Introduction
Rebiopsy is considered an option for specific cancer
types, such as breast cancer, non-small cell lung cancer (NSCLC),
and prostate cancer, due to the ability of gene profiling to detect
hormonal changes (1–3). However, rebiopsy is not usually
considered in the treatment of colorectal cancer (4,5).
The present study reports a case of a 68-year-old
man who was initially diagnosed with metastatic adenocarcinoma, but
was subsequently rediagnosed with metastatic neuroendocrine
carcinoma (NEC) from a primary rectal cancer following rebiopsy.
The patient was unresponsive to the standard chemotherapy regimen
of 5-fluorouracil, leucovorin, and oxaliplatin (FOLFOX), but
responsive to the treatment for NEC.
NEC is an atypical type of colorectal cancer that
accounts for <1% of all colorectal cancer cases (6). The 5-year survival rate of stage IV
colorectal NEC is poor, at ~3% (7).
While colorectal adenocarcinoma is usually treated with the FOLFOX
or 5-fluorouracil, leucovorin and irinotecan (FOLFIRI) regimens,
colorectal NEC is treated with cisplatin (CDDP)/carboplatin and
etoposide (VP-16). In the current case report, the clinical
significance of rebiopsy as it applied to the treatment of this
colorectal cancer patient is discussed. Written informed consent
was obtained from the patient for the publication of this case
report and any accompanying images.
Case report
A 68-year-old man was diagnosed with colorectal
cancer and underwent lower anterior resection and D3 lymph node
