Introduction
Cervical cancer is the fourth most common malignant
tumor in women and is responsible for the fourth-highest mortality
rate (1). Early-stage cervical cancer
can be cured with comprehensive treatment, which can include
surgery, radiotherapy and chemotherapy, among others. However,
approximately one-third of patients suffer recurrence, 75% of whom
experience it within 2 years of treatment (2). Treatment of recurrent cervical cancer is
effective in only 25% of cases and the median survival time is only
12 months (3). If the recurrence is
local and the patient has no history of prior radiotherapy and
cannot undergo surgical resection, radiotherapy is be used in
combination with platinum-based chemotherapy. Brachytherapy can
also be used, for cases where the tumor did not entirely subside
(4).
Cervical cancer brachytherapy primarily involves
intracavitary afterloading and the interstitial implantation of a
radiation source. Broadly speaking, interstitial radioactive seed
implantation is also included within the scope of brachytherapy,
although it is not recommended as a routine treatment of cervical
cancer under certain guidelines. The present case concerning
non-central recurrence of cervical cancer was treated with
intensity-modulated radiotherapy (IMRT) in combination with
chemotherapy and interstitial iodine-125 (125I) seed
implantation. At the point of submission of the present manuscript,
the progression-free survival (PFS) time had reached 26 months.
This suggests that interstitial 125I seed implantation
can be used as a complementary treatment for recurrent cervical
cancer and, as the patient had characteristics similar to primary
cervical cancer, may even have potential as a treatment for primary
cervical cancer.
Case report
A 47-year-old woman presented to the Department of
Gynecology of Changchun Central Hospital (Changchun, China) in July
2011 with contact vaginal bleeding. A diagnosis of cervical
squamous cell carcinoma was reached by cervical biopsy and
pathological analysis. The analysis revealed irregular cell
morphology, large and irregular cell nuclei and cancer cell nests
