Introduction
Platinum-based two-drug combination chemotherapy is
currently the first-line chemotherapy regimen for non-small cell
lung cancer (NSCLC) (1). Platinum
would be hydrolysed in the tumor cells and form DNA-platinum
complex, which prevents the DNA replication to exert cytotoxic
effects. However, the resistance of tumor cells against cisplatin
would seriously influence the treatment efficacy for NSCLC. Up to
now, nucleotide excision repair (NER) has been recognized as one of
the important mechanisms for the cisplatin resistance, in which
excision repair cross-complementation 1 (ERCC1) is known to be
critically involved.
ERCC1 is a single-strand DNA endonuclease, which is
located on the 19q13.2 chromosome in human beings. ERCC1 is the
rate-limiting enzyme for the NER pathway, which plays an important
role in the DNA repairing process. The expression level of ERCC1
reflects the DNA repair capability (DRC). Downregulated DRC
expression delays the DNA repairing process and results in
increased susceptibility to lung cancers. In contrast,
overexpression of ERCC1 contributes to the repairing of
DNA-platinum complex, leading to platinum resistance. At present,
retrospective clinical studies concerning ERCC1 mainly focus on
NSCLC patients at advanced stage and/or after surgery. Due to the
differences in experimental design and result evaluation, it is
still controversial for the effects of ERCC1 expression on the
prognosis of NSCLC (2–5). Several studies show that the expression
of ERCC1 can be used as a predictor for the sensitivity to
cisplatin (6–9). The mRNA level of ERCC1 in the tumor
tissue from patients with advanced NSCLC has been shown to be
closely associated with the response to the two-drug combination
chemotherapy (10). A meta-analysis
further shows that ERCC1 is the important indicator for the
response rate of patients with advanced NSCLC to platinum and the
overall survival (OS) rate (11).
The p38 signaling pathway is an important branch of
the MAPK pathway, which is involved in various physiological
processes, including inflammation, cell proliferation, and
apoptosis (12). As stress signals,
chemotherapeutic drugs can activate the p38 signaling pathway in a
variety of tumor cells. The p38 signaling pathway has been shown to
be closely related to the resistance of tumors, and its inhibitor
could enhance the tumor sensitivity to chemotherapeutic agents
(13,14). The chemotherapeutic agent-activated
p38 MAPK signal breaks the dynamic balance between the p38 and PERK
signaling pathways to inhibit the proliferation of tumor cells and
force the cells into dormancy, resulting in drug resistance
(15,16). Our previous study has shown that the
p38 inhibitor BIRB796 could specifically suppress the function of
membrane transporter ABCB1, and thereby reverse the drug resistance
to chemotherapeutic agents, such as doxorubicin, paclitaxel, and
vincristine (17).
Clinical studies concerning the advanced NSCLC
patients have confirmed the association between the ERCC1
expression and the cisplatin response rate and disease prognosis.
However, inconsistent findings have been obtained for the patients
at stages I–III after surgery, and relationship between the p38
signaling pathway and the tumor drug resistance still needs to be
elucidated. In this study, the effects of ERCC1 expression on the
prognosis of NSCLC were investigated, especially concerning its
association with the p38 signaling pathway.
Materials and methods
Cell line and cell culture
Human lung carcinoma cell line A549 was purchased
from ATCC. These cells were cultured with the RPMI-1640 complete
medium (Gibco-BRL, Grand Island, NY, USA) containing 10% fetal calf
serum (FCS), supplemented with 2.2% (w/v) sodium bicarbonate, 0.03%
(w/v) L-glutamine, as well as 100 U/ml penicillin and 100 mg/ml
streptomycin, in a 37°C, 5% CO2 incubator.
Study subjects
Totally 343 patients with NSCLC (squamous carcinoma
or adenocarcinoma) were screened in this study, who had admitted to
the Affiliated Tumor Hospital, Xinjiang Medical University and
received standard lung cancer resection (i.e., lobectomy and
systematic mediastinal lymph node dissection), from January 1, 2010
to December 31, 2013. Inclusion criteria were as follows: i)
patients with complete follow-up data; ii) patients who had not
received chemotherapy before surgery; iii) patients with cancer at
stage II or III who received systematic platinum-based two-drug
combination chemotherapy for four cycles or radiotherapy after
surgery; iv) patients with cancer at stage I who did not receive
systematic chemotherapy until tumor recurrence during the follow-up
period; and v) patients from whom the tumor and adjacent tissues
were obtained. After screening, 140 patients were finally included
in this study. Prior written and informed consent were obtained
from every patient and the study was approved by the ethics review
board of the Affiliated Tumor Hospital, Xinjiang Medical
University.
Post-operative follow-up
These 140 patients with NSCLC were followed up by
telephone, which begun from the date of surgery and ended at Jun 1,
2015. Endpoint events included tumor recurrence and patient death.
Evaluation indexes included the 1-, 2-, and 3-year disease-free
survival (DFS) and OS rates.
Immunohistochemistry
The expression of ERCC1 was detected with
immunohistochemistry, according to Hubner et al (18). The tumor and adjacent tissues from
NSCLC patients were obtained and cut into 5-µm sections. After
dewaxing and rehydration, these sections were treated with 3%
H2O2 for 20 min, followed by antigen
retrieval for 10 min. After blocked with serum blocking solution at
room temperature for 30 min, these sections were incubated with
mouse anti-human anti-ERCC1 monoclonal antibody (1:100 dilution;
ab2356; Abcam, Cambridge, MA, USA) at 4°C overnight. Then the
sections were incubated with biotinylated secondary antibody at
37°C for 1 h. After washing, these sections were treated with SABC
agent and subjected to DAB colorization. After hematoxylin
staining, dehydration, xylene clearing, and neutral resin sealing,
the sections were observed under microscope.
Immunohistochemical assessment was performed by two
independent senior physicians from the Department of Pathology,
according to the evaluation and scoring criteria from Planchard
et al (19). Five fields with
high magnification (×400) were randomly selected from each section,
and totally 100 cells were counted. Semi-quantitative H-score
indicating the relative protein expression level was obtained as
the product of the staining intensity score and the positive tumor
cell percentage. For the staining intensity score: 0, negative (no
staining); 1, weak positive (light yellow staining); 2, positive
(dark yellow staining); and 3, strong positive (brown staining).
For the positive tumor cell percentage: 0, no positive tumor cells;
0.1, 1–9%; 0.5, 10–50%, and 1.0, >50%.