dissection with ileostomy at a public hospital in Nagasaki, Japan
in October 2013. The pathological stage was IIIB (T2N2M0), and the
tumor was determined to be a moderately differentiated tubular
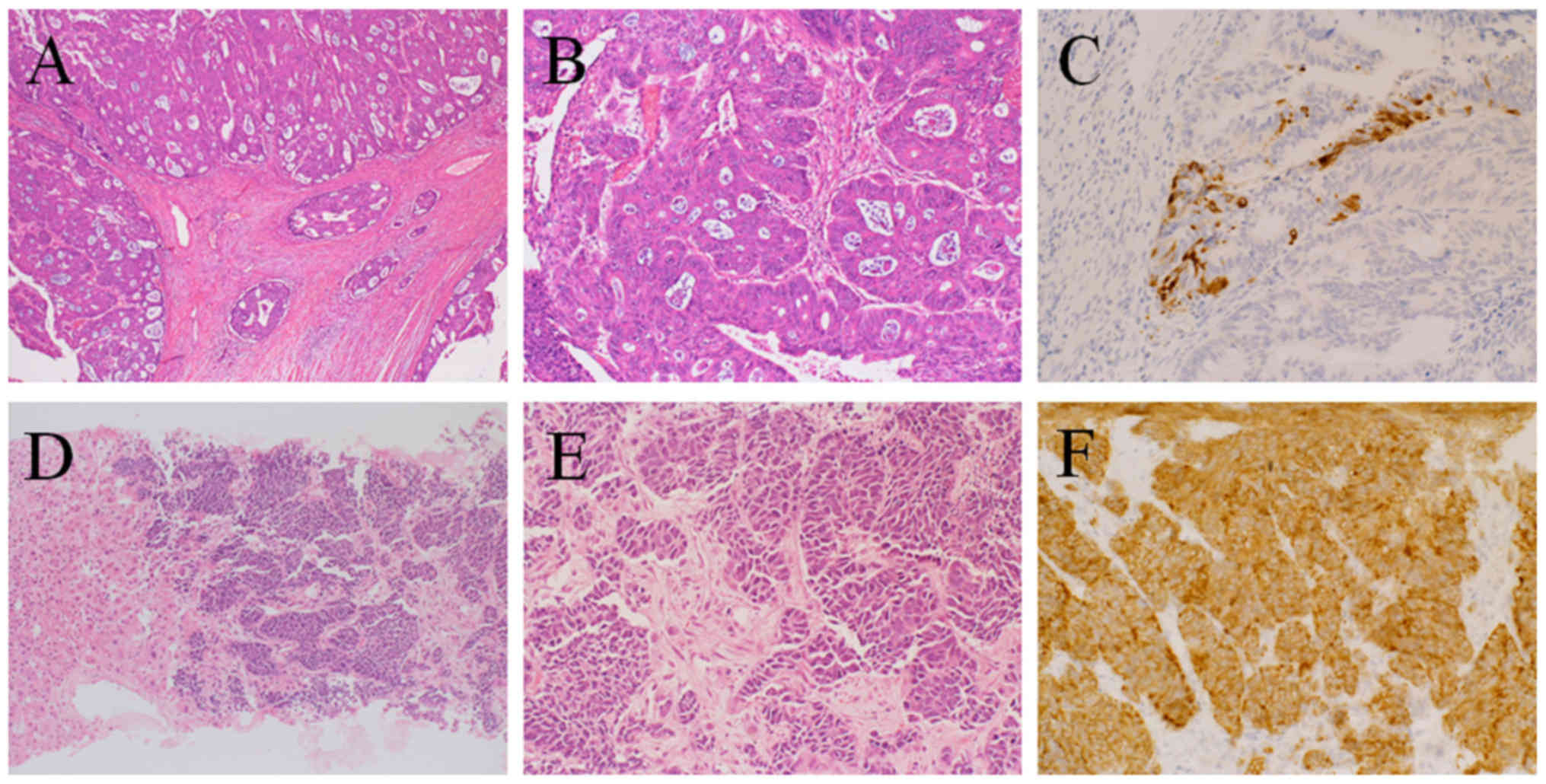
adenocarcinoma with positive G12A KRAS mutation (Fig. 1A-C). The lymph node metastasis
exhibited the same adenocarcinoma histology as the primary lesion.
The patient received modified FOLFOX6 [400 mg/m2 bolus
5-fluorouracil (day 1 of each cycle); 200 mg/m2
leucovorin (day 1 of each cycle); 100 mg/m2 oxaliplatin
(day 1 of each cycle); 2,400 mg/m2 continuous
5-fluorouracil (day 1–2 of each cycle); all intravenously
administered every 2 weeks] as post-operative chemotherapy;
however, after receiving two cycles, the patient was found to be
suffering from liver dysfunction. A subsequent computed tomography
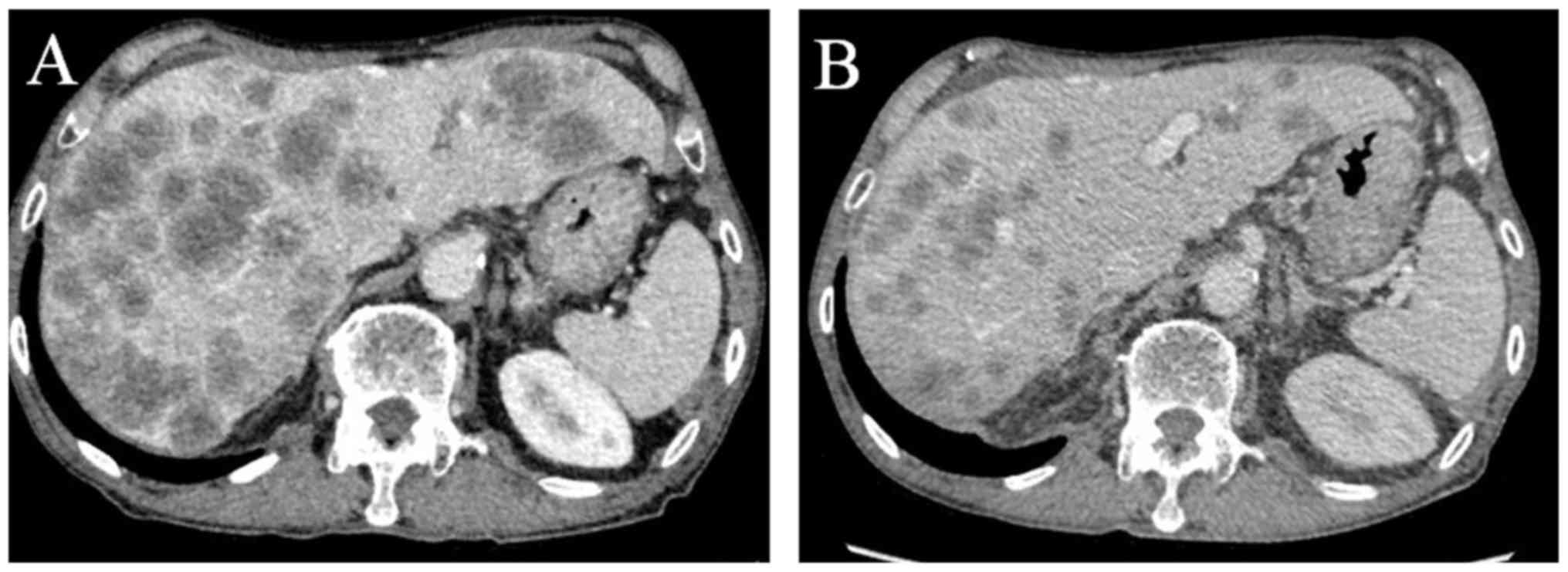
(CT) scan showed several large masses in the liver (Fig. 2A). Relapse of rectal cancer was
considered; however, the elevated neuron-specific enolase (NSE)
level (155.2 ng/ml) and non-elevated carbohydrate antigen 19–9 (12
U/ml) and carcinoembryonic antigen levels (3.6 ng/ml), together
with the cancer's resistance to FOLFOX treatment, indicated a more
unusual type of rectal cancer. Therefore, a liver biopsy was
performed.
From the biopsy, the patient was rediagnosed with
NEC that was synaptophysin-positive, chromogranin A-positive,
CD56-positive, and Ki-67-positive (>80% of cells) (Fig. 1D-F). Pathologically, ductal formation