in stroma. The patient underwent a radical hysterectomy, left
lateral adnexectomy and pelvic lymph node dissection on August 4,
2011. A postoperative pathological microscopic examination of a
hematoxylin and eosin (H&E)-stained tissue sample revealed an
irregular cell shape, with large, irregular and deeply stained
nuclei, and single keratinocytes, further confirming the diagnosis
of squamous cell carcinoma (Fig. 1A and
B). No cancerous cells were found in the vaginal stump or
parametrial tissue and no evidence of metastasis was found in
selected lymph node samples (5 lymph nodes were sampled per group).
No further treatments, including radiotherapy and chemotherapy,
were administered following surgery.
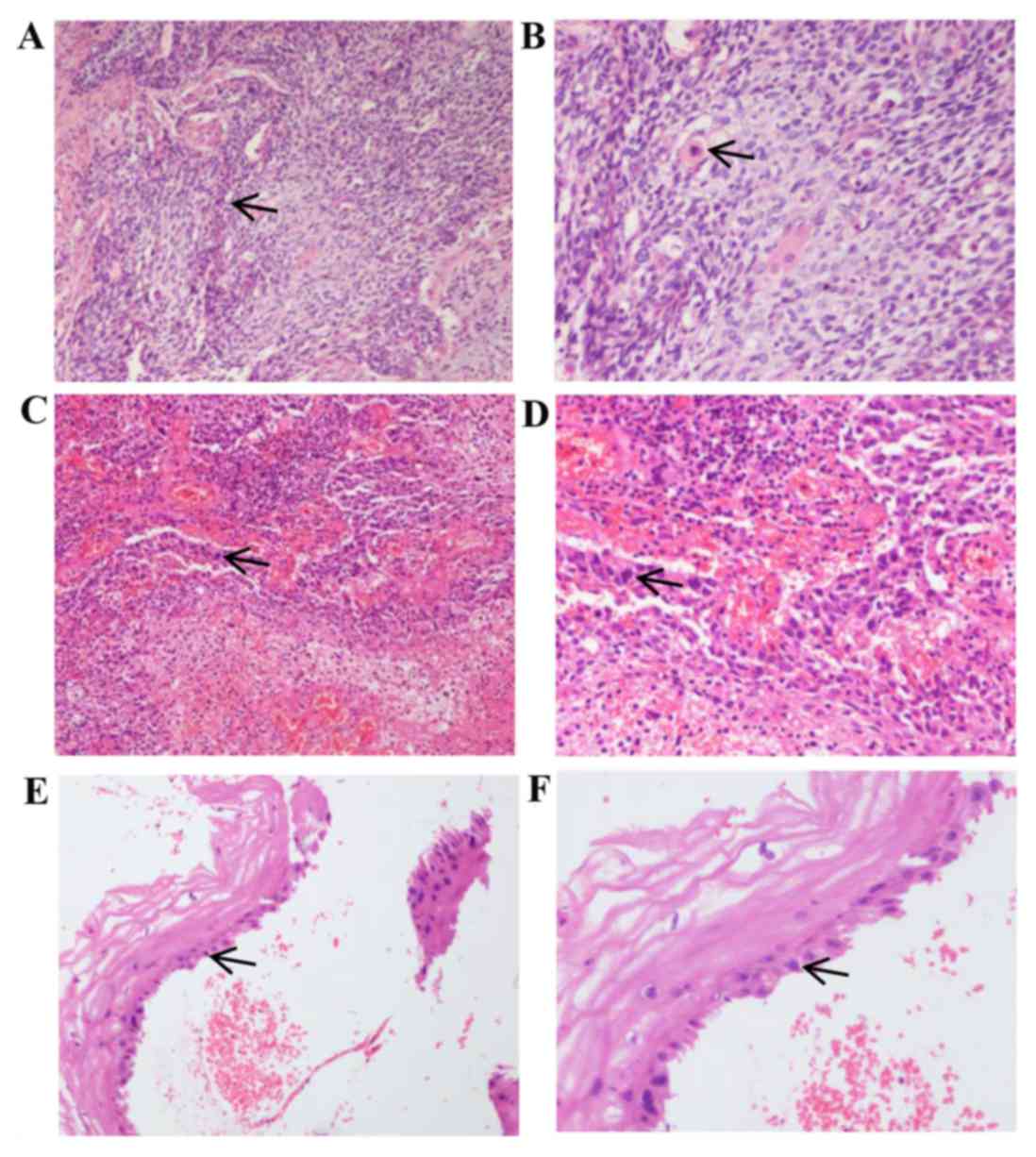 | Figure 1.H&E staining of cervical and
recurrent tumor tissues. (A) Magnification ×100 and (B) ×200 images
of H&E staining of initially diagnosed cervical tissues.
Microscopy reveals an irregular cell shape, large, irregular and
deeply stained nuclei, and single keratinocytes, confirming the
diagnosis of squamous cell carcinoma. (C) Magnification ×100 and
(D) ×200 images of H&E staining of cervical stump tissue from
recurrent cancer. At the higher magnification in (D), tumor cells
can be observed to invade the stroma of the cervix. The cells have
atypia and dark nuclei, supporting the diagnosis of squamous cell
carcinoma. (E) Magnifcation ×100 and (F) ×200 images of H&E
staining of the cervical stump following external-beam irradiation,
biopsy and chemotherapy. The squamous cells exhibit large, blurred
nuclei, which are considered to be reactive changes caused by
radiotherapy. Arrows indicate squamous cells in each image.
H&E, hematoxylin and eosin. |
Written informed consent was obtained from the
patient for publication of this case report and any accompanying
images, in addition to permission from the Ethics Committee of the
Second Hospital of Jilin University (Changchun, China).
The patient presented to the Department of
Radiotherapy of the Second Hospital of Jilin University in July
2013 (23 months after surgery) with lower abdominal pain and
abnormal vaginal discharge containing small amounts of blood.
