Introduction
Urinary bladder cancer (UBC) is one of the most
common types of malignant tumor in the United States, with an
estimated 58,950 new cases and 11,820 UBC-associated mortalities in
2016 (1). Data between 2005 and 2011
in USA revealed that the 5-year survival rate for localized UBC was
~70%, whereas the rate for patients with UBC with distant lesions
was ~5% (1). In China, bladder cancer
prevalence ranks the 9th and the 2nd positions for the entire
population, and people >65 years, respectively (2). However, current treatments, including
chemotherapy and radiotherapy possess limited effects on muscle
invasive bladder cancer (>stage 2). Therefore, studies
investigating the underlying molecular mechanisms of UBC
development and the development of efficacious therapeutic reagents
for UBC, particularly for patients with invasive cancer are
warranted.
Steroid receptor coactivator-3 (SRC-3) and alias
amplification in breast cancer 1 belong to the p160 steroid
receptor coactivator family (3).
Amplification and/or overexpression of SRC-3 have been implicated
in steroid-targeted tissues, including in breast and prostate
cancer (4–6), and in non-steroid-targeted tissues,
including lung and bladder cancer (7–10).
Accumulating evidence indicates that SRC-3 can activate steroid and
non-steroid receptors. For example, SRC-3 serves as a co-activator
for transcription factors ETS variant 4 (PEA3) and JunB
proto-oncogene AP-1 transcription factor subunit, which leads to
the upregulation of matrix metalloproteinase (MMP)-2, and −13 in
androgen receptor-null PC3 prostate cancer cells (11). Furthermore, SRC-3 facilitates E2F
transcription factor 1 (E2F1) to promote the proliferation of
breast cancer cells (12). Previous
studies, including our previous study, have demonstrated that SRC-3
cooperates with hypoxia-inducible factor 1-α and E2F1, thus
promoting the survival and proliferation of UBC cells (9,13).
However, whether SRC-3 serves a role in cell migration and invasion
of UBC remains unclear.
Honokiol is the major active component derived from
the stem and bark of the plant Magnolia officinalis, a
traditional Chinese medicine. As one of the major lignans with high
bioavailability, honokiol exhibits multiple biological properties,
including muscle relaxant, neuroprotective, anti-inflammatory and
anticancer effects (14–19). However, whether honokiol exhibits an
effect UBC cell migration and invasion remains unclear. The present
study demonstrated that honokiol inhibited UBC cell invasion by
repressing the process of epithelial-mesenchymal transition (EMT).
It was further revealed that honokiol downregulated Twist1 (an
EMT-associated transcription factor) and MMP-2 (an enzyme
associated with cell invasion) via suppressing SRC-3 expression.
However, overexpression of SRC-3 reversed the honokiol-mediated
inhibition of UBC cell migration and invasion.
Materials and methods
Human bladder cancer cell line and
reagents
The human bladder cancer J82 cell line was obtained
from the Type Culture Collection of the Chinese Academy of Sciences
Cell Bank (Shanghai, China) and maintained in RPMI-1640 medium (cat
no. 31800-022; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% fetal bovine serum (FBS-12A; Capricorn
Scientific GmbH, Ebsdorfergrund, Germany). Cells were cultured at
37°C in a humidified atmosphere with 5% CO2. HonoPure
(98% honokiol; EcoNugenics, Santa Rosa, CA, USA) was dissolved in
dimethyl sulfoxide and further diluted with RPMI-1640 medium
immediately prior to use. For each protocol, cells treated with
DMSO vehicle were used as the negative control.
Luciferase assay
Luciferase assays were performed using a
Dual-Luciferase Reporter Assay System kit (cat no. 1910; Promega
Corporation, Madison, WI, USA) according to the manufacturer's
protocol. The Twist1 gene promoter was inserted into the pGL3-basic
vector (Promega Corporation) to generate the 100 ng Twist1 reporter
plasmid (Twist1-Luc) (20).
Subsequent to the J82 cells reaching 60% confluency in 24-well
plates, Twist1-Luc plasmid was co-transfected into cells with 100
ng SRC-3 expression plasmid, which was constructed by the insertion
of the open reading frame of the human SRC-3 gene into
pCMV10-3xFLAG (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
(9). Honokiol at various
concentrations (0–4.8 µg/ml) were added 1 day following plasmid
transfection. After 24 h of incubation at 37°C, the cells were
lysed for use in the luciferase assay. A total of 100 µl 1X Passive
Lysis Buffer (Promega Corporation) were used to lyse the cells, and
then they were tested for luciferase activity according to the
protocol. Renilla luciferase activity was used for
normalization.
RNA isolation and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was isolated using TRIzol reagent (cat no.
15596018; Invitrogen; Thermo Fisher Scientific, Inc.). RT was
conducted with random primers in the Takara PrimeScript™ RT reagent
system (Takara Biotechnology Co., Ltd., Dalian, China) according to
the manufacturer's protocol. The expression levels of genes were
measured using SYBR-Green-based qPCR (Takara Biotechnology Co.,
Ltd.). The thermocycler protocol was 95°C for 10 sec, then 95°C for
5 sec, 60°C for 31 sec for 40 cycles from step 2 to step 3. The
formula 2−ΔΔCq (Cq cycle threshold) was used to
determine the expression levels of target genes normalized by
β-actin (21). qPCR was performed in
triplicate for each sample. The primer sequences used were as
follows: SRC-3 forward, 5′-GGGACTAAGCAACAGGTGTTT-3′ and reverse,
5′-TTTGGCCCACCCATACTTGAG-3′; MMP-2 forward,
5′-CCGTCGCCCATCATCAAGTT-3′ and reverse, 5′-CTGTCTGGGGCAGTCCAAAG-3′;
Twist1 forward, 5′-TGGAGGACCTGGTAGAGGAA-3′ and reverse,
5′-GTCCGCAGTCTTACGAGGAG-3′; β-actin forward,
5′-CATGTACGTTGCTATCCAGGC-3′ and reverse,
5′-CTCCTTAATGTCACGCACGA-3′.