MTT assay
Cell proliferation was assessed with the MTT assay
(20). Briefly, cells were seeded
onto 96-well plates and cultured overnight. The cells were
pre-incubated with or without the p38MAPK inhibitor BIRB796
(SelleckChem, Houston, TX, USA) for 1 h and then with
diamminedichloroplatinum (DDP; Sigma-Aldrich, St. Louis, MO, USA)
at indicated concentrations. After 68 h, these cells were treated
with 20 µl MTT (4 mg/ml) for 4 h. After the medium was discarded,
120 µl dimethylsulfoxide (DMSO) was added into each well. The
absorbance at 655 nm was read by the Model 550 microplate reader
(Bio-Rad, Hercules, CA, USA).
Western blot analysis
After treatment, cells were lysed with lysis buffer.
The protein concentration was determined, and protein sample was
subjected to SDS-PAGE and then transferred onto the nitrocellulose
membrane. After blocked with 5% non-fat milk in TBST at room
temperature for 2 h, the membrane was incubated with primary
antibodies against p38 (ab170099), p-p38 (ab178867), β-tubulin
(ab6046) and ERCC1 (ab2356) (all from Abcam) at 4°C overnight. The
membrane was then incubated with HRP-conjugated secondary antibody
(1:5,000 dilution; Abcam) at room temperature for 2 h. After
washing with TBST, the protein bands were visualized by the
enhanced Phototope™-HRP Detection kit (Cell Signaling Technology,
Beverly, MA, USA) and exposed to Kodak medical X-ray processor
(Carestream Health, Rochester, NY, USA). The p38 was used as
loading control.
Quantitative real-time PCR
After treatment, total RNA was extracted with the
TRIzol reagent RNA Extraction kit (Molecular Research Center,
Cincinnati, OH, USA), following the manufacturer's instructions.
The first-strand cDNA was synthesized by RevertAid™ Premium
First-Strand cDNA Synthesis kit (Fermentas International Inc.,
Burlington, ON, Canada). The PCR primer sequences were as follows:
ERCC1 forward, 5′-TGTCCAGGTGGATGTGAAAGAT-3′ and reverse,
5′-GGCCTTGTAGGTCTCCAGGTA −3′; and GAPDH forward,
5′-TGTTGCCATCAATGACCCCTT-3′ and reverse, 5′-CTCCACGACGTACTCAGCG-3′.
PCR was performed on the Gene Amp PCR system 9700 (PE Applied
Biosystems, Foster City, CA, USA), with the following conditions:
Denaturation at 94°C for 2 min; then 95°C for 30 sec, 61°C for 30
sec, and 72°C for 1 min, for totally 30 cycles; followed by
extension at 72°C for 10 min. Products were resolved and examined
by 1.0% agarose gel electrophoresis. Quantitative real-time PCR was
performed on the Bio-Rad CFX96™ real-time system (Applied
Biosystems, Framingham, MA, USA). Relative mRNA expression levels
of ERCC1 were determined with the 2−ΔΔCt method.
Statistical analysis
SPSS20.0 software was used for statistical analysis.
The χ2 test and non-parametric Wilcoxon rank sum test
were performed for the comparison of clinical data. The
Kaplan-Meier analysis with Breslow test was used for the survival
analysis. Cox regression model was applied for the multivariate
analysis. P<0.05 was considered as statistically
significant.
Results
ERCC1 expression is elevated in NSCLC
tumor tissue
To investigate the expression levels of ERCC1 in the
NSCLC tumor and adjacent tissues, immunohistochemistry was
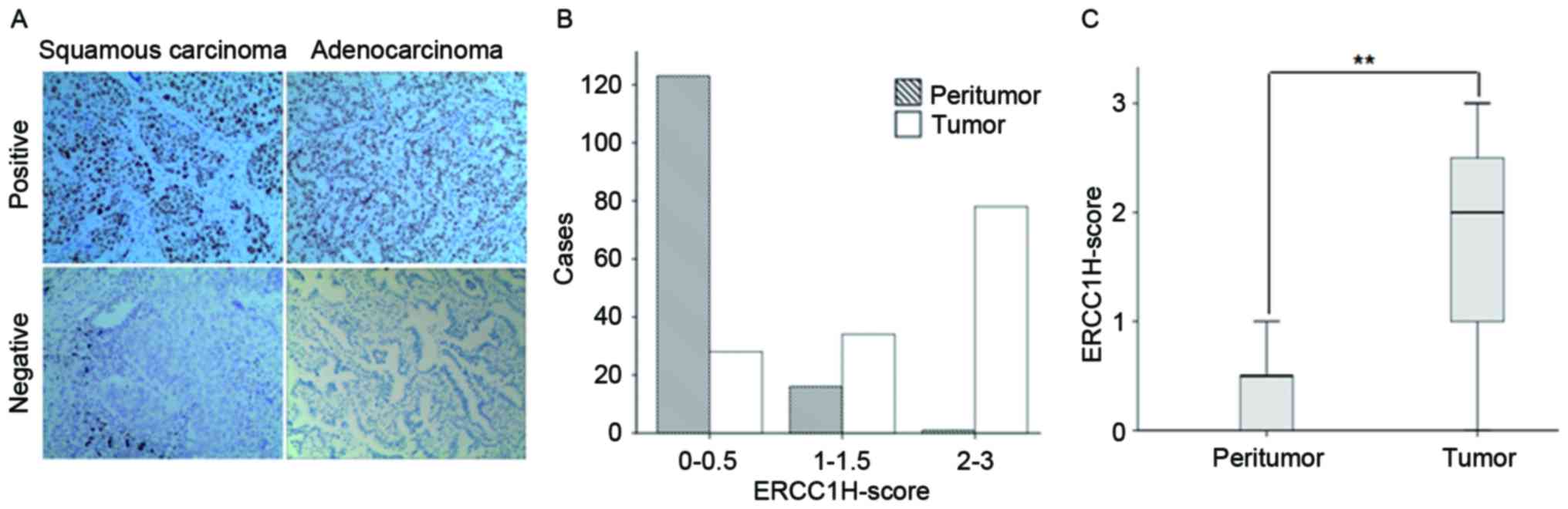
performed. Positive and negative staining of ERCC1 in the tissues
from NSCLC (squamous carcinoma and adenocarcinoma) was shown in
Fig. 1A. The positive staining of
ERCC1 was mainly located in the nucleus. The H-score assessment
showed that for the tumor tissues, the median H-score was 2, which
was higher than the median H-score for the adjacent tissues (0)
(P<0.01) (Fig. 1B and C). When
H-score ≥2.0 in the tumor tissue was considered as positive (i.e.,
the staining intensity score >2 while the positive tumor cell
percentage >50%), the positive staining percentage for the tumor
tissue was 46.4% (65/140). These results suggest that the
expression level of ERCC1 is upregulated in the NSCLC tumor
tissue.