Liquid-based cervical cytology indicated a high-grade squamous
intraepithelial lesion. An H&E-stained cervical stump biopsy
revealed cells with atypia and dark nuclei, supporting the
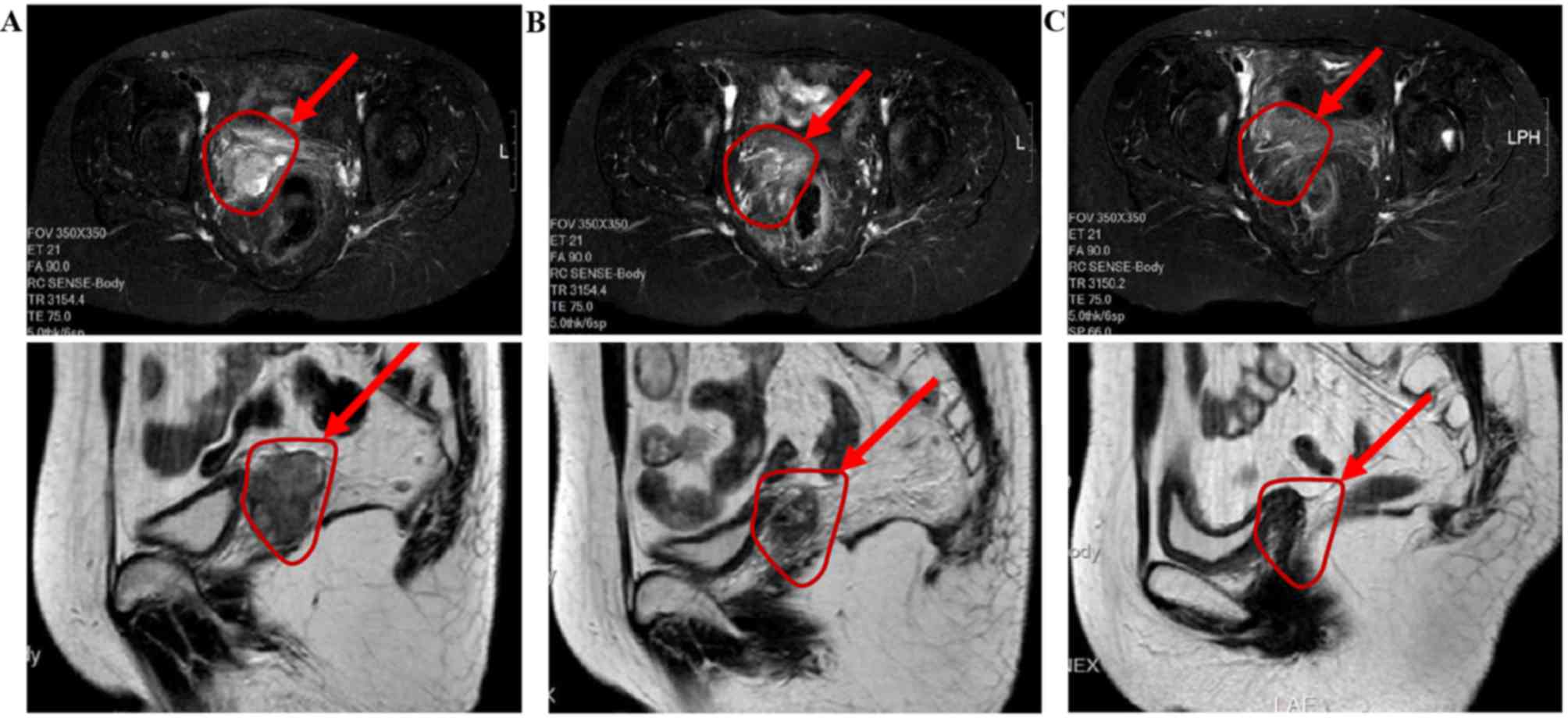
diagnosis of squamous cell carcinoma (Fig. 1C and D). Pelvic magnetic resonance
imaging (MRI) revealed an oval iso-T1 and long T2 signal shadow at
the vaginal level, with a maximum lesion diameter of ~38 mm,
departing from the center of the vagina by ~40 mm. An enhanced scan
showed an evident heterogeneous enhancement (Fig. 2A). Levels of the serum tumor marker
cancer antigen 125 (CA125) were 286.3 U/ml, >8 times higher than
the normal limit (≤35 U/ml). The most common sites of metastases,
including the lungs, liver and bone, exhibited no abnormalities.
Thus, the case was diagnosed as recurrent cervical cancer.
Between July and August 2013 (23–24 months after
surgery), the patient was treated with 2 cycles of intravenous
docetaxel (120 mg) and nedaplatin (100 mg) at a 21-day interval.
Following this, pelvic MRI was performed, which showed a round
iso-T1 iso-T2 signal shadow at the cervical stump level. The
diameter of the shadow was ~22 mm, departing from the center of the
vagina by ~30 mm. Enhanced MRI showed a heterogeneous enhancement
with an uneven distribution of microvessels (Fig. 2B). The size of the shadow was markedly
decreased when compared with that prior to treatment.
Between September and November 2013 (25–27 months
after surgery), the patient was treated with external-beam
radiation therapy, which ran concurrent with the previously
described chemotherapy cycles. Following this, the patient was
treated with another 2 cycles of chemotherapy. Again, the
chemotherapy program was 2 cycles of intravenous docetaxel (120 mg)
plus nedaplatin (100 mg) with a 21-day interval. The patient was
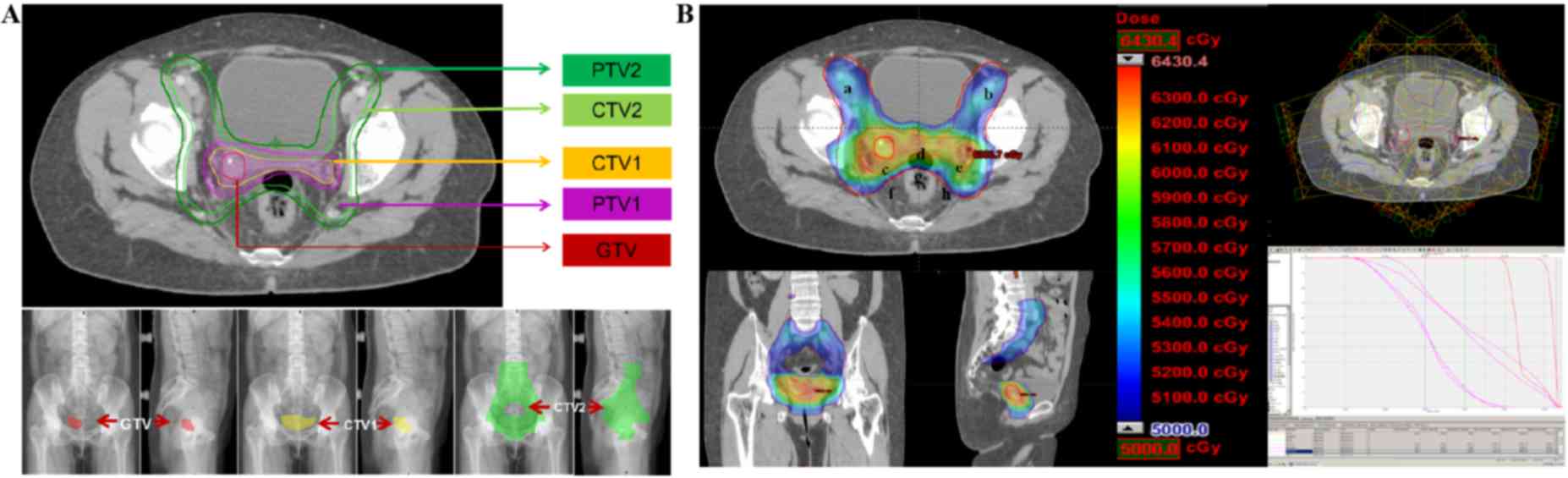
treated with 6 MV X-ray IMRT. The total dose of prophylactic
radiation in the pelvic lymph drainage area was 50.4 Gy using 1.8
Gy/F for 28F. The radiation doses for the cervical stump and
non-central isolated lesion were synchronously increased to 59.92
Gy using 2.14 Gy/F for 28F (Fig.