and mucus production were not observed. On positron emission
tomography (PET), extrahepatic lesions, which could have been
considered primary cancer, were not observed. Therefore, liver
metastasis from primary rectal cancer was diagnosed. To confirm
this, immunostaining was performed, which revealed sporadic
synaptophysin-positive cells in the primary adenocarcinoma of the
rectum (Fig. 1C). Based on this
diagnosis, the chemotherapy regimen was changed from FOLFOX to CDDP
(80 mg/m2 on day 1 of each cycle) and VP-16 (100
mg/m2 on day 1–3 of each cycle), which were
intravenously administered every 3 weeks.
After the first cycle in March 2014, the patient was
admitted to Keio University Hospital (Tokyo, Japan) with febrile
neutropenia. A CT scan was performed to investigate the site of the
infection, and a remarkable decrease of 45.1% [Response Evaluation
Criteria In Solid Tumors (RECIST), version 1.1; Fig. 2B] (8)
was observed, which was associated with a decrease in the serum
concentration of NSE (Fig. 3). The
patient was then evaluated as having a partial response (RECIST,
version 1.1) (8). Following four
cycles of chemotherapy, an ileostomy closure was performed. Within
6 months, the metastatic lesions had enlarged and the patient was
treated with 40 mg/m2 amrubicin, which was intravenously
administered every 3 weeks (day 1–3 of each cycle) for seven cycles
before he was reevaluated as having progressive disease (PD). The
patient opted for best supportive care; however, the patient
succumbed in June 2015.
