been
demonstrated to be potent inhibitor against certain prostate,
ovarian and colon cancer (20,28,29).
Based on the results of the present study, it was revealed that Que
is able to inhibit the proliferation, invasion and migration
ability, and induces apoptosis in RB cells. In addition, it was
demonstrated that Que downregulated angiogenesis in RB cells, which
may be the major mechanism through which Que exerted its treatment
effect on RB.
It is well known that tumor growth and the formation
of hematogenous metastasis depend on angiogenesis, and as a
response, tumor cells possessing metastatic potential have
accumulated mutations to induce angiogenesis (30,31).
Therefore, suppression of angiogenesis may be an important target
to inhibit tumor growth and metastasis. Several anti-angiogenic
strategies have been developed by targeting various components of
tumor angiogenesis (32,33). Of all the potential treatment
modalities, numerous phytochemicals have demonstrated potency as
antiangiogenic agents during the investigation of cancer
development and metastasis (20). In
the present study, the cytotoxicity of Que on the human RB Y79 cell
line was assessed. The results revealed that Que reduced cell
viability and mobility of RB cells, and also induced apoptosis,
leading to tumor cell death. These results supported the hypothesis
that Que may be a promising agent in the treatment of RB.
Among types of pro-angiogenic mechanisms, the VEGF
signaling pathway has been implicated as the central mediator of
tumor neovascularization (34). As an
attractive therapeutic target, VEGF has been demonstrated to be
associated with initiation of angiogenesis by regulating
proliferation, migration and differentiation of endothelial cells
(35). The function of VEGF is
mediated through the activation of receptor tyrosine kinases. In
the present study, Que significantly inhibited the level of VEGFR
in Y79 cells, which represented the blockade of the VEGF signaling
pathway. The present data reported a similar result to a previous
study by Pratheeshkumar et al (20) based on prostate cancer, in which the
authors reported that Que administration inhibited the activation
of VEGFR2, and thereby suppressed the downstream Akt/mechanistic
target of rapamycin/P70S6K-mediated angiogenesis signal
transduction pathways. A dose-dependent effect of Que on RB cells
and VEGFR expression was also observed. However, the suitable
concentration of Que for treating RB in the clinic requires
additional study.
In conclusion, Que inhibited RB growth and invasion
in vitro in a dose-dependent manner. In addition, Que
blocked angiogenesis in RB by targeting VEGF. Thus, it may be
proposed that Que is a potential anti-RB therapy based on its
anti-angiogenic effect. Although the inhibitory effects of Que
in vitro were evident, additional studies are required to
achieve a comprehensive understanding of the effect and mechanism
of this compound on angiogenesis in RB.
Acknowledgements
The present study was supported by the Youth
Foundation of the Second Hospital of Shandong University (grant no.
Y2013010064).
References
|
1
|
Menon BS, Alagaratnam J, Juraid EA,
Mohamed M, Ibrahim H and Naing NN: Late presentation of
retinoblastoma in Malaysia. Pediatr Blood Cancer. 52:215–217. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bowman RJ, Mafwir MI, Luther P, Luande J
and Wood M: Outcome of retinoblastoma in east Africa. Pediatr Blood
Cancer. 50:160–162. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Abramson DH, Frank CM, Susman M, Whalen
MP, Dunkel IJ and Boyd NW: Presenting signs of retinoblastoma. J
Pediatr. 132:505–508. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kivelä T: The epidemiological challenge of
the most frequent eye cancer: Retinoblastoma, an issue of birth and
death. Brit J Ophthalmol. 93:1129–1131. 2009. View Article : Google Scholar
|
|
5
|
Leal-Leal C, Flores-Rojo M, Medina-Sansón
A, Cerecedo-Díaz F, Sánchez-Félix S, González-Ramella O,
Pérez-Pérez F, Gómez-Martínez R, Quero-Hernández A,
Altamirano-Alvarez E, et al: A multicentre report from the Mexican
Retinoblastoma Group. Br J Ophthalmol. 88:1074–1077. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Comings DE: A general theory of
carcinogenesis. Proc Natl Acad Sci USA. 70:3324–3328. 1973.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Friend SH, Bernards RA, Rogelj S, Weinberg
RA, Rapaport JM, Albert DM and Dryja TP: A human DNA segment with
properties of the gene that predisposes to retinoblastoma and
osteosarcoma. Nature. 323:643–646. 1986. View Article : Google Scholar
|
|
8
|
Alfred G and Knudson AG Jr: Mutation and
cancer: Statistical study of retinoblastoma. Proc Natl Acad Sci
USA. 68:820–823. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dimaras H, Kimani K, Dimba EA, Gronsdahl
P, White A, Chan HS and Gallie BL: Retinoblastoma. Lancet.
379:1436–1446. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shields CL and Shields JA: Basic
understanding of current classification and management of
retinoblastoma. Curr Opin Ophthalmol. 17:228–234. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Saxena P and Kaur J: Differential
expression of genes in retinoblastoma. Clin Chim Acta.