ERCC1 is associated with NSCLC
pathological type, histological grade, and patient smoking
status
The association between the ERCC1 expression and
clinicopathological features of NSCLC was next investigated. As
shown in Table I, the expression of
ERCC1 is associated with the NSCLC pathological type. The
expression level of ERCC1 in the squamous carcinoma (59.3%, 35/59)
was significantly higher than the adenocarcinoma (37%, 30/81)
(P<0.01). Moreover, significant differences were observed in the
ERCC1 expression levels between different histological stages
(P<0.05), and the expression level of ERCC1 showed an increasing
trend along with the decreasing differentiation degrees.
Furthermore, the expression level of ERCC1 for the smoking patients
(60.3%, 41/68) was significantly higher than the non-smokers
(33.3%, 24/72) (P<0.01). On the other hand, the ERCC1 expression
was not significantly associated with the other investigated
clinicopathological features, including age, sex, pathological
staging, T staging, N staging, and history of radio- and/or
chemotherapy. These results suggest that the expression of ERCC1 is
significantly associated with the NSCLC pathological type,
histological grade, and patient smoking status.
 | Table I.Relationship between the ERCC1
expression in tumor tissue and clinicopathological features of
NSCLC. |
Table I.
Relationship between the ERCC1
expression in tumor tissue and clinicopathological features of
NSCLC.
|
|
| Tumor ERCC1
expression |
|
|
|---|
|
|
|
|
|
|
|---|
|
| Total cases, n
(%) | Positive, n (%) | Negative, n (%) | χ2 | P-value |
|---|
| Age (years) |
|
|
| 5.596 | 0.061 |
|
<50 | 20 (14.3) | 14 (70.0) | 6 (30.0) |
|
|
|
50–70 | 95 (67.8) | 39 (41.0) | 56 (59.0) |
|
|
|
>70 | 25 (17.9) | 12 (48.0) | 13 (52.0) |
|
|
| Sex |
|
|
| 0.161 | 0.689 |
| Male | 88 (62.9) | 42 (47.7) | 46 (52.3) |
|
|
|
Female | 52 (37.1) | 23 (44.2) | 29 (55.8) |
|
|
| Histological
type |
|
|
| 6.816 | 0.009 |
| Squamous
cancer | 59 (42.1) | 35 (59.3) | 24 (40.7) |
|
|
|
Adenocarcinoma | 81 (57.9) | 30 (37.0) | 51 (63.0) |
|
|
| Histological
grade |
|
|
| 7.665 | 0.022 |
| G1 | 59 (42.1) | 35 (59.3) | 24 (40.7) |
|
|