3B).
Following external-beam radiation and chemotherapy,
pelvic MRI was performed to examine the effect of treatment on
November 24, 2013 (27 months after surgery). No clear swollen
shadow was observed at the pelvic lymph nodes, indicating that the
nodes were normal. The lesion was ill-defined with its surrounding
tissue and had become substantially smaller (Fig. 2C) compared with that on the MRI from
August 26, 2013 (Fig. 2B). A
gynecological examination revealed vaginal patency, no abnormal
secretions, no mucous membrane congestion and a smooth vaginal wall
with no palpable nodules. However, a cervical stump biopsy revealed
squamous cell hyperplasia, cell heterogeneity and the formation of
granulation tissue (Fig. 1E and F).
Levels of the tumor marker CA125 were 6.1 U/ml, recovering to a
normal level. Following chemotherapy and external-beam radiation, a
residual tumor shadow remained present upon MRI. However, no cancer
cells were observed in the cervical stump and the size of the
parametrial isolated tumor lesion was markedly reduced. Thus,
brachytherapy was continued following the end of external-beam
radiotherapy.
On December 2, 2013 (28 months after surgery and 2
weeks after the cessation of external-beam radiation and
chemotherapy), the patient was admitted to the Department of
Radiotherapy of the Sino-Japanese Friendship Hospital of Jilin
University for 125I radioactive seed-implantation
therapy. A total of 14 125I seeds were implanted, with
seed radiation covering the recurrence area. Seed-implantation
treatment plans are shown in Table I.
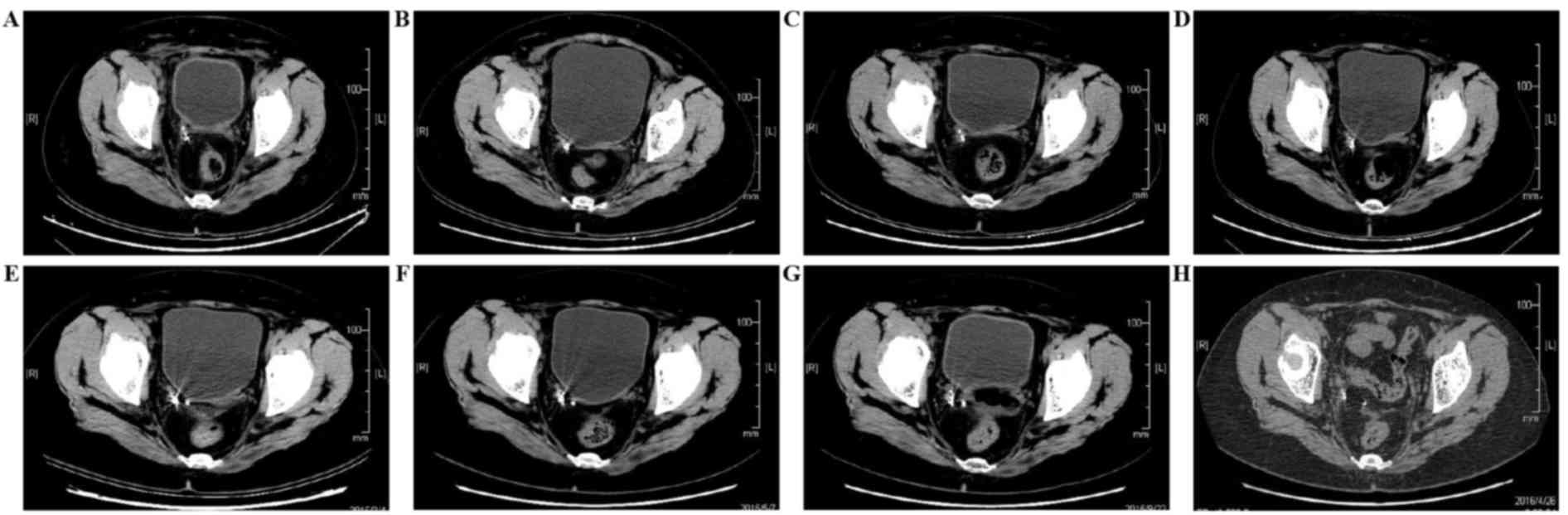
Examinations were performed at 1, 6, 10, 15, 17, 21 and 28 months
after implantation (Fig. 4). The
images in the first month following implantation showed radiation
particle aggregation and effective seed distribution. At 28 months
after implantation, a computed tomography (CT) scan revealed that
pelvic seed particles were scattered and were not fixed at the
designated location. No evident mass had appeared at the location
of tumor recurrence (Fig. 4H). The
physical condition of the patient was good; the performance status
score was 0 points and the patient did not complain of any
discomfort.