Western blotting
Cells were lysed in RIPA buffer containing a
phosphatase inhibitor cocktail I (Sigma-Aldrich; Merck KGaA) and a
protease inhibitor cocktail mini-tablet (Roche Diagnostics,
Indianapolis, IN, USA). Subsequently, Bradford regent was used to
determine protein concentration, and 20 µg protein/lane were
separated using 10% SDS-PAGE and transferred onto a polyvinylidene
difluoride membrane. The membrane was blocked by 5% non-fat milk at
room temperature for 1 h. Primary antibodies directed against
E-cadherin (cat no. BS1098; 1:1,000; Bioworld Technology, Inc., St.
Louis Park, MN, USA), N-cadherin (cat no. 22018-1-AP; 1:1,000;
ProteinTech Group, Inc., Chicago, IL, USA), SRC-3 (cat no. 611104;
1:1,000; BD Biosciences, San Jose, CA, USA), MMP-2 (cat no. 29090;
1:1,000), Twist1 (cat no. 21642; 1:1,000) (both from Signalway
Antibody, College Park, MA, USA), and β-actin (cat no. 05-0079;
1:1,000; AbMax Biotechnology Co., Ltd., Beijing, China) were
incubated with the membrane overnight at 4°C. Subsequent to washing
three times with 1X PBST [1 ml Tween-20 diluted in 1,000 ml 1X PBS
(140 mM NaCl, 2.7 mM KCl, 1.8 mM KH2PO4, 10 mM
Na2HPO4)], corresponding mouse and rabbit
secondary antibodies conjugated with horseradish peroxidase (cat
nos. 7076 and 7074; Cell Signaling Technology, Inc., Danvers, MA,
USA) were then used at room temperature for 2 h. The western blots
were visualized using enhanced chemiluminescence reagents (cat no.
180-501; Tanon Science and Technology Co., Ltd., Shanghai,
China).
Wound healing assay
Cells were seeded at a density of 5×105 cells/well
into 35-mm dishes and treated with 0, 2.4 or 4.8 µg/ml honokiol.
After 24 h, a wound scratch was made with a 100 µl pipette tip on
cell monolayer and images were captured after 24 h to estimate the
area occupied by migratory cells. Cells were maintained at 37°C
throughout the protocol.
Transwell invasion assay
Following treatment with different concentrations of
honokiol, 1×105 J82 cells were diluted in 500 µl serum-free
RPMI-1640 medium and inoculated in the upper Transwell chamber
coated with growth factor-reduced Matrigel. RPMI-1640 medium
containing 10% FBS was added to the lower chamber as a
chemoattractant. Following 16 h, cells on upper surface of the
membrane were removed using a Q-tip, and invaded cells were fixed
with 4% formaldehyde for 10 min at room temperature followed by
0.5% crystal violet staining (Sigma-Aldrich; Merck KGaA) for
another 10 min at room temperature. The numbers of invaded cells
were counted in five randomly chosen fields under a light
microscope at ×20 magnification.
Cell viability assay
J82 cells were seeded into 96-well plates at a
density of 1×104 cells/well. Honokiol at various concentrations
(0–4.8 µg/ml) were added 1 day after cell inoculation. Following
treatment with honokiol for 16 h, cells were washed with PBS and 5
mg/ml MTT was added for 3 h at 37°C. Subsequently, 100 µl DMSO/well
was loaded to dissolve the formazan crystals. Plates were incubated
at 37°C for 15 min. Absorbance at 490 nm was examined using a
microplate reader (BioTek Instruments, Inc., Winooski, VT, USA) and
absorbance at 680 nm was used as reference.
Immunofluorescence staining
Cells growing on the coverslips in 24-well plates
were fixed by 4% paraformaldehyde for 15 min and washed with PBS
three times. After blocking with 5% BSA in PBS for 60 min, the
coverslips were incubated in the primary antibodies against
E-cadherin (Bioworld Technology) and N-cadherin (ProteinTech Group,
Inc.) were used overnight at 4°C. Fluorescein-conjugated secondary
antibodies were applied, followed by DAPI counterstaining.
Statistical analysis
Each experiment was repeated three times. Data are
represented as the mean ± standard deviation following experiments
performed in triplicate. The significant difference between control
and experimental groups was analyzed using the Student's t-test.
P<0.05 was considered to indicate a statistically significant
difference. All of the statistical analyses were performed with
Graphpad Prism 5 software (GraphPad Software, Inc., La Jolla, CA,
USA).