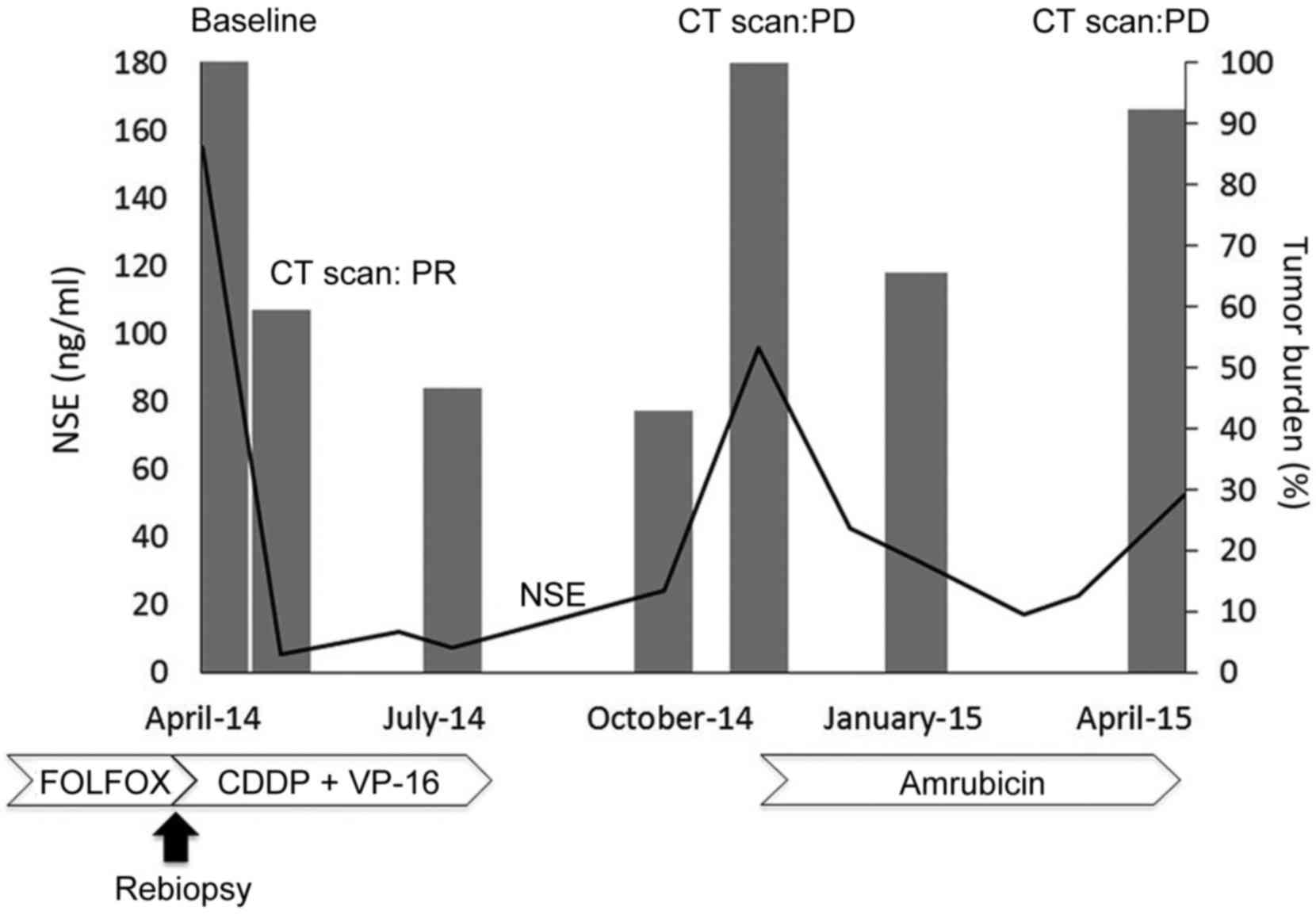 | Figure 3.Time course of the patient, including
NSE, tumor burden and treatment. Tumor burden, shown as a bar
graph, was calculated as the sum of two metastatic liver lesions
according to the Response Evaluation Criteria In Solid Tumors,
version 1.1. The line graph shows the NSE levels over time. NSE,
neuron-specific enolase; CT, computed tomography; PR, partial
response; PD, progressive disease; FOLFOX, 5-fluorouracil,
leucovorin, and oxaliplatin; CDDP, cisplatin; VP-16, carboplatin
and etoposide. |
Discussion
The present study reports the case of a
FOLFOX-resistant metastatic rectal cancer, which was diagnosed as
NEC from rebiopsy. According to the National Comprehensive Cancer
Network (NCCN) guidelines, for metachronous unresectable
metastases, as in this case, continuation of intensive chemotherapy
for colorectal cancer is recommended (4,5). Thus,
rebiopsy was not indicated for this patient, and he would be
characterized as having PD (4,5). However,
rebiopsy was undertaken and revealed the existence of NEC in the
metastatic lesion. It is notable that the primary cancer was an
adenocarcinoma which had a completely different pathological status
compared with the metastatic site. The result of the PET scan
revealed no other primary sites. It is possible that the
pathological analysis results from the primary adenocarcinoma were
not representative of the whole tumor. As shown in Fig. 1C, sporadic synaptophysin-positive
cells were detected in the primary adenocarcinoma, and these
sporadic cells may have been the origin of the liver metastasis.