 | Table I.Seed implantation treatment plan. |
Table I.
Seed implantation treatment plan.
|
|
|
| Volume of the tumor
receiving dose, cc (%) | Dose received by
fraction of the tumor, cGy | Dose, cGy |
|---|
|
|
|
|
|
|
|
|---|
| Region | Total volume, cc | Dose level volume, cc
(%) | 150 Gy | 100 Gy | 90 Gy | 100% | 90% | 80% | Min | Max | Mean | Median | Mode |
|---|
| Tumor | 65.1 | 59.3 (91.2) | 39.9 (61.4) | 59.3 (91.2) | 61.5 (94.5) | 4,400.0 | 14,992.1 | 18,085.8 | 4,338.8 | 172,906.6 | 19,947.8 | 21,750.0 | 21,750.0 |
| Vessel | 27.1 | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) |
300.0 |
439.8 |
545.0 |
263.1 |
2,474.9 |
963.6 |
850.0 |
550.0 |
| Spinal cord | 7.5 | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) |
600.0 |
743.3 |
833.4 |
518.0 |
2,851.0 |
1,256.0 |
1,150.0 |
950.0 |
Discussion
The present patient was a 47-year-old woman in good
health with a performance status score of 1. The primary disease
was diagnosed as International Federation of Gynecology and
Obstetrics (FIGO) stage IB1 cervical cancer. The postoperative
pathological examination was negative for cancerous tissue, with no
vascular invasion and no high-risk factors identified. Therefore,
no further treatment was administered following surgery. However,
the patient presented with recurrent disease 23 months after
surgery. MRI did not reveal pelvic lymph node metastasis, but a
solitary lesion with a maximum diameter of ~38 mm was found towards
the right side of the cervical stump level. A biopsy of the
cervical stump revealed squamous cell carcinoma, consistent with
the diagnosis of the primary tumor. MRI images did not reveal
evident space occupation in the cervical stump (Fig. 2A).
The case was diagnosed as the non-central recurrence
of cervical squamous cell carcinoma. The patient had not received
chemoradiotherapy following surgery and had only one single
recurred lesion without metastasis. According to 2016 NCCN
Guidelines for cervical cancer (4),
patients with a localized recurrence of cervical cancer following
initial treatment may be candidates for radical retreatment.
Treatment options include tumor-directed radiotherapy,
brachytherapy and/or chemotherapy (if no prior radiotherapy has
been administered or the lesion is present outside of the
previously treated field) and surgery. As the patient refused
surgery, following repeated discussions a treatment regimen of
IMRT, chemotherapy and 125I seed implantation was
chosen.
In clinical practice, the local or regional
recurrence of cervical cancer, if it is unresectable and the
patient has no history of radiotherapy, can be treated with
radiotherapy in combination with platinum-based chemotherapy.
Brachytherapy can be used in addition, according to different
disease conditions (4).
A phase III clinical trial performed by Monk et
al (5) revealed that in FIGO
stage IVB recurrent or refractory cervical cancer, a dual-drug
regimen of vinorelbine plus cisplatin, gemcitabine plus cisplatin
or topotecan plus cisplatin chemotherapy did not produce superior
overall survival, PFS or response rates compared with paclitaxel
with cisplatin. A phase III clinical trial by Moore et al
(6) also supported the dual-drug
regimen of paclitaxel plus cisplatin, finding that it produced
significantly better PFS than cisplatin monotherapy (P<0.001).
On the basis of these studies and the toxicity of various
chemotherapy drugs, docetaxel (as it is similar to paclitaxel, with
a reduced likelihood of an allergic reaction) plus nedaplatin (as
it is similar to cisplatin, with a reduced likelihood of digestive
adverse reactions) was selected for this case. Evaluation following
2 cycles of chemotherapy showed that the size of the tumor had
decreased from 38 to 22 mm, confirming the effectiveness of the
chemotherapy. Thus, this chemotherapy was applied for a total of 4
cycles.