412:2015–2021. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rossler J, Dietrich T, Pavlakovic H,
Schweigerer L, Havers W, Schüler A, Bornfeld N and Schilling H:
Higher vessel densities in retinoblastoma with local invasive
growth and metastasis. Am J Pathol. 164:391–394. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Marback EF, Arias VEA, Paranhos A Jr,
Soares FA, Murphree AL and Erwenne CM: Tumour angiogenesis as a
prognostic factor for disease dissemination in retinoblastoma. Br J
Ophthalmol. 87:1224–1228. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Houston SK, Murray TG, Wolfe SQ and
Fernandes CE: Current update on retinoblastoma. Int Ophthalmol
Clin. 51:77–91. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Slusarz A, Shenouda NS, Sakla MS,
Drenkhahn SK, Narula AS, MacDonald RS, Besch-Williford CL and
Lubahn DB: Common botanical compounds inhibit the hedgehog
signaling pathway in prostate cancer. Cancer Res. 70:3382–3390.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Comalada M, Camuesco D, Sierra S,
Ballester I, Xaus J, Gálvez J and Zarzuelo A: In vivo quercitrin
anti-inflammatory effect involves release of quercetin, which
inhibits inflammation through down-regulation of the NF-kappaB
pathway. Eur J Immunol. 35:584–592. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Murakami A, Ashida H and Terao J:
Multitargeted cancer prevention by quercetin. Cancer Lett.
269:315–325. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Granado-Serrano AB, Martin MA, Bravo L,
Goya L and Ramos S: Quercetin induces apoptosis via caspase
activation, regulation of Bcl-2 and inhibition of PI-3-kinase/Akt
and ERK pathways in a human hepatoma cell line (HepG2). J Nutr.
136:2715–2721. 2006.PubMed/NCBI
|
|
19
|
Duraj J, Zazrivcova K, Bodo J, Sulikova M
and Sedlak J: Flavonoid quercetin, but not apigenin or luteolin,
induced apoptosis in human myeloid leukemia cells and their
resistant variants. Neoplasma. 52:273–279. 2004.
|
|
20
|
Pratheeshkumar P, Budhraja A, Son YO, Wang
X, Zhang Z, Ding S, Wang L, Hitron A, Lee JC, Xu M, et al:
Quercetin inhibits angiogenesis mediated human prostate tumor
growth by targeting VEGFR-2 regulated AKT/mTOR/P70S6K signaling
pathways. PLoS One. 7:e475162012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Dela Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Merriam GR Jr: Retinoblastoma; analysis of
17 autopsies. Arch Ophthal. 44:71–108. 1950. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Carbajal UM: Metastasis in retinoblastoma.
Am J Ophthalmol. 48:47–69. 1959. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Taktikos A: Investigation of
retinoblastoma with special reference to histology and prognosis.
Br J Ophthalmol. 50:225–234. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rubin CM, Robison LL, Cameron JD, Woods
WG, Nesbit ME Jr, Krivit W, Kim TH, Letson RD and Ramsay NK:
Intraocular retinoblastoma group V: An analysis of prognostic
factors. J Clin Oncol. 3:680–685. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kopelman JE, McLean IW and Rosenberg SH:
Multivariate analysis of risk factors for metastasis in
retinoblastoma treated by enucleation. Ophthalmology. 94:371–377.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu Q, Wang Y, Wang H, Liu Y, Liu T and
Kunda PE: Tandem therapy for retinoblastoma: Immunotherapy and
chemotherapy enhance cytotoxicity on retinoblastoma by increasing
apoptosis. J Cancer Res Clin Oncol. 139:1357–1372. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ren MX, Deng XH, Ai F, Yuan GY and Song
HY: Effect of quercetin on the proliferation of the human ovarian
cancer cell line SKOV-3 in vitro. Exp Ther Med. 10:579–583.
2015.PubMed/NCBI
|
|
29
|
Park CH, Chang JY, Hahm ER, Park S, Kim HK
and Yang CH: Quercetin, a potent inhibitor against beta-catenin/Tcf
signaling in SW480 colon cancer cells. Biochem Biophys Res Commun.
328:227–234. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Folkman J, Watson K, Ingber D and Hanahan
D: Induction of angiogenesis during the transition from hyperplasia
to neoplasia. Nature. 339:58–61. 1989. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liotta LA, Steeg PS and Stetler-Stevenson
WG: Cancer metastasis and angiogenesis: An imbalance of positive
and negative regulation. Cell. 64:327–336. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tang N, Shi L, Yu Z, Dong P, Wang C, Huo
X, Zhang B, Huang S, Deng S, Liu K, et al: Gamabufotalin, a major
derivative of bufadienolide, inhibits VEGF-induced angiogenesis by
suppressing VEGFR-2 signaling pathway. Oncotarget. 7:3533–3547.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang Z, Zhang H, Peng T, Li D and Xu J:
Melittin suppresses cathepsin S-induced invasion and angiogenesis
via blocking of the VEGF-A/VEGFR-2/MEK1/ERK1/2 pathway in human
hepatocellular carcinoma. Oncol Lett. 11:610–618. 2016.PubMed/NCBI
|
|
34
|
Keck PJ, Hauser SD, Krivi G, Sanzo K,
Warren T, Feder J and Connolly DT: Vascular permeability factor, an
endothelial cell mitogen related to PDGF. Science. 246:1309–1312.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tie J and Desai J: Antiangiogenic
therapies targeting the vascular endothelia growth factor signaling
system. Crit Rev Oncog. 17:51–67. 2012. View Article : Google Scholar : PubMed/NCBI
|