Results
Honokiol inhibits UBC cell migration
and invasion
Patients with bladder cancer with metastatic lesions
have poor prognosis. Thus, an investigation into whether honokiol,
an anticancer traditional medicine, has any effects on bladder
cancer cell migration and invasion was performed. The highly
metastatic human bladder cancer J82 cell line was chosen for the
present study. J82 cells were treated with different concentrations
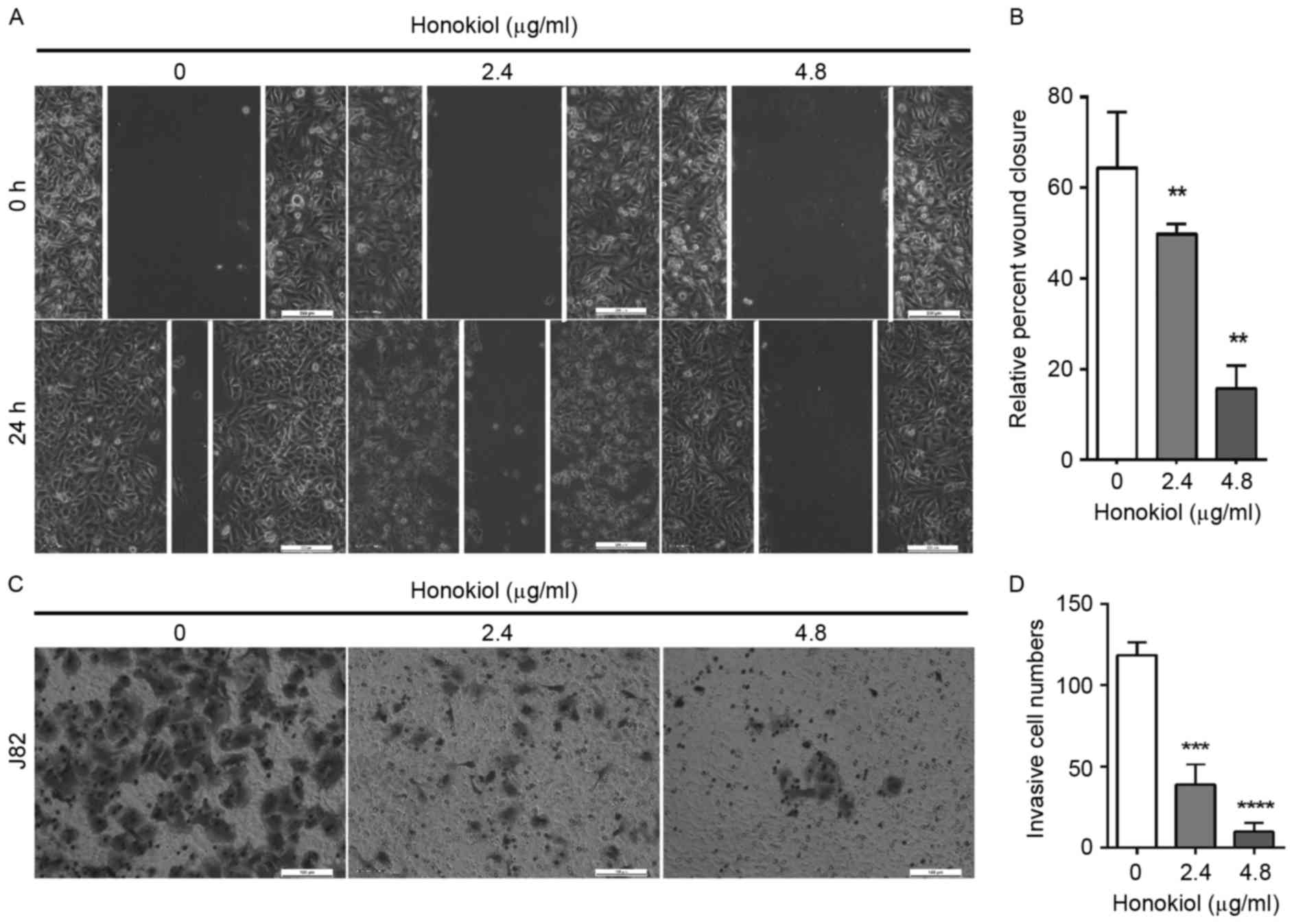
of honokiol (0, 2.4 and 4.8 µg/ml). The results from the wound
healing assay demonstrated that honokiol was capable of inhibiting
the migratory capacity of J82 cells in a dose-dependent manner
(Fig. 1A). Following 24 h treatment
with honokiol at 2.4 and 4.8 µg/ml, the wound closures were
significantly reduced by 23, and 75%, respectively, compared with
that in the vehicle-treated cell group (Fig. 1B). Invasion capacity of UBC cells was
measured using Transwell assays. The cells invading into the lower
chambers were significantly decreased upon treatment with honokiol
for 16 h compared with the vehicle-treated control group (Fig. 1C and D). The numbers of the invading
cells significantly reduced by 67 and 92% upon 2.4, and 4.8 µg/ml
honokiol treatment, respectively (Fig.
1D). In order to confirm that the honokiol-induced decrease in
migration and invasion ability was not merely due to the decrease
of cell number associated with honokiol-induced cell growth arrest,
a MTT assay was applied to determine UBC cell viability. J82 cell
viability was significantly decreased by 17 and 33% when treated
with 2.4, and 4.8 µg/ml honokiol for 16 h, respectively, compared
with the vehicle control group (data not shown). The inhibition on
cell viability observed was less compared with the effects on cell
invasion demonstrated using the Transwell assay. These data suggest
that honokiol can inhibit UBC cell migration and invasion.