On the basis of the radiotherapy target for cervical
cancer recommended by the US Radiation Therapy Oncology Group and
our own clinical practice, the present patient was treated with
external-beam radiation using IMRT. The radiation area involved the
lymphatic drainage area of the internal iliac, external iliac,
presacral and obturator foramen, and a region of the vaginal and
parametrial tissue (7). The total
dose was 50.4 Gy (Fig. 3B).
As described in Cancer Radiation Therapy by Gu et
al (8), the most common
radiotherapy treatment for cervical cancer is the combined use of
external-beam and intracavitary radiation. The proper coordination
of the two techniques can partly compensate for the disadvantages
that result from the uneven distribution of a brachytherapy dose.
If permitted, the radiation dose can be appropriately increased to
complement the insufficient radiation in the parametrial tissue
from intracavitary radiation. Therefore, in uncertain conditions
(with a high-risk target, for example), the radiation dose is
synchronously increased to 60 Gy in the tumor area and parametrial
tissue using external-beam radiotherapy (Fig. 3B).
In clinical practice, conventional internal
radiotherapy primarily includes intracavitary afterloading and
interstitial implantation. According to the linear quadratic
formula and its derived formula, the basic formula of equivalent
transformation of different segmentation scheme is
n2d2[1+d2/(α/β)]=n1d1(1+
d1/(α/β)], with n, d and nd refering to the radiation
number, dose of one radiation and the total dose, respectively. α/β
is defined as the tissue-specific α/β value. When the prescribed
dose is 6 Gy and the total number of fractions is 5, the equivalent
biological dose is five fractions of 8 Gy, giving a total of 40 Gy
(α/β is set as 10). Thus, the total curative dose of external beam
radiation plus afterloading should be 85–90 Gy. In other words,
once patients have received external-beam radiation, they then
require 4–6 treatments of intracavitary afterloading or
interstitial implantation. However, this dose could increase the
radiation in the bladder posterior wall and the rectal anterior
wall, which may increase the likelihood of long-term adverse
reactions in these two organs.
As the present case concerned non-central recurrent
cervical cancer with a lesion that deviated from the vaginal center
by ~30 mm, conventional intracavitary afterloading would lead to an
uneven dose distribution in the target area, perhaps even missing
it altogether. If the target area is to be covered by the isodose
curve of the dose reference point, the dose and volume of radiation
received by the rectum and bladder will be increased. Toita et
al (9) reported a
three-dimensional interstitial implantation and brachytherapy
system, in which the three-dimensional position of an implantation
needle was reconstructed, and the gross tumor volume, clinical
target volume and radiation dose in the relevant organs were
redefined. Consequently, the dose curve of the reference point
closely conformed to that of the treatment target, meaning it is
possible that the tumor area was accurately radiated by high-dose
radiation (9).
According to Sharma et al (10), interstitial implantation-guided
afterloading radiotherapy is notably superior to conventional
afterloading radiotherapy. In interstitial implantation, the
radiation dose to the target area is homogeneously distributed with
high coverage, reducing the volume and dose of radiation in normal
tissues, including the bladder and rectum. However, when compared
with conventional intracavitary afterloading, interstitial
implantation-guided afterloading radiotherapy is relatively
invasive. The implantation process requires the coordination of
multiple implantation needles and repeated CT scans to confirm and
adjust the location and depth of the needles. The total treatment
plan requires 4–6 implantation procedures, which increases the risk
of bleeding, infection and radiation hazards, and the possibility
of iatrogenic tumor seeding and metastasis.
125I seed implantation is a minimally
invasive, widely used treatment that has unique advantages in the
treatment of locally advanced tumors (11,12). In
the US, radioactive seed implantation has been used as the standard
treatment for early prostate cancer (13). However, the use of 125I
seed implantation has rarely been reported for the treatment of
non-central recurrent cervical cancer. The 125I seed has
a half-life of 59.6 days and has similar biological characteristics
to hyperfractionated radiotherapy; 125I seeds
continuously irradiate cells in different phases of the cell cycle
through the continuous release of low-energy γ-rays, increasing
their sensitivity to radiation and promoting recurrent cervical
cancer cell death (14).
Once the size of the tumor is confirmed by a CT scan
and the treatment plan is decided upon using a three-dimensional
treatment-planning system, the radioactive seeds are implanted into
the tumor using ultrasound-guidance. The photons released from the
radioactive seeds produce continuous, low-dose radiation at the
position of the lesion (14). This
treatment method achieves the purpose of conventional radiotherapy,
while protecting the surrounding normal organs as much as
possible.
Although the initial radiation dose-rate of the
125I seeds was low in the present study, it did not
cause uncontrolled tumor growth, as the present case was of
squamous cell carcinoma, in which tumor cell proliferation is slow.
In the present study, the patient and her family were provided
detailed information on the treatment regimen and consented to
interstitial 125I seed-implantation therapy following
the end of external-beam radiotherapy and chemotherapy.