| G2 | 57 (40.7) | 23 (40.4) | 34 (59.6) |
|
|
| G3 | 24 (17.2) | 7 (29.2) | 17 (70.8) |
|
|
| pTNM |
|
|
| 2.094 | 0.351 |
| Stage
I | 50 (35.7) | 23 (46.0) | 27 (54.0) |
|
|
| Stage
II | 35 (25.0) | 13 (37.1) | 22 (62.9) |
|
|
| Stage
III | 55 (39.3) | 29 (52.7) | 26 (47.3) |
|
|
| T stage |
|
|
| 1.264 | 0.532 |
| T1 | 34 (24.2) | 16 (47.1) | 18 (52.9) |
|
|
| T2 | 81 (57.9) | 35 (43.2) | 46 (56.8) |
|
|
| T3 | 25 (17.9) | 14 (56.0) | 11 (44) |
|
|
| N stage |
|
|
| 1.129 | 0.569 |
| N0 | 74 (52.9) | 33 (44.6) | 41 (55.4) |
|
|
| N1 | 18 (12.9) | 7 (38.9) | 11 (61.1) |
|
|
| N2 | 48 (34.2) | 25 (52.1) | 23 (47.9) |
|
|
| Smoking status |
|
|
| 10.22 | 0.001 |
|
Non-smoker | 72 (51.4) | 24 (33.3) | 48 (66.7) |
|
|
|
Smoker | 68 (48.6) | 41 (60.3) | 27 (39.7) |
|
|
|
Chemoradiotherapy |
|
|
| 0.838 | 0.36 |
| No | 61 (43.6) | 31 (50.8) | 30 (49.2) |
|
|
|
Yes | 79 (56.4) | 34 (43.0) | 45 (57.0) |
|
|
ERCC1 affects postoperative prognosis
of NSCLC patients
The association between the ERCC1 expression and
postoperative prognosis of NSCLC was next investigated. Our results
showed that in the term of DFS, for the ERCC1-positive NSCLC
patients, the 1-, 2-, and 3-year DFS rates were 79.8, 69.1, and
53.4%, respectively, with the median DFS of 22.17 m (ranging from
9.85 to 34.49 m). On the other hand, the 1-, 2-, and 3-year DFS
rates for the ERCC1-negative NSCLC patients were 85.9, 76.8, and
66.8%, respectively, with the median DFS of 28.4 m (ranging from
19.34 to 37.46 m) (Table II). The
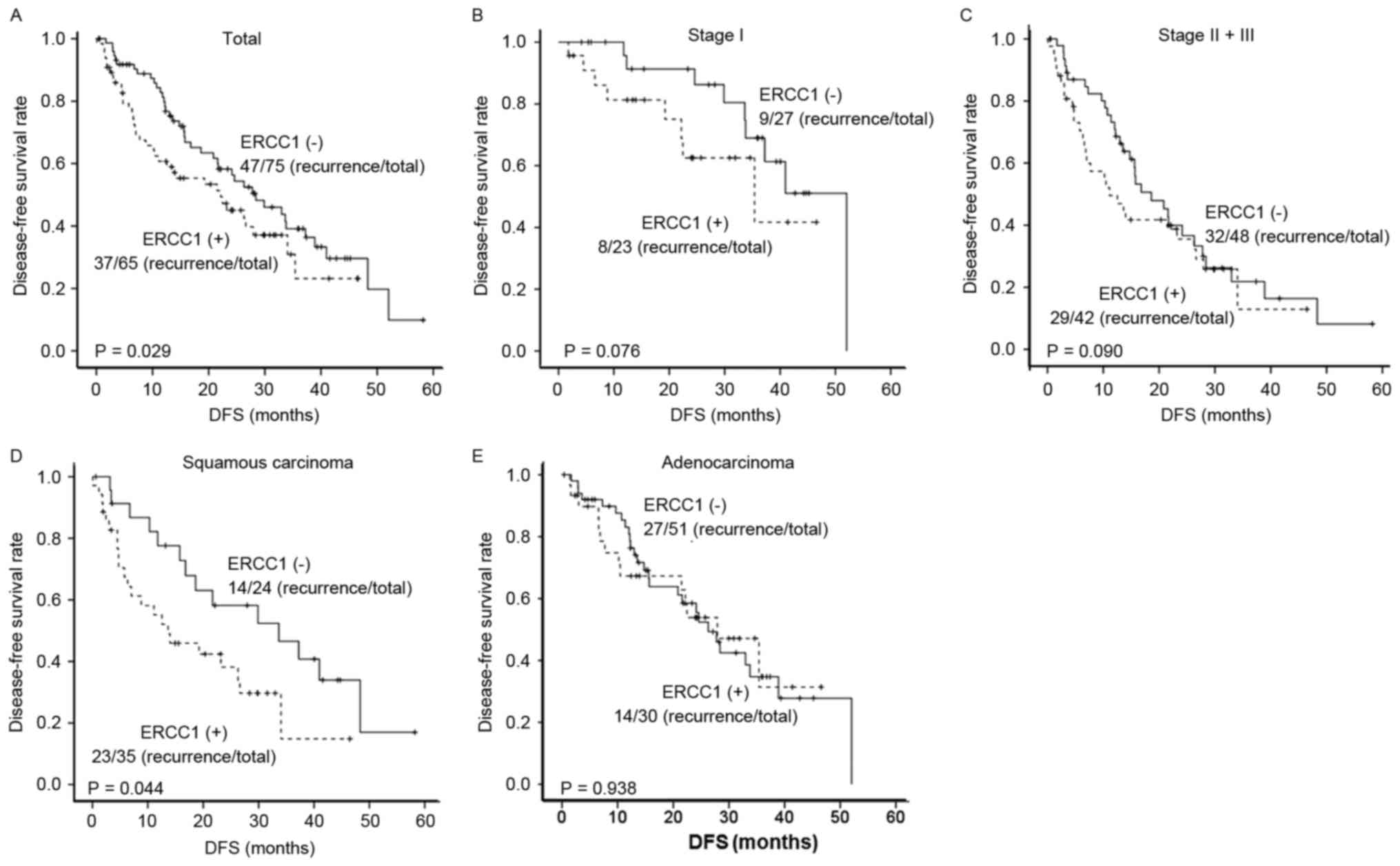
Kaplan-Meier survival analysis showed that the DFS for
ERCC1-negative patients was superior to the ERCC1-positive patients
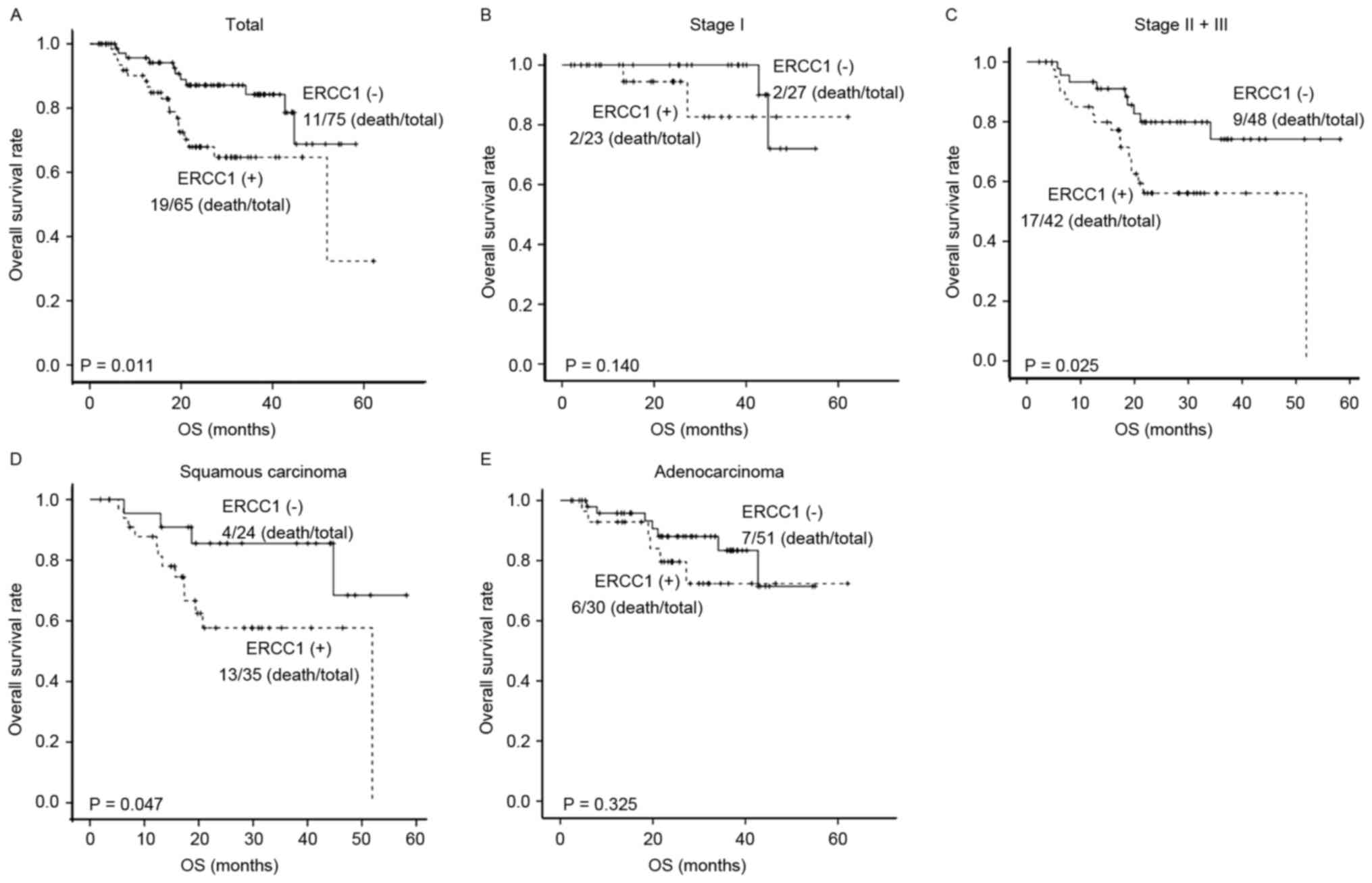
(Fig. 2A). In the term of the OS, for
the ERCC1-positive NSCLC patients, the 1-, 2-, and 3-year OS rates
were 88.4, 67.9, and 64.7%, respectively, with the median OS of
51.93 m (ranging from 41.69 to 62.17 m). On the other hand, the 1-,
2-, and 3-year OS rates for the ERCC1-negative NSCLC patients were
94.2, 87.1, and 84.2%, respectively (Table II). The survival analysis showed that
the OS for ERCC1-negative patients was superior to the
ERCC1-positive patients (Fig.