Honokiol suppresses EMT of bladder
cancer cells
Since EMT has been implicated in cancer cell
invasion, whether EMT could be suppressed by honokiol treatment
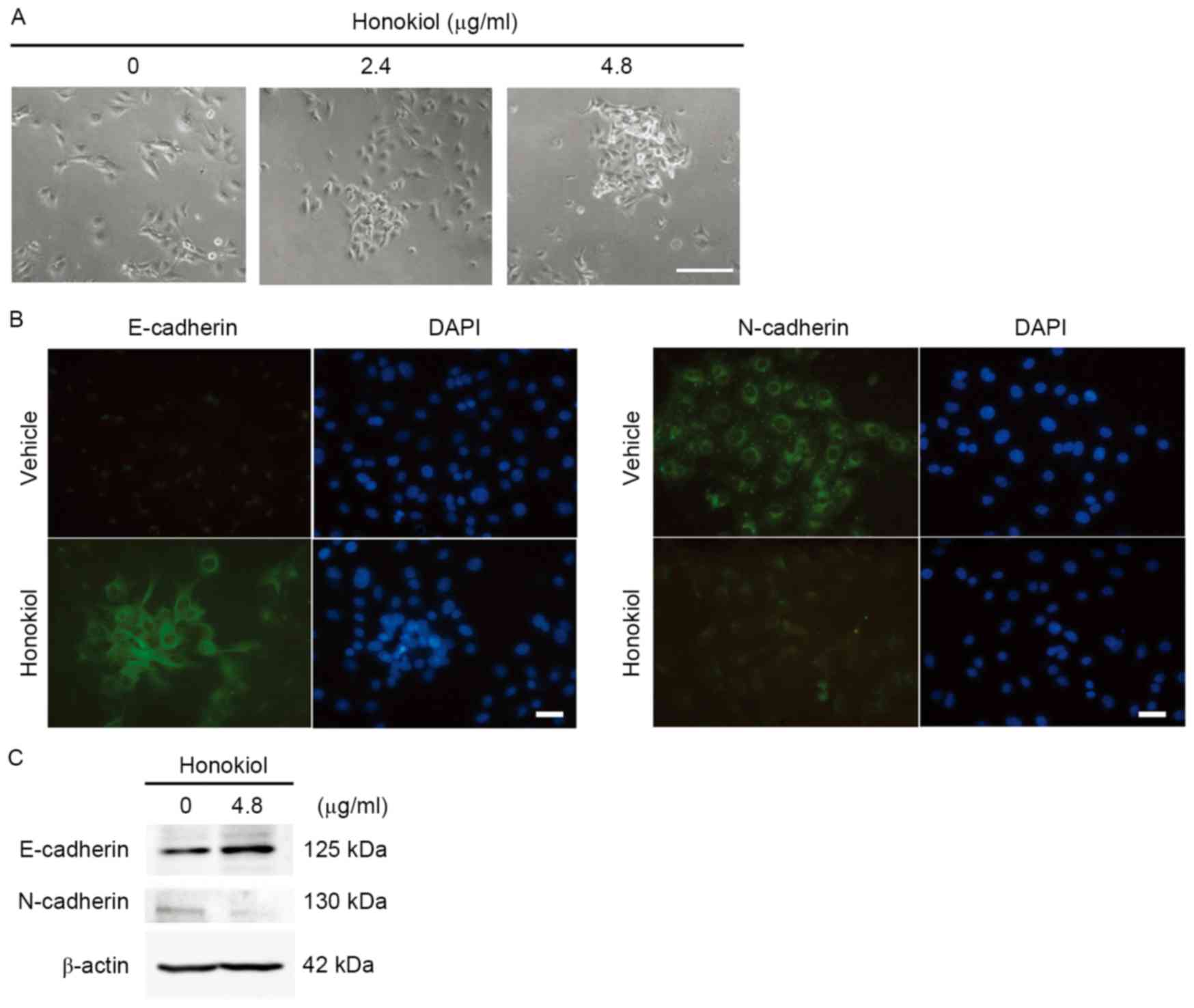
(4.8 µg/ml) was investigated in J82 cells. Morphological changes,
including cell-cell adhesion, were observed upon honokiol treatment
(Fig. 2A). Loss of E-cadherin and
gain of N-cadherin are considered to be the fundamental events of
EMT (22–24). Immunofluorescence staining assays
revealed increased expression of E-cadherin and the decreased
expression of N-cadherin (Fig. 2B),
which were further confirmed by the western blotting assay
(Fig. 2C). These results suggest that
honokiol suppresses EMT of UBC cells via regulating the expression
levels of E- and N-cadherin.
Honokiol downregulates expression
levels of cancer cell invasion-associated genes
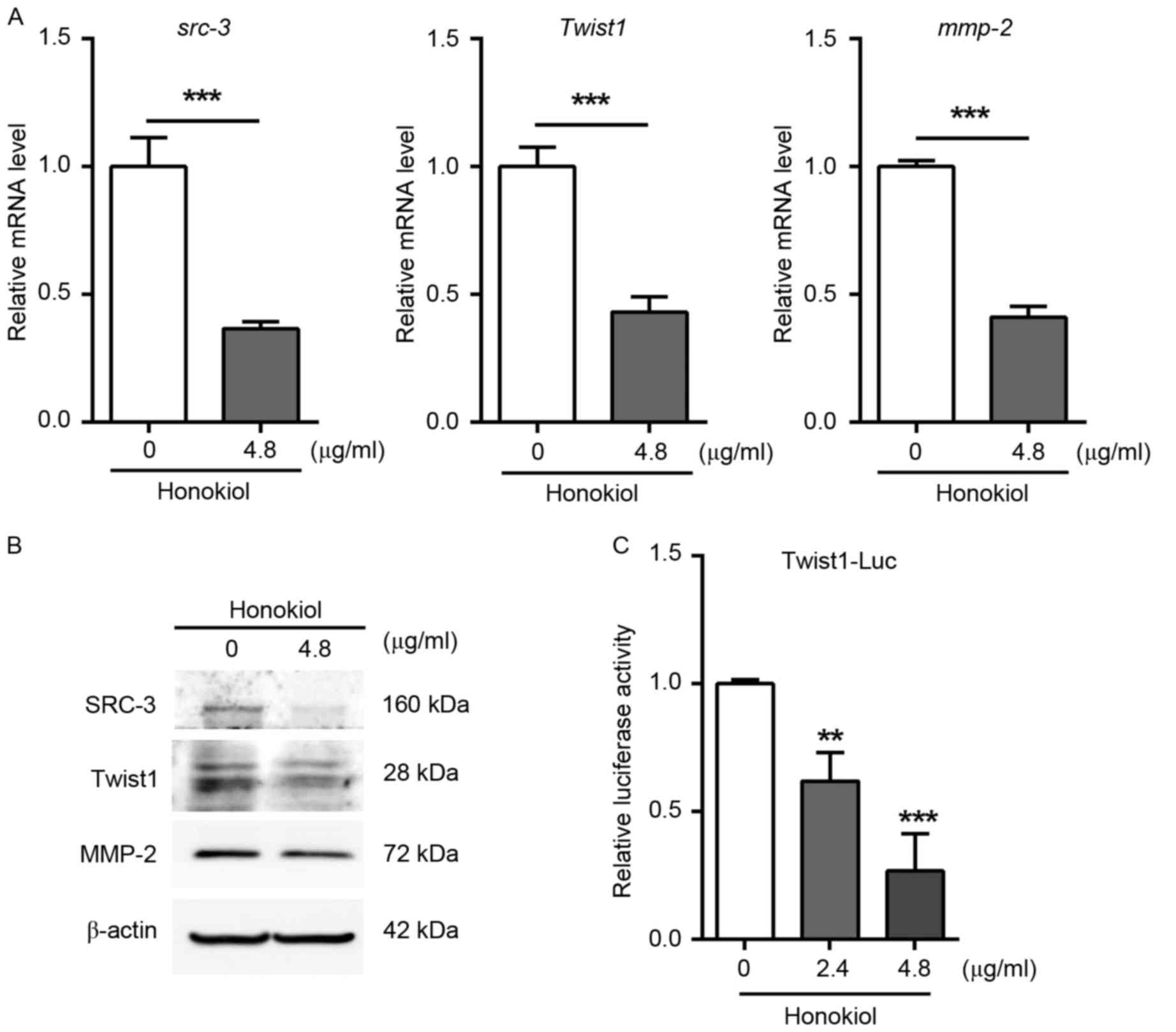
To investigate the mechanism underlying the
inhibition of UBC cell invasion induced by honokiol, the expression
levels of genes associated with cell invasion were determined using
RT-qPCR and western blotting assays. The results demonstrated that
the expression of SRC-3, MMP-2 and Twist1 was significantly
downregulated by honokiol at the mRNA (Fig. 3A) and protein (Fig. 3B) level compared with the
vehicle-treated control group. The positive association between