Another possibility is that the adenocarcinoma changed into NEC
subsequent to the initial diagnosis. One previous report has shown
that neuroendocrine differentiation is more frequently observed in
metastatic cancers compared with primary site tumors (9), whereas other reports have described
cases wherein chemotherapy was shown to cause histological
conversions (10,11). This phenomenon has also been observed
in cases of prostate cancer and NSCLC, but the underlying mechanism
is unknown (12).
Rebiopsy is indicated in the NCCN guidelines for
NSCLC, breast and prostate cancers in clinical trials and in
practice (Table I). With regard to
clinical benefit, an alteration in the gene profile of the tumor
may arise from the development of drug resistance, or from hormonal
changes (13,14). For breast cancer, the NCCN, the
American Society of Clinical Oncology, and the European Society for
Medical Oncology guidelines all recommend rebiopsy of the
metastasis (1,15,16). The
guidelines are supported by a number of reports that indicate that
rebiopsy can reveal changes in the tumor for 14–20% of patients
(13,14). This is determined from the hormonal
status of the tumor and any differences between the primary tumor
and its distant metastases (13,14). For
NSCLC, tumor rebiopsy is recommended if deemed suitable (17), and in clinical practice it has been
reported that >80% of patients undergo rebiopsy (18). Recently, drug-resistant cell lines
derived from rebiopsy specimens have been established (19). This strategy can be used in directing
the selection of the most appropriate treatment, which has been
demonstrated to be important in the treatment of NSCLC (19). The NCCN guidelines also recommend
rebiopsy of prostate cancer, but only in cases where small cell
prostate cancer has been suspected (3). Treatment-related NEC has been reported,
and the necessity for rebiopsy has been clearly demonstrated in
numerous cases (12,20). The prevalence of prostate NEC is
<2% of all prostate malignancies, which is similar to that of
colorectal NEC (20).
 | Table I.Indications for rebiopsy in different
cancer types according to various guidelines. |
Table I.
Indications for rebiopsy in different
cancer types according to various guidelines.
| Indication | Breast cancer | Non-small cell lung
cancer | Prostate cancer | Colorectal
cancer |
|---|
| NCCN guidelines | First recurrence of
disease should be biopsied (1) | Consider rebiopsy if
appropriate (2) | Consider biopsy if
small cell carcinoma is suspected (3) | None |
| ASCO guidelines | Based on the
discordance of results between primary and metastatic tissues,
rebiopsy is recommended (15) | None | None | None |
| ESMO guidelines | Biopsy of metastasis
should be performed (16) | Rebiopsy at disease
progression may be considered (26) | None | None |
| Clinical benefit | Change in hormonal
status in 5–40% of patients (13,14) | Different molecular
mutation and resistance in 30-50% of patients (17,18) | Small cell carcinoma
can be detected in 0.5–2% of patients (27) | Unknown |
For colorectal cancer, rebiopsy is not indicated for
metachronous metastases (4,5) and the clinical benefit is unknown.
Rebiopsy is supported where there is evidence of differing genetic
mutations between the primary tumor and the metastasis, including
those in KRAS, BRAF, PTEN and PIK3CA
(21–24).