3A).
 | Table II.Prognostic analysis of the tumor
ERCC1 expression in NSLCL patients. |
Table II.
Prognostic analysis of the tumor
ERCC1 expression in NSLCL patients.
|
|
| Tumor ERCC1
expression |
|
|
|---|
|
|
|
|
|
|
|---|
|
| Total | Positive | Negative | χ2 | P-value |
|---|
| DFS |
|
|
| 4.758 | 0.029 |
| Recurrence/total,
n | 78/140 | 37/65 | 41/75 |
|
|
| 1 year
DFS rate | 70.9% | 79.8% | 85.9% |
|
|
| 2 years
DFS rate | 51.0% | 69.1% | 76.8% |
|
|
| 3 years
DFS rate | 33.7% | 53.4% | 66.8% |
|
|
| Median
DFS, m (95%CI) | 24.57
(19.6–29.54) | 22.17
(9.85–34.49) | 28.40
(19.34–37.46) |
|
|
| OS |
|
|
| 6.502 | 0.011 |
| Death/total, n | 30/140 | 19/65 | 11/75 |
|
|
| 1 year
OS rate | 97.7% | 88.4% | 94.2% |
|
|
| 2 years
OS rate | 92.3% | 67.9% | 87.1% |
|
|
| 3 years
OS rate | 89.8% | 64.7% | 84.2% |
|
|
| Median
OS, m (95%CI) | – | 51.93
(41.686–62.174) | – |
|
|
Considering the impact of other factors from the
adjuvant chemotherapy, subgroup analysis was performed for the
combined data of NSCLC patients at stages II–III. As shown in
Table III, no significant effects
of the ERCC1 expression on DFS or OS were observed for the NSCLC
patients at stage I (Figs. 2B and
3B). For the NSCLC patients at stages
II–III, no significant association was observed between the ERCC1
expression and DFS (Fig. 2C), while
the 3-year OS for ERCC1-negative patients (74.2%) was significantly
higher than the ERCC1-positvie patients (56.1%) (P<0.05)
(Fig. 3C). These results suggest that
the elevated expression of ERCC1 in the NSCLC patients who need the
postoperative adjuvant chemotherapy may indicate the tumor
resistance.
 | Table III.Prognostic analysis of the tumor
ERCC1 expression in NSLCL patients at stages I and II–III. |
Table III.
Prognostic analysis of the tumor
ERCC1 expression in NSLCL patients at stages I and II–III.
|
|
| Tumor ERCC1
expression |
|
|
|---|
|
|
|
|
|
|
|---|
| Variable | Total | Positive | Negative | χ2 | P-value |
|---|
| Stage I |
|
|
|
|
|
| Recurrence/total,
n | 17/50 | 8/23 | 9/27 | 3.143 | 0.076 |
| 3-years
DFS rate | 59.7% | 41.7% | 69.0% |
|
|
| Median
DFS, m (95%CI) | 40.97
(35.213–46.727) | 35.4
(12.702–58.098) | 52.03 |
|
|
| Death/total, n | 4/50 | 2/23 | 2/27 | 2.180 | 0.140 |
| 3-years
OS rate | 85.8% | 82.6% | 90.0% |
|
|
| Median
OS, m (95%CI) | – | – | – |
|
|
| Stage II–III |
|
|
|
|
|
| Recurrence/total,
n | 61/90 | 29/42 | 32/48 | 2.869 | 0.090 |
| 3-years
DFS rate | 19.9% | 12.9% | 21.8% |
|
|
| Median
DFS, m (95%CI) | 15.67
(9.122–22.218) | 11.1
(3.972–18.228) | 18.6
(11.762–25.438) |
|
|
| Death/total, n | 26/90 | 17/42 | 9/48 | 5.001 | 0.025 |
| 3-years
OS rate | 64.7% | 56.1% | 74.2% |
|
|
| Median
OS, m (95%CI) |
51.93(22.646–81.214) | – | – |
|
|
Since the expression level of ERCC1 for the squamous
carcinoma was higher than the adenocarcinoma, according subgroup
analysis was further conducted. As shown in Table IV, the 3-year DFS and OS rates for
the patients with ERCC1-negative squamous carcinoma were 40.7 and
68.4%, respectively, with the median DFS of 13.63 m (ranging from
2.73 to 24.53 m). On the other hand, the 3-year DFS and OS rates
for the patients with ERCC1-positie squamous carcinoma were 14.8
and 57.7%, respectively, with the median DFS of 33.63 m (ranging
from 13.95 to 53.31 m). The Kaplan-Meier survival analysis showed
that the DFS and OS rates of the patients with ERCC1-negative
squamous carcinoma were superior to the ERCC1-positive patients
(both P<0.05) (Figs. 2D and
3D). For the adenocarcinoma, the
3-year DFS and OS rates for the ERCC1-negative patients were 27.8
and 83.4%, respectively, with the median DFS of 27.93 m (ranging
from 17.55 to 38.31 m). The 3-year DFS and OS rates for the
patients with ERCC1-positive adenocarcinoma were 31.4 and 72.4%,
respectively, with the median DFS of 26.27 m (ranging from 13.95 to
53.31 m). No significant effects of ERCC1 expression on the DFS and
OS were observed for the patients with adenocarcinoma (Figs. 2E and 3E). In addition, the COX regression
multivariate survival analysis showed that the pathologic staging
was the only independent factor affecting the patient DFS, while
the pathologic staging, T staging, and ERCC1 expression were
independent factors affecting the OS of NSCLC patients (Table V).
 | Table IV.Prognostic analysis of the tumor
ERCC1 expression in patients with squamous carcinoma and
adenocarcinoma. |
Table IV.
Prognostic analysis of the tumor
ERCC1 expression in patients with squamous carcinoma and
adenocarcinoma.
|
|
| Tumor ERCC1
expression |
|
|
|---|
|
|
|
|
|
|
|---|
| Variable | Total | Positive | Negative | χ2 | P-value |
|---|
| Squamous
carcinoma |
|
|
|
|
|
| Recurrence/total,
n | 37/59 | 23/35 | 14/24 | 4.042 | 0.044 |
| 3-years
DFS rate | 28.1% | 14.8% | 40.7% |
|
|
| Median
DFS, m (95%CI) |
21.70(10.611–32.789) |
33.63(13.948–53.312) |
13.63(2.731–24.529) |
|
|
| Death/total, n | 17/59 | 13/35 | 4/24 | 3.948 | 0.047 |
| 3-years
OS rate | 69.8% | 57.7% | 68.4% |
|
|
| Median
OS, m (95%CI) |
51.93(41.686–62.174) | – | – |
|
|
| Adenocarcinoma |
|
|
|
|
|
| Recurrence/total,
n | 41/81 | 14/30 | 27/51 | 0.006 | 0.938 |
| 3-years
DFS rate | 29.8% | 31.4% | 27.8% |
|
|
| Median
DFS, m (95%CI) |
27.77(21.861–33.679) |
26.27(13.948–53.312) |
27.93(17.549–38.311) |
|
|
| Death/total, n | 13/81 | 6/30 | 7/51 | 0.97 | 0.325 |
| 3-years
OS rate | 79.1% | 72.4% | 83.4% |
|
|
| Median
OS, m (95%CI) | – | – | – |
|
|
 | Table V.COX regression multivariate survival
analysis for NSCLC patients. |
Table V.