The efficacy of the treatment was evaluated using
MRI or CT images, based on the Response Evaluation Criteria In
Solid Tumors (15). Complete
remission (CR) refers to the disappearance of all target lesions,
the presence of no new lesions, and levels of tumor markers within
the normal range. All these should be maintained for at least 4
weeks. Partial remission (PR) is achieved when the sum of the
maximum diameters of target lesions are reduced by ≥30% for at
least 4 weeks. A pelvic CT scan was examined 1 month after seed
implantation, at which point partial remission had been achieved. A
further CT scan 21 months after the treatment revealed there was
CR. Evaluation of treatment efficacy was performed every 3 months
using pelvic MRI or CT scans and no progression was observed
(Fig. 4). The latest evaluation of
the patient revealed that the PFS time had reached 33 months.
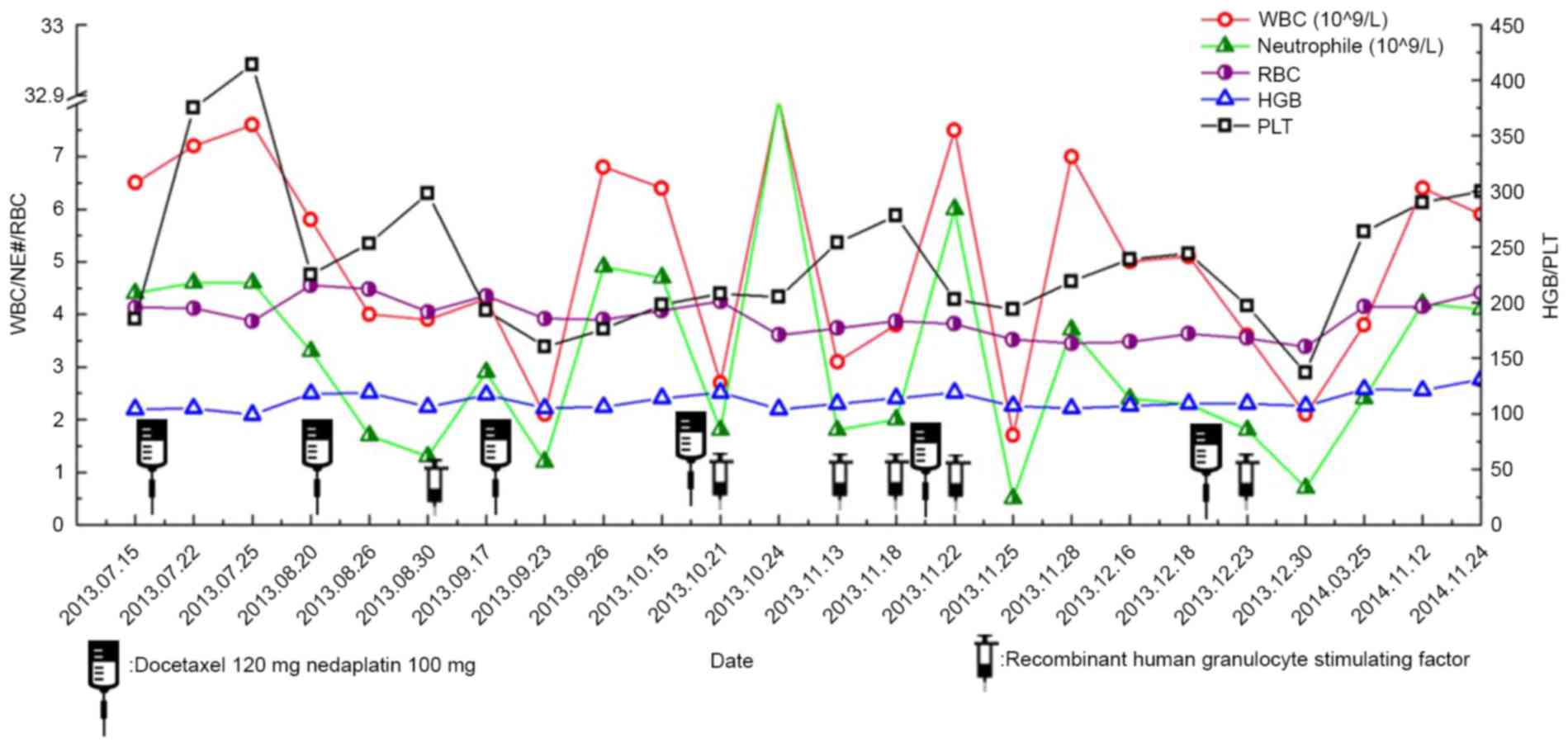
Different degrees of bone marrow suppression
occurred during treatment, including four incidences of grade 1
bone marrow suppression, two incidences of grade 2 and two
incidences of grade 3. No incidences of grade 4 occurred (Fig. 5). Following the administration of
recombinant human granulocyte colony stimulating factor, bone
marrow suppression was improved. No infection event occurred.