When considering rebiopsy, it is important to
determine whether it will improve the patient's quality of life and
overall survival compared with the burden and the potential risks
associated with additional biopsy (25). From the present case study, we
consider rebiopsy to have been beneficial for the patient, as the
correct diagnosis resulted in the selection of a treatment regimen
that produced a better response.
In conclusion, for cases in which a patient with
colorectal cancer is unresponsive to a standard treatment, it may
be beneficial to consider an atypical histological type, and, if
appropriate, to perform rebiopsy.
References
|
1
|
National Comprehensive Cancer Network, .
NCCN Clinical Practice Guidelines in OncologyInvasive Breast
Cancer. version 3; BINV-17, MS-45. Fort Washington: 2015
|
|
2
|
National Comprehensive Cancer Network
(NCCN), . NCCN Clinical Practice Guidelines in OncologyNon Small
Cell Lung Cancer. version 1. Fort Washington: NSCL-16; 2016
|
|
3
|
National Comprehensive Cancer Network, .
NCCN Clinical Practise Guidelines in OncologyProstate Cancer.
version 1; PROS-9, MS-32. Fort Washington: 2016
|
|
4
|
National Comprehensive Cancer Network, .
NCCN Clinical Practice Guidelines in OncologyColon Cancer. version
1; COL-9, 11, COL-C 1 of 9. Fort Washington: 2016
|
|
5
|
National Comprehensive Cancer Network, .
NCCN Clinical Practice Guidelines in OncologyRectal Cancer. version
1. REC-9, 11, MS37-MS38. Fort Washington: 2016
|
|
6
|
Bernick PE, Klimstra DS, Shia J, Minsky B,
Saltz L, Shi W, Thaler H, Guillem J, Paty P, Cohen AM and Wong WD:
Neuroendocrine carcinomas of the colon and rectum. Dis Colon Retum.
47:163–169. 2004. View Article : Google Scholar
|
|
7
|
Shafqat H, Ali S, Salhab M and Olszewski
AJ: Survival of patients with neuroendocrine carcinoma of the colon
and rectum: A population-based analysis. Dis Colon Rectum.
58:294–303. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van
Oosterom AT, Christian MC and Gwyther SG: New guidelines to
evaluate the response to treatment in solid tumors. J Natl Cancer
Inst. 92:205–216. 2000. View Article : Google Scholar
|
|
9
|
Volante M, Marci V, Andrejevic-Blant S,
Tavaglione V, Sculli MC, Tampellini M and Papotti M: Increased
neuroendocrine cells in resected metastases compared to primary
colorectal adenocarcinomas. Virchows Arch. 457:521–527. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shia J, Tickoo SK, Guillem JG, Qin J,
Nissan A, Hoos A, Stojadinovic A, Ruo L, Wong WD, Paty PB, et al:
Increased endocrine cells in treated rectal adenocarcinomas: A
possible reflection of endocrine differentiation in tumor cells
induced by chemotherapy and radiotherapy. Am J Surg Pathol.
26:863–872. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tampellini M, Brizzi MP, Bitossi R,
Alabiso I, Sculli CM, Chiusa L, Papotti M and Dogliotti L: Six-year
stabilisation of a relapsed pelvic mass from rectal cancer after
oxaliplatin-containing chemotherapy. J Cancer Res Clin Oncol.
133:783–785. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tagawa ST: Neuroendocrine prostate cancer
after hormonal therapy: Knowing is half the battle. J Clin Oncol.
32:3360–3364. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Amir E, Clemons M, Purdie CA, Miller N,
Quinlan P, Geddie W, Coleman RE, Freedman OC, Jordan LB and
Thompson AM: Tissue confirmation of disease recurrence in breast
cancer patients: Pooled analysis of multi-centre,
multi-disciplinary prospective studies. Cancer Treat Rev.