SRC-3 and MMP-2 is consistent with the fact that MMP-2 is a direct
target of SRC-3 gene (11). Twist1 is
a basic helix-loop-helix transcription factor and serves an
essential role in cancer metastasis (20,25). To
examine whether Twist1 is downregulated by honokiol through
inhibition of its promoter activity, J82 cells were transfected
with a reporter plasmid, firefly luciferase driven by human Twist1
promoter (Twist1-Luc). Following 24 h of transfection, cells with
were treated honokiol for another 24 h. As a result, honokiol (2.4
and 4.8 µg/ml) significantly reduced the luciferase activity of
Twist1-reporter in a dose-dependent manner (Fig. 3C). Overall, these data indicate that
honokiol represses the expression of genes involved in cancer cell
invasion, including SRC-3, MMP-2 and Twist1.
Overexpression of SRC-3 inhibits the
effects of honokiol on cell migration and invasion
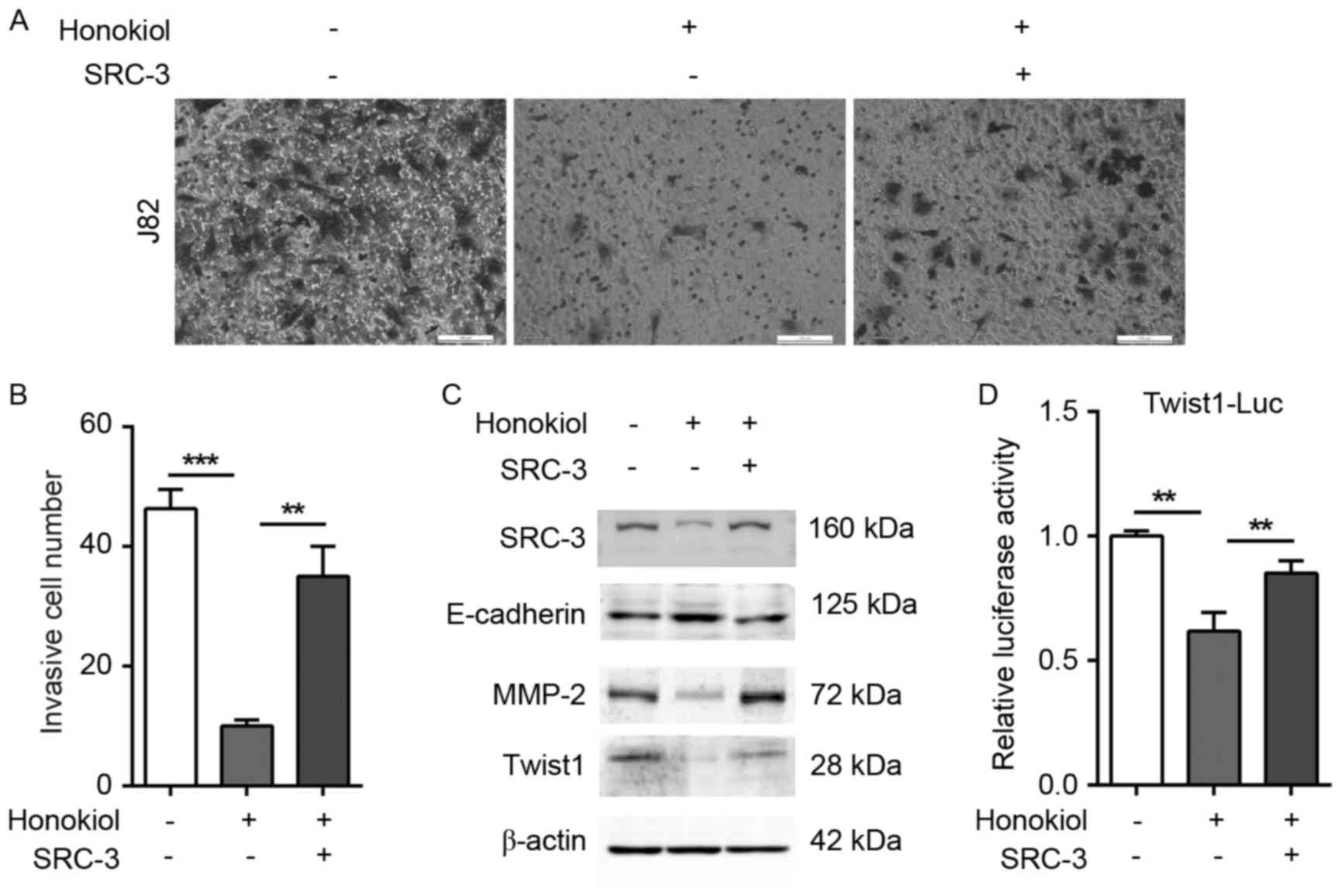
To further investigate whether honokiol inhibits
bladder cancer cells migration and invasion through SRC-3, SRC-3
expression was reintroduced into honokiol-treated J82 cells. Empty
vector-transfected J82 cells (mock transfectants) were used as a
control. In the presence of honokiol (4.8 µg/ml), the ectopic
expression of SRC-3 in J82 cells significantly increased the number
of invading cells to lower chamber in the Transwell assay compared
with that of the mock transfectants (Fig.