COX regression multivariate survival
analysis for NSCLC patients.
|
|
|
|
|
|
|
|
| 95.0% CI for
Exp(β) |
|---|
|
|
|
|
|
|
|
|
|
|
|---|
| Survival | Variables | β | SE | Wald | df | P-value | Exp(β) | Up | Down |
|---|
| DFS | pTNM | 0.747 | 0.146 | 26.304 | 1 | 0.000 | 2.110 | 1.586 | 2.807 |
| OS | pTNM | 0.972 | 0.293 | 11.042 | 1 | 0.001 | 2.644 | 1.490 | 4.691 |
|
| T stage | 0.402 | 0.199 | 4.057 | 1 | 0.044 | 1.494 | 1.011 | 2.209 |
|
| ERCC1 | 0.803 | 0.385 | 4.352 | 1 | 0.037 | 2.231 | 1.050 | 4.742 |
Inhibition of p38 enhances A549 cell
sensitivity to DDP and downregulates ERCC1 expression
Both p38MAPK and ERCC1 are closely related to the
tumor resistance. Studies have shown that positive correlation
could be observed between the ERCC1 and p-p38 levels in the NSCLC
tissues (21). The relationship
between p38MAPK and ERCC1, and its effect on the cell tumor
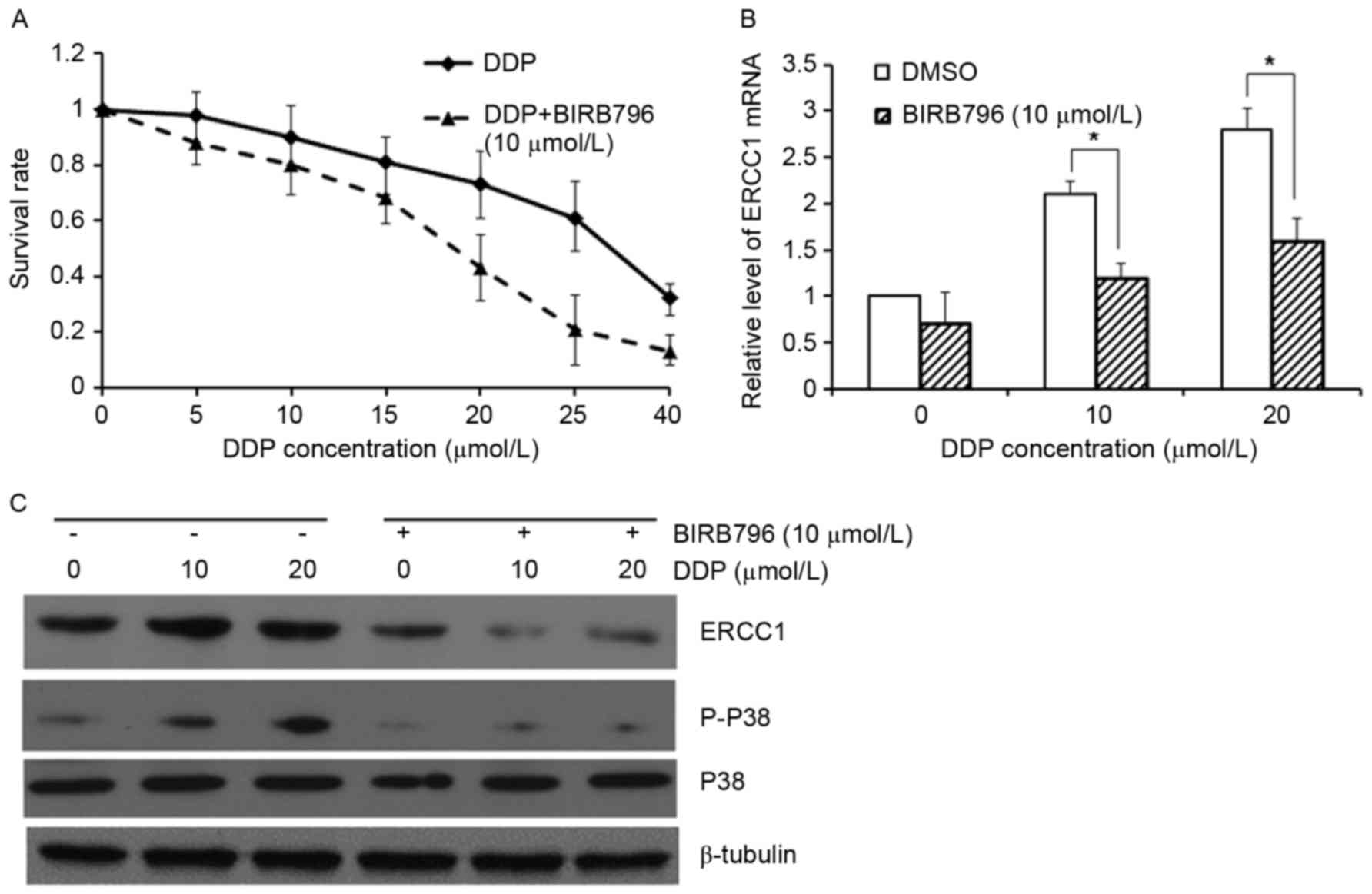
resistance, were then investigated. Our results from the MTT assay
showed that the treatment of p38 inhibitor, BIRB796, significantly
enhanced the sensitivity of A549 cells to cisplatin (Fig. 4A). The mRNA and protein expression
levels of ERCC1, and the performance of the p38 signaling pathway,
were then investigated. Our results showed that along with the
increasing treatment concentrations of cisplatin, the mRNA and
protein expression levels of ERCC1, and the level of p-p38, were
gradually elevated, which could be significantly declined by the
treatment of 10µmol/LBIRB796 (Fig. 4B and
C). These results suggest that the p38 signaling pathway could
exert direct or indirect regulatory effects on the expression of
ERCC1.