38:708–714. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Simmons C, Miller N, Geddie W, Gianfelice
D, Oldfield M, Dranitsaris G and Clemons MJ: Does confirmatory
tumor biopsy alter the management of breast cancer patients with
distant metastases? Ann Oncol. 20:1499–1504. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Van Poznak C, Somerfield MR, Bast RC,
Cristofanilli M, Goetz MP, Gonzalez-Angulo AM, Hicks DG, Hill EG,
Liu MC, Lucas W, et al: Use of biomarkers to guide decisions on
systemic therapy for women with metastatic breast cancer: American
Society of Clinical Oncology Clinical Practice Guideline. J Clin
Oncol. 33:2695–2704. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cardoso F, Costa A, Norton L, Senkus E,
Aapro M, André F, Barrios CH, Bergh J, Biganzoli L, Blackwell KL,
et al: ESO-ESMO 2nd international consensus guidelines for advanced
breast cancer (ABC2)†. Ann Oncol. 25:1871–1888. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun JM, Ahn MJ, Choi YL, Ahn JS and Park
K: Clinical implications of T790M mutation in patients with
acquired resistance to EGFR tyrosine kinase inhibitors. Lung
Cancer. 82:294–298. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chouaid C, Dujon C, Do P, Monnet I,
Madroszyk A, Le Caer H, Auliac JB, Berard H, Thomas P, Lena H, et
al: Feasibility and clinical impact of re-biopsy in advanced non
small-cell lung cancer: a prospective multicenter study in a
real-world setting (GFPC study 12–01). Lung Cancer. 86:170–173.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Crystal AS, Shaw AT, Sequist LV, Friboulet
L, Niederst MJ, Lockerman EL, Frias RL, Gainor JF, Amzallag A,
Greninger P, et al: Patient-derived models of acquired resistance
can identify effective drug combinations for cancer. Science.
346:1480–1486. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Beltran H, Tagawa ST, Park K, MacDonald T,
Milowsky MI, Mosquera JM, Rubin MA and Nanus DM: Challenges in
recognizing treatment-related neuroendocrine prostate cancer. J
Clin Oncol. 30:e386–e389. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tórtola S, Steinert R, Hantschick M,
Peinado MA, Gastinger I, Stosiek P, Lippert H, Schlegel W and
Reymond MA: Discordance between K-ras mutations in bone marrow
micrometastases and the primary tumor in colorectal cancer. J Clin
Oncol. 19:2837–7843. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zauber P, Sabbath-Solitare M, Marotta SP
and Bishop DT: Molecular changes in the Ki-ras and APC genes in
primary colorectal carcinoma and synchronous metastases compared
with the findings in accompanying adenomas. Mol Pathol. 56:137–140.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Albanese I, Scibetta AG, Migliavacca M,
Russo A, Bazan V, Tomasino RM, Colomba P, Tagliavia M and La Farina
M: Heterogeneity within and between primary colorectal carcinomas
and matched metastases as revealed by analysis of Ki-ras and p53
mutations. Biochem Biophys Res Commun. 325:784–791. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Baas JM, Krens LL, Guchelaar HJ, Morreau H
and Gelderblom H: Concordance of predictive markers for EGFR
inhibitors in primary tumors and metastases in colorectal cancer: A
review. Oncologist. 16:1239–1249. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Khasraw M, Brogi E and Seidman AD: The
need to examine metastatic tissue at the time of progression of
breast cancer: Is rebiopsy a necessity or a luxury? Curr Oncol Rep.
13:17–25. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Reck M, Popat S, Reinmuth N, De Ruysscher
D, Kerr KM and Peters S: ESMO Guidelines Working Group: Metastatic
non-small-cell lung cancer (NSCLC): ESMO clinical Practice
guidelines for diagnosis, treatment and follow-up. Ann Oncol.
25:(Suppl 3). iii27–iii39. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Palmgren JS, Karavadia SS and Wakefield
MR: Unusual and underappreciated: Small cell carcinoma of the
prostate. Semin Oncol. 34:22–29. 2007. View Article : Google Scholar : PubMed/NCBI
|