4A and B). Furthermore, the ectopic expression of SRC-3
reversed the honokiol-induced changes to E-cadherin, MMP-2 and
Twist1 expression (Fig. 4C).
Consistently, SRC-3 overexpression almost restored the
Twist1-reporter activity under honokiol treatment, suggesting that
Twist1 could be a target gene of SRC-3 (Fig. 4D). Taken together, these data suggest
that honokiol inhibits UBC cell invasion via repression of EMT and
regulation of the expression of cell invasion-associated genes,
including SRC-3, MMP-2 and Twist1.
Discussion
Cell invasion is a highly coordinated cellular
process, including secretion of MMPs for degradation of
extracellular matrix and morphological changes to facilitate EMT.
The cadherin switch from E-cadherin to N-cadherin in EMT has been
demonstrated to be essential for bladder cancer-associated
mortality (24,26). In the present study, it was
demonstrated that a low concentration of honokiol (4.8 µg/ml) was
capable of inhibiting UBC cell migration and invasion, which was
accompanied with the induction of the epithelial marker E-cadherin,
and the reduction of two mesenchymal markers Twist-1 and
N-cadherin. Mechanistically, SRC-3, the transcriptional factor
coactivator, is indispensable in honokiol-mediated cell invasion
inhibition.
SRC-3 is a bona fide oncoprotein in multiple types
of solid tumor, including in breast and prostate cancer (5). It was reported that SRC-3 overexpression
and amplification occurred in 32.5, and 7.0% human UBC specimens
(n=163), respectively (10). The
expression levels of SRC-3 in patients with UBC have been suggested
to be an independent prognostic marker (10). In addition, data from the present
study and other studies indicate that overexpression of SRC-3 is
essential for UBC cell survival and proliferation (9,10).
Therefore, SRC-3 is an important oncoprotein and serves essential
roles in UBC development.
Multiple lines of evidence suggest that different
mechanisms are used in SRC-3-mediated cancer cell migration and
invasion in a cancer-specific manner (10,11,27–29).
An inverse correlation between SRC-3 and E-cadherin has been
reported in human pancreatic adenocarcinoma, implying that SRC-3
regulates E-cadherin directly or indirectly (27). By co-activating estrogen receptor α
(ERα) in T47D breast cancer cells, SRC-3 also transcriptionally
upregulates Snail, which directly represses E-cadherin (28). However, SRC-3 overexpression is not
associated with the levels of ERα in UBC tissue samples (10), and urothelial specific ERα-knockout
enhanced carcinogen-induced UBC development, suggesting that ERα
behaves as a tumor suppressor in UBC (30). Therefore, SRC-3 is likely to induce
EMT through transcription factors other than ERα in UBC cells. It
has been reported that SRC-1, another member of the p160 family,
can induce EMT-associated transcription factor Twist1 by
co-activating PEA3 (29). In prostate
cancer cells, SRC-3 is capable of upregulating MMP-2 by
co-activating the PEA3/activator protein-1 complex (11). In the present study, it was
demonstrated that SRC-3 is essential for the expression of MMP-2
and Twist1. Notably, the overexpression of SRC-3 in UBC cells
reversed honokiol-mediated invasion repression, and upregulated
MMP-2 and EMT-associated marker Twist1 expression. Therefore, it
was hypothesized that SRC-3 enhances UBC cell invasion by
co-activating transcriptional factors similar to those activated by
PEA3, in order to upregulate Twist1 and MMP-2.
Intensive screening for small molecular inhibitors
targeting the oncoprotein SRC-3 is ongoing (31–33).
Several potential agents from different chemical libraries,
including gossypol, bufalin and verrucarin, have been identified,
which all induce the instability of SRC-3 protein (31–33). In
the current study, it was demonstrated that honokiol is capable of
downregulating the mRNA expression of SRC-3. However, the
suppressive effect of honokiol on cancer cell migration and
invasion may not be limited to target SRC-3. It has been reported
that honokiol targets multiple signaling pathways, including KiSS-1
metastasis-suppressor (KISS1)/KISS1 receptor in renal cell
carcinoma (18), signal transducer
and activator of transcription 3 signaling in breast cancer
(34), epidermal growth factor
receptor signaling in head and neck squamous cell carcinoma
(35), and the
inflammation-associated nuclear factor κB pathway in other cancer
cells (36,37). Penetration through the endothelial
cell layer is one of the prerequisite steps in metastasis. Joo
et al demonstrated that by reducing the interaction between
cancer and endothelial cells, honokiol suppresses EMT and
transendothelial invasion of glioblastoma cells via targeting
vascular cell adhesion molecule 1 (38). Taken together, these data suggest that
honokiol serves a range of inhibitory roles in cancer cell invasion
and metastasis, therefore further in vivo studies are
warranted to confirm the results presented.