Discussion
Platinum-based two-drug combined chemotherapy is an
important therapeutic method for the treatment of NSCLC, however,
with the overall efficiency rate of only 20–40%. With the rapid
development of molecular biology in recent years, the interference
of ERCC1 expression has been shown to be able to enhance the
sensitivity of a variety of tumor cells to platinum drugs,
including lung, colon, liver, and ovarian cancers (22–25).
Olaussen et al (26) have
shown that according to the immunohistochemical staining for the
NSCLC patients at stages I–III after radical resection, the
ERCC1-negatie patients could benefit from the platinum-based
chemotherapy, while ERCC1-overexpressing patients could not. These
findings suggest that ERCC1 overexpression might be associated with
the platinum resistance. Sad et al (7) have investigated the chemotherapy for
locally progressed NSCLC, and they suggest that the OS and
progression-free survival for the ERCC1-negative patients would be
longer for the ERCC1-positive patients. Hubner et al
(18) have also shown that the
elevated ERCC1 expression in NSCLC patients who have received
platinum-based treatment would imply poor prognosis, which is
however not the case for the naïve patients. Moreover, NSCLC
patients overexpressing ERCC1 exhibit resistance against
platinum-based therapy. Therefore, it has been accepted that the
expression of ERCC1 is an important predictor for the prognosis of
platinum-based chemotherapy for locally progressed lung cancer. Our
results demonstrated that for the NSCLC patients after surgery, the
ERCC1-negative patients have longer DFS and OS compared with the
ERCC1-positive patients. Subgroup analysis showed that the OS for
ERCC1-negative patients at stages II–III was significantly longer
than the ERCC1-positive patients. However, no significant
differences in DFS were observed between the ERCC1-negative and
-positive NSCLC patients. These findings suggest that the NSCLC
patients at stages II–III who receive platinum-based adjuvant
chemotherapy would benefit from the negative expression of ERCC1.
However, these is still no widely accepted objective criteria for
the expression of ERCC1, and the majority of investigations are
retrospective studies using the median split method, which would
inevitably result in subjective bias in sampling, limiting its
clinical application.
Our results showed that the expression levels of
ERCC1 were elevated in the tumor tissues from the patients with
squamous carcinoma and those had long-term smoking habit. The
expression of ERCC1 in the lung squamous carcinoma tissue was
significantly higher than the adenocarcinoma tissue, which was in
line with the findings from Olaussen et al (26). The prognosis analysis also showed that
in the patients with squamous carcinoma, the 3-year DFS and OS
rates for the ERCC1-negative patients were significant higher than
the ERCC1-positive patients. Moreover, the expression rate of ERCC1
was significantly higher for the long-term smoking patients than
the non-smokers. Studies have shown that smoking could not only
cause DNA damage and stimulate DNA repairing (27), but also activate p-p38 in the lung
tissue of mice (28,29) and induce bronchial squamous metaplasia
(30,31). The present study preliminarily
confirmed that the p38 inhibitor could suppress the expression of
ERCC1 via blocking the p38 signaling pathway. Based on these
findings, we hypothesize that as exogenous stimuli, tobacco could
activate the p38 signaling pathway to enhance the expression of
ERCC1. Of course, further studies are still needed to confirm the
hypothesis.
In conclusion, our results showed that the
expression of ERCC1 in the NSCLC tumor tissue was an important
indicator for the disease prognosis, especially for the patients at
stages II–III who received systematic platinum-based chemotherapy.
Moreover, the expression rates of ERCC1 were elevated for patients
with squamous carcinoma and with smoking habit. Prognosis of
ERCC1-negative patients with squamous carcinoma was superior to the
ERCC1-positive patients. Furthermore, the p38 signaling pathway may
directly or indirectly regulate the expression of ERCC1. These
findings suggest a promising role of ERCC1 expression as the
indicator for the clinical treatment and prognostic prediction of
NSCLC.
Acknowledgements
This work was supported by the China National
Natural Sciences Foundation (no. 81460354) and the Xinjiang Medical
University Innovation Fund (XJC201376).
References
|
1
|
Le Chevalier T: Adjuvant chemotherapy for
resectable non-small-cell lung cancer: Where is it going? Ann
Oncol. 7:(Suppl 7). vii196–vii198. 2010.
|
|
2
|
He YW, Zhao ML, Yang XY, Zeng J, Deng QH
and He JX: Prognostic value of ERCC1, RRM1 and TS proteins in
patients with resected non-small cell lung cancer. Cancer Chemother
Pharmacol. 75:861–867. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Geredeli C, Artac M, Yildirim S, Inal A,
Dede I, Guler T, Boruban MC, Koral L, Karaagac M, Zamani AG, et al:
Prognostic value of ERCC1, ERCC2, XRCC1, and TP53 single nucleotide
polymorphisms in patients with early-stage non-small cell lung
cancer. Tumour Biol. 36:4279–4285. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pierceall WE, Olaussen KA, Rousseau V,
Brambilla E, Sprott KM, Andre F, Pignon JP, Le Chevalier T, Pirker
R, Jiang C, et al: Cisplatin benefit is predicted by
immunohistochemical analysis of DNA repair proteins in squamous
carcinoma but not adenocarcinoma: Theranostic modeling by NSCLC
constituent histological subclasses. Ann Oncol. 23:2245–2252. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bepler G, Zinner RG, Moon J, Calhoun R,
Kernstine K, Williams CC, Mack PC, Oliveira V, Zheng Z, Stella PJ,
et al: A phase 2 cooperative group adjuvant trial using a
biomarker-based decision algorithm in patients with stage I
non-small cell lung cancer (SWOG-0720, NCT00792701). Cancer.
120:2343–2351. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kalikaki A, Voutsina A, Koutsopoulos A,
Papadaki C, Sfakianaki M, Yachnakis E, Xyrafas A, Kotsakis A,
Agelaki S, Souglakos J, et al: ERCC1 SNPs as potential predictive
biomarkers in non-small cell lung cancer patients treated with
platinum-based chemotherapy. Cancer Invest. 33:107–113. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sad LM, Younis SG and Elity MM: Prognostic
and predictive role of ERCC1 protein expression in locally advanced
stage III non-small cell lung cancer. Med Oncol. 31:582014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sullivan I, Salazar J, Majem M, Pallarés
C, Del Río E, Páez D, Baiget M and Barnadas A: Pharmacogenetics of
the DNA repair pathways in advanced non-small cell lung cancer
patients treated with platinum-based chemotherapy. Cancer Lett.
353:160–166. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Papadaki C, Sfakianaki M, Ioannidis G,
Lagoudaki E, Trypaki M, Tryfonidis K, Mavroudis D, Stathopoulos E,
Georgoulias V and Souglakos J: ERCC1 and BRAC1 mRNA expression
levels in the primary tumor could predict the effectiveness of the
second-line cisplatin-based chemotherapy in pretreated patients
with metastatic non-small cell lung cancer. J Thorac Oncol.