At 11 months after seed implantation, the patient
suffered diarrhea and a grade 2 gastrointestinal adverse effect. A
colonoscopy revealed mucosa congestion, edema and poorly defined
vasculature in the large intestine (Fig.
6). After 1 week of administration of intestinal mucosal
protective agents and active symptomatic treatment, these symptoms
completely disappeared.
Data from the present study show that IMRT combined
with 125I seed implantation, as a supplement for the
treatment of recurrent cervical cancer, can achieve the desired
curative effect. As the patient in the present study had similar
characteristics to primary cervical cancer, it may have the
potential for use as a reliable radical radiotherapy in newly
diagnosed cervical cancer.
Glossary
Abbreviations
Abbreviations:
|
IMRT
|
intensity-modulated radiotherapy
|
|
PFS
|
progression-free survival
|
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goncalves A, Fabbro M, Lhommé C, Gladieff
L, Extra JM, Floquet A, Chaigneau L, Carrasco AT and Viens P: A
phase II trial to evaluate gefitinib as second- or third-line
treatment in patients with recurring locoregionally advanced or
metastatic cervical cancer. Gynecol Oncol. 108:42–46. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Elit LM and Hirte H: Management of
advanced or recurrent cervical cancer: Chemotherapy and beyond.
Expert Rev Anticancer Ther. 14:319–332. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
National Comprehensive Cancer Network
(NCCN): NCCN Clinical Practice Quidelines in Oncology: Cervical
Cancer. version 2. 2015 http://www.nccn.org/professionals/physician_gls/f_guidelines.aspAccessed.
March 11–2017.
|
|
5
|
Monk BJ, Sill MW, McMeekin DS, Cohn DE,
Ramondetta LM, Boardman CH, Benda J and Cella D: Phase III trial of
four cisplatin-containing doublet combinations in stage IVB,
recurrent, or persistent cervical carcinoma: A gynecologic oncology
group study. J Clin Oncol. 27:4649–4655. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Moore DH, Blessing JA, McQuellon RP,
Thaler HT, Cella D, Benda J, Miller DS, Olt G, King S, Boggess JF
and Rocereto TF: Phase III study of cisplatin with or without
paclitaxel in stage IVB, recurrent, or persistent squamous cell
carcinoma of the cervix: A gynecologic oncology group study. J Clin
Oncol. 22:3113–3119. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Japan Clinical Oncology Group, . Toita T,
Ohno T, Kaneyasu Y, Uno T, Yoshimura R, Kodaira T, Furutani K,
Kasuya G, Ishikura S, et al: A consensus-based guideline defining
the clinical target volume for pelvic lymph nodes in external beam
radiotherapy for uterine cervical cancer. Jpn J Clin Oncol.
40:456–463. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gu X, Yin W, Hu Y, et al: Tumor radiation
therapy. Pecking Union Medical College Press; Beijing: 2007
|
|
9
|
Toita T, Kitagawa R, Hamano T, Umayahara
K, Hirashima Y, Aoki Y, Oguchi M, Mikami M and Takizawa K: Cervical
Cancer (Vulva Cancer) Committee of Japanese Gynecologic Oncology
Group (JGOG): Phase II study of concurrent chemoradiotherapy with
high-dose-rate intracavitary brachytherapy in patients with locally
advanced uterine cervical cancer: Efficacy and toxicity of a low
cumulative radiation dose schedule. Gynaecol Oncol. 126:211–216.
2012. View Article : Google Scholar
|
|
10
|
Sharma DN, Subramani V, Rath GK, Ganesh T,
Julka PK, Basu Jyothi KS, Bahl A and Gopishankar N: Interstitial
brachytherapy guided intensity modulated radiation therapy in
cervical cancer: A dosimetric study. Int J Radiation Oncol Biol
Phys. 69:(Suppl). S731–S732. 2007. View Article : Google Scholar
|
|
11
|
Shi L, Wu C, Wu J, Zhou W, Ji M, Zhang H,
Zhao J, Huang Y, Pei H, Li Z, et al: Computed tomography-guided
permanent brachytherapy for locoregional recurrent gastric cancer.
Radiat Oncol. 7:1142012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang J, Jiang Y, Li J, Tian S, Ran W and
Xiu D: Intraoperative ultrasound-guided iodine-125 seed
implantation for unresectable pancreatic carcinoma. J Exp Clin
Cancer Res. 28:882009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nag S, Ellis RJ, Merrick GS, Bahnson R,
Wallner K and Stock R: American Brachytherapy Society: American
brachytherapy society recommendations for reporting morbidity after
prostate brachytherapy. Int J Radiat Biol Phys. 54:462–470. 2002.
View Article : Google Scholar
|
|
14
|
Yao L, Wang J, Jiang Y, Li J, Lin L, Ran W
and Liu C: Permanent interstitial 125I seed implantation as a
salvage therapy for pediatric recurrent or metastatic soft tissue
sarcoma after multidisciplinary treatment. World J Surg Oncol.
13:3352015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|