In conclusion, to the best of our knowledge, this is
the first study to demonstrate that honokiol inhibits UBC cell
migration and invasion via suppression of oncoprotein SRC-3, and
two SRC-3 downstream targets, MMP-2 and Twist1. Further clinical
trials are required to confirm whether honokiol is a
chemotherapeutic agent for patients with UBC, particularly for the
muscle invasive subtype.
Dr. Yan once received a research fund from
EcoNugenics, which provided honokiol for the present study.
However, the current study was not supported using that fund and
there was no influence from the EcoNugenics on the study design,
data collection and interpretation.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81372168, 81572519
to J.Y. and 81470116 to B.S.), the Natural Science Foundation for
Universities in Jiangsu Province of China (grant no. BK20151396 to
J.Y.); Wu Jieping Medical Foundation (320.6750.16051 to B.S.); the
‘One Hundred Talent Program’ of Chinese Academy of Sciences (to
R.H.); fund from the State Key Laboratory of Drug Research (grant
no. SIMM1705KF-06 to R.H.) and the Shanghai Natural Science
Foundation of China (grant no. 14ZR1433200 to B.S.).
Glossary
Abbreviations
Abbreviations:
|
UBC
|
urinary bladder cancer
|
|
MMP
|
matrix metalloproteinase
|
|
EMT
|
epithelial-mesenchymal transition
|
|
SRC-3
|
steroid receptor coactivator-3
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zheng R, Zeng H, Zhang S, Chen T and Chen
W: National estimates of cancer prevalence in China, 2011. Cancer
Lett. 370:33–38. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Anzick SL, Kononen J, Walker RL, Azorsa
DO, Tanner MM, Guan XY, Sauter G, Kallioniemi OP, Trent JM and
Meltzer PS: AIB1, a steroid receptor coactivator amplified in
breast and ovarian cancer. Science. 277:965–968. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhou HJ, Yan J, Luo W, Ayala G, Lin SH,
Erdem H, Ittmann M, Tsai SY and Tsai MJ: SRC-3 is required for
prostate cancer cell proliferation and survival. Cancer Res.
65:7976–7983. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yan J, Tsai SY and Tsai MJ: SRC-3/AIB1:
Transcriptional coactivator in oncogenesis. Acta Pharmacol Sin.
27:387–394. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liao L, Kuang SQ, Yuan Y, Gonzalez SM,
O'Malley BW and Xu J: Molecular structure and biological function
of the cancer-amplified nuclear receptor coactivator SRC-3/AIB1. J
Steroid Biochem Mol Biol. 83:3–14. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cai D, Shames DS, Raso MG, Xie Y, Kim YH,
Pollack JR, Girard L, Sullivan JP, Gao B, Peyton M, et al: Steroid
receptor coactivator-3 expression in lung cancer and its role in
the regulation of cancer cell survival and proliferation. Cancer
Res. 70:6477–6485. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Long W, Foulds CE, Qin J, Liu J, Ding C,
Lonard DM, Solis LM, Wistuba II, Qin J, Tsai SY, et al: ERK3
signals through SRC-3 coactivator to promote human lung cancer cell
invasion. J Clin Invest. 122:1869–1880. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao W, Chang C, Cui Y, Zhao X, Yang J,
Shen L, Zhou J, Hou Z, Zhang Z, Ye C, et al: Steroid receptor
coactivator-3 regulates glucose metabolism in bladder cancer cells
through coactivation of hypoxia inducible factor 1α. J Biol Chem.
289:11219–11229. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Luo JH, Xie D, Liu MZ, Chen W, Liu YD, Wu
GQ, Kung HF, Zeng YX and Guan XY: Protein expression and
amplification of AIB1 in human urothelial carcinoma of the bladder
and overexpression of AIB1 is a new independent prognostic marker
of patient survival. Int J Cancer. 122:2554–2561. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yan J, Erdem H, Li R, Cai Y, Ayala G,
Ittmann M, Yu-Lee LY, Tsai SY and Tsai MJ: Steroid receptor
coactivator-3/AIB1 promotes cell migration and invasiveness through
focal adhesion turnover and matrix metalloproteinase expression.
Cancer Res. 68:5460–5468. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Louie MC, Zou JX, Rabinovich A and Chen
HW: ACTR/AIB1 functions as an E2F1 coactivator to promote breast
cancer cell proliferation and antiestrogen resistance. Mol Cell
Biol. 24:5157–5171. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tong ZT, Wei JH, Zhang JX, Liang CZ, Liao
B, Lu J, Fan S, Chen ZH, Zhang F, Ma HH, et al: AIB1 predicts
bladder cancer outcome and promotes bladder cancer cell
proliferation through AKT and E2F1. Br J Cancer. 108:1470–1479.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Watanabe K, Watanabe HY, Goto Y, Yamamoto
N and Yoshizaki M: Studies on the active principles of magnolia
bark. Centrally acting muscle relaxant activity of magnolol and
hōnokiol. Jpn J Pharmacol. 25:605–607. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Arora S, Singh S, Piazza GA, Contreras CM,
Panyam J and Singh AP: Honokiol: A novel natural agent for cancer
prevention and therapy. Curr Mol Med. 12:1244–1252. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Esumi T, Makado G, Zhai H, Shimizu Y,
Mitsumoto Y and Fukuyama Y: Efficient synthesis and
structure-activity relationship of honokiol, a neurotrophic
biphenyl-type neolignan. Bioorg Med Chem Lett. 14:2621–2625. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang Q, Zhao W, Ye C, Zhuang J, Chang C,
Li Y, Huang X, Shen L, Li Y, Cui Y, et al: Honokiol inhibits
bladder tumor growth by suppressing EZH2/miR-143 axis. Oncotarget.