7:663–671. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cobo M, Isla D, Massuti B, Montes A,
Sanchez JM, Provencio M, Viñolas N, Paz-Ares L, Lopez-Vivanco G,
Muñoz MA, et al: Customizing cisplatin based on quantitative
excision repair cross-complementing 1 mRNA expression: A phase III
trial in non-small-cell lung cancer. J Clin Oncol. 25:2747–2754.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Roth JA and Carlson JJ: Prognostic role of
ERCC1 in advanced non-small-cell lung cancer: A systematic review
and meta-analysis. Clin Lung Cancer. 12:393–401. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li CF, Cao S and Meng SD: Tumor dormancy
and identification of therapeutic targets. Ai Zheng. 28:555–558.
2009.(In Chinese). PubMed/NCBI
|
|
13
|
Paillas S, Boissière F, Bibeau F, Denouel
A, Mollevi C, Causse A, Denis V, Vezzio-Vié N, Marzi L, Cortijo C,
et al: Targeting the p38 MAPK pathway inhibits irinotecan
resistance in colon adenocarcinoma. Cancer Res. 71:1041–1049. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wen J, Cheng HY, Feng Y, Rice L, Liu S, Mo
A, Huang J, Zu Y, Ballon DJ and Chang CC: P38 MAPK inhibition
enhancing ATO-induced cytotoxicity against multiple myeloma cells.
Br J Haematol. 140:169–180. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ranganathan AC, Adam AP, Zhang L and
Aguirre-Ghiso JA: Tumor cell dormancy induced by p38SAPK and
ER-stress signaling: An adaptive advantage for metastatic cells?
Cancer Biol Ther. 5:729–735. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ranganathan AC, Zhang L, Adam AP and
Aguirre-Ghiso JA: Functional coupling of p38-induced up-regulation
of BiP and activation of RNA-dependent protein kinase-like
endoplasmic reticulum kinase to drug resistance of dormant
carcinoma cells. Cancer Res. 66:1702–1711. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He D, Zhao XQ, Chen XG, Fang Y, Singh S,
Talele TT, Qiu HJ, Liang YJ, Wang XK, Zhang GQ, et al: BIRB796, the
inhibitor of p38 mitogen-activated protein kinase, enhances the
efficacy of chemotherapeutic agents in ABCB1 overexpression cells.
PLoS One. 8:e541812013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hubner RA, Riley RD, Billingham LJ and
Popat S: Excision repair cross-complementation group 1 (ERCC1)
status and lung cancer outcomes: A meta-analysis of published
studies and recommendations. PLoS One. 6:e251642011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Planchard D, Domont J, Taranchon E, Monnet
I, Tredaniel J, Caliandro R, Validire P, Besse B, Soria JC and
Fouret P: The NER proteins are differentially expressed in ever
smokers and in never smokers with lung adenocarcinoma. Ann Oncol.
20:1257–1263. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shi Z, Tiwari AK, Shukla S, Robey RW,
Singh S, Kim IW, Bates SE, Peng X, Abraham I, Ambudkar SV, et al:
Sildenafil reverses ABCB1- and ABCG2-mediated chemotherapeutic drug
resistance. Cancer Res. 71:3029–3041. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Planchard D, Camara-Clayette V, Dorvault
N, Soria JC and Fouret P: p38 mitogen-activated protein kinase
signaling, ERCC1 expression, and viability of lung cancer cells
from never or light smoker patients. Cancer. 118:5015–5025. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Seetharam RN, Sood A, Basu-Mallick A,
Augenlicht LH, Mariadason JM and Goel S: Oxaliplatin resistance
induced by ERCC1 up-regulation is abrogated by siRNA-mediated gene
silencing in human colorectal cancer cells. Anticancer Res.
30:2531–2538. 2010.PubMed/NCBI
|
|
23
|
Ueda S, Shirabe K, Morita K, Umeda K,
Kayashima H, Uchiyama H, Soejima Y, Taketomi A and Maehara Y:
Evaluation of ERCC1 expression for cisplatin sensitivity in human
hepatocellular carcinoma. Ann Surg Oncol. 18:1204–1211. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu GY, Qu QX, Mi RR and Qi J:
Relationship between nucleotide excision repair gene ERCC1 and
resistance to cisplatin in ovarian cancer. Zhonghua Zhong Liu Za
Zhi. 30:184–187. 2008.(In Chinese). PubMed/NCBI
|
|
25
|
Cheong HT, Hui CW, Xu F, Mok TSK and Wong
CH: Abstract 2558: The mechanistic study on the effect of
platinum-based chemotherapy efficacy imposed by EGFR-TKI regulated
ERCC1 in non-small cell lung cancer (NSCLC). Cancer Res.
75:25582015. View Article : Google Scholar
|
|
26
|
Olaussen KA, Dunant A, Fouret P, Brambilla
E, André F, Haddad V, Taranchon E, Filipits M, Pirker R, Popper HH,
et al: DNA repair by ERCC1 in non-small-cell lung cancer and
cisplatin-based adjuvant chemotherapy. N Engl J Med. 355:983–991.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wei Q, Cheng L, Amos CI, Wang LE, Guo Z,
Hong WK and Spitz MR: Repair of tobacco carcinogen-induced DNA
adducts and lung cancer risk: A molecular epidemiologic study. J
Natl Cancer Inst. 92:1764–1772. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Marwick JA, Kirkham PA, Stevenson CS,
Danahay H, Giddings J, Butler K, Donaldson K, Macnee W and Rahman
I: Cigarette smoke alters chromatin remodeling and induces
proinflammatory genes in rat lungs. Am J Respir Cell Mol Biol.
31:633–642. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yao H, Edirisinghe I, Rajendrasozhan S,
Yang SR, Caito S, Adenuga D and Rahman I: Cigarette smoke-mediated
inflammatory and oxidative responses are strain-dependent in mice.
Am J Physiol Lung Cell Mol Physiol. 294:L1174–L1186. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhong CY, Zhou YM, Douglas GC, Witschi H
and Pinkerton KE: MAPK/AP-1 signal pathway in tobacco smoke-induced
cell proliferation and squamous metaplasia in the lungs of rats.
Carcinogenesis. 26:2187–2195. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bolton SJ, Pinnion K, Oreffo V, Foster M
and Pinkerton KE: Characterisation of the proximal airway squamous
metaplasia induced by chronic tobacco smoke exposure in
spontaneously hypertensive rats. Respir Res. 10:1182009. View Article : Google Scholar : PubMed/NCBI
|