6:37335–37348. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cheng S, Castillo V, Eliaz I and Sliva D:
Honokiol suppresses metastasis of renal cell carcinoma by targeting
KISS1/KISS1R signaling. Int J Oncol. 46:2293–2298. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jeong JJ, Lee JH, Chang KC and Kim HJ:
Honokiol exerts an anticancer effect in T98G human glioblastoma
cells through the induction of apoptosis and the regulation of
adhesion molecules. Int J Oncol. 41:1358–1364. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Khan MA, Tania M, Wei C, Mei Z, Fu S,
Cheng J, Xu J and Fu J: Thymoquinone inhibits cancer metastasis by
downregulating TWIST1 expression to reduce epithelial to
mesenchymal transition. Oncotarget. 6:19580–19591. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) methods. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gheldof A and Berx G: Cadherins and
epithelial-to-mesenchymal transition. Prog Mol Biol Transl Sci.
116:317–336. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tania M, Khan MA and Fu J: Epithelial to
mesenchymal transition inducing transcription factors and
metastatic cancer. Tumour Biol. 35:7335–7342. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
McConkey DJ, Choi W, Marquis L, Martin F,
Williams MB, Shah J, Svatek R, Das A, Adam L, Kamat A, et al: Role
of epithelial-to-mesenchymal transition (EMT) in drug sensitivity
and metastasis in bladder cancer. Cancer Metastasis Rev.
28:335–344. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Khan MA, Chen HC, Zhang D and Fu J: Twist:
A molecular target in cancer therapeutics. Tumour Biol.
34:2497–2506. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jäger T, Becker M, Eisenhardt A, Tilki D,
Tötsch M, Schmid KW, Romics I, Rübben H, Ergün S and Szarvas T: The
prognostic value of cadherin switch in bladder cancer. Oncol Rep.
23:1125–1132. 2010.PubMed/NCBI
|
|
27
|
Guo S, Xu J, Xue R, Liu Y and Yu H:
Overexpression of AIB1 correlates inversely with E-cadherin
expression in pancreatic adenocarcinoma and may promote lymph node
metastasis. Int J Clin Oncol. 19:319–324. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang M, Zhao F, Li S, Chang AK, Jia Z,
Chen Y, Xu F, Pan H and Wu H: AIB1 cooperates with ERα to promote
epithelial mesenchymal transition in breast cancer through SNAI1
activation. PLoS One. 8:e655562013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qin L, Liu Z, Chen H and Xu J: The steroid
receptor coactivator-1 regulates twist expression and promotes
breast cancer metastasis. Cancer Res. 69:3819–3827. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hsu I, Yeh CR, Slavin S, Miyamoto H, Netto
GJ, Tsai YC, Muyan M, Wu XR, Messing EM, Guancial EA and Yeh S:
Estrogen receptor alpha prevents bladder cancer via INPP4B
inhibited akt pathway in vitro and in vivo. Oncotarget.
5:7917–7935. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang Y, Lonard DM, Yu Y, Chow DC, Palzkill
TG and O'Malley BW: Small molecule inhibition of the steroid
receptor coactivators, SRC-3 and SRC-1. Mol Endocrinol.
25:2041–2053. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang Y, Lonard DM, Yu Y, Chow DC, Palzkill
TG, Wang J, Qi R, Matzuk AJ, Song X, Madoux F, et al: Bufalin is a
potent small-molecule inhibitor of the steroid receptor
coactivators SRC-3 and SRC-1. Cancer Res. 74:1506–1517. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yan F, Yu Y, Chow DC, Palzkill T, Madoux
F, Hodder P, Chase P, Griffin PR, O'Malley BW and Lonard DM:
Identification of verrucarin a as a potent and selective steroid
receptor coactivator-3 small molecule inhibitor. PLoS One.
9:e952432014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Avtanski DB, Nagalingam A, Bonner MY,
Arbiser JL, Saxena NK and Sharma D: Honokiol inhibits
epithelial-mesenchymal transition in breast cancer cells by
targeting signal transducer and activator of transcription
3/Zeb1/E-cadherin axis. Mol Oncol. 8:565–580. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Leeman-Neill RJ, Cai Q, Joyce SC, Thomas
SM, Bhola NE, Neill DB, Arbiser JL and Grandis JR: Honokiol
inhibits epidermal growth factor receptor signaling and enhances
the antitumor effects of epidermal growth factor receptor
inhibitors. Clin Cancer Res. 16:2571–2579. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ahn KS, Sethi G, Shishodia S, Sung B,
Arbiser JL and Aggarwal BB: Honokiol potentiates apoptosis,
suppresses osteoclastogenesis, and inhibits invasion through
modulation of nuclear factor-kappaB activation pathway. Mol Cancer
Res. 4:621–633. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu SH, Lee WJ, Lai DW, Wu SM, Liu CY,
Tien HR, Chiu CS, Peng YC, Jan YJ, Chao TH, et al: Honokiol confers
immunogenicity by dictating calreticulin exposure, activating ER
stress and inhibiting epithelial-to-mesenchymal transition. Mol
Oncol. 9:834–849. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Joo YN, Eun SY, Park SW, Lee JH, Chang KC
and Kim HJ: Honokiol inhibits U87MG human glioblastoma cell
invasion through endothelial cells by regulating membrane
permeability and the epithelial-mesenchymal transition. Int J
Oncol. 44:187–194. 2014. View Article : Google Scholar : PubMed/NCBI
|