Introduction
Angiogenesis, which is the formation, recruitment
and growth of new blood capillaries from existing neighboring
vasculature, is known to serve important roles in pathological
conditions, including cancer growth, progression, rheumatoid
arthritis and diabetic retinopathy (1,2).
Angiogenesis is associated with the stimulation of endothelial cell
proliferation, migration, adhesion, invasion and tube formation by
a variety of angiogenic and anti-angiogenic factors, and is
regulated by a variety of signaling pathways within the tissue
microenvironment (3,4). Numerous angiogenic factors such as
vascular endothelial growth factor (VEGF)-A and subsequent
signaling pathways, including extracellular signal-regulated kinase
(ERK), phosphatidylinositol 3-kinase (PI3K)/Akt and p70 ribosomal
S6 kinase (p70S6K), stimulate endothelial cells, thus inducing cell
proliferation, migration and survival, indicating that these
factors may be targeted as a therapeutic strategy for a variety of
angiogenesis-associated diseases (5–8).
PI3K/Akt, one of the key signaling enzymes in cell
mitogenesis, is closely associated with various types of cell
growth, cell survival and cancer progression (9,10). The
serine/threonine kinase Akt is activated by a PI3K-dependent
signaling pathway and serves a pivotal role in angiogenesis
(11,12). A previous study demonstrated that the
PI3K/Akt signaling pathway promoted retinal angiogenesis by
cooperation with cysteine-rich protein 61 in retinopathy of
prematurity (13,14). In addition, the PI3K/Akt signaling
pathway is essential to hypoxia-induced expression of
hypoxia-inducible factor-1a and VEGF in choroidal
neovascularization (12). Inhibition
of the PI3K/Akt pathway usually results in substantial antitumor
and anti-angiogenic effects (15–17),
indicating that targeting PI3K/AKT may be a strategy for blocking
angiogenesis-associated diseases.
Trigonostemon reidioides (TR) Craib
(Euphorbiaceae) has been used as a Thai traditional medicine for
the treatment of drug addiction, asthma, food poisoning,
constipation and snake bites (18).
TR is a native species to Southeast Asia, including Vietnam,
Cambodia and Myanmar (19). Numerous
previous studies have demonstrated that the bioactive compounds of
TR have cytotoxic activity against a number of cell lines,
including bile duct cancer, cervical cancer and liver cancer cell
lines (20,21); however, the effects and signaling
pathways of the ethanolic extract of TR (ETR) on angiogenesis
remain unknown. Therefore, the present study evaluated the effects
and molecular mechanisms of ETR on cell proliferation, adhesion,
migration, invasion and tube formation in human umbilical vein
endothelial cells (HUVECs).
Materials and methods
Cell culture conditions
Primary cultures of HUVECs were purchased from Lonza
(Walkersville, MD, USA) and used between passages 4 and 6 for all
experiments. Cells were cultured in EGM-2® BulletKit
medium, containing endothelial basal medium-2 (EBM-2) and growth
supplements (EGM-2® SingleQuots kit, human epidermal
growth factor, VEGF, R3-insulin-like growth factor-1, human
fibroblast growth factor, ascorbic acid, hydrocortisone, heparin,
fetal bovine serum and gentamicin/amphotericin B), which was
designated as complete medium. Cell culture was performed according
to the manufacturer's protocol (Lonza).
Reagents
The following antibodies were purchased from
commercial sources: Anti-phosphorylated (p)-ERK (T202/Y204; catalog
no., 9101), anti-p-Akt (S473; catalog no., 4060), anti-p-p70S6K
(T421/S424; catalog no., 9204), anti-retinoblastoma protein (pRb;
S780; catalog no., 9307) and anti-p-pRb (S811; catalog no., 9308),
which were all purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA), and anti-ERK (catalog no., 9102), anti-Akt
(catalog no., 9272), anti-cyclin-dependent kinase (Cdk) 4 (catalog
no., sc-260), anti-Cdk2 (catalog no., sc-6248), anti-cyclin D
(catalog no., sc-20044), anti-cyclin E (catalog no., sc-247) and
anti-β-actin (catalog no., sc-47778) antibodies, in addition to
mouse and rabbit immunoglobulin G-horseradish peroxidase
conjugates, which were all purchased from Santa Cruz Biotechnology
Inc. (Dallas, TX, USA).
Preparation of ETR
Dried TR (175 g) was pulverized and extracted using
70% ethanol for 24 h at room temperature. The extract was filtered
and concentrated under vacuum at reduced pressure using a rotary
flash evaporator (BÜCHI Labortechnik AG, Flawil, Switzerland), and
ethanol was allowed to completely evaporate. The remaining aqueous
solution was concentrated under vacuum and freeze dried
(ilShinBioBase Co., Ltd., Dongducheon, Korea). The crude extract
yield was 4% (w/v).
Cell viability and proliferation
assay
Subconfluent HUVECs were plated at a density of
1×105 cells/well on 6-well plates (BD Biosciences,
Franklin Lakes, NJ, USA) and serum-starved for 14 h at 37°C in
EBM-2 medium to synchronize cells in the
G1/G0 cell cycle phase, prior to incubation
for 24 h at 37°C in EGM-2 BulletKit medium in the presence or
absence of ETR (1–25 µg/ml). Following incubation for 24 h, cell
viability was determined using an Invitrogen™
Countess™ Automated Cell Counter (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). The results from triplicate
determinations (mean ± standard deviation) are presented as the
numbers of cells per culture.
Western blot analysis
Quiescent HUVECs were plated at density of
1×106 cells/dish on 100-mm dishes (BD Biosciences),
serum-starved for 14 h in EBM-2 medium and incubated for 15 min or
24 h at 37°C in EGM-2 BulletKit medium in the presence or absence
of ETR (1–25 µg/ml). Cells were rinsed twice with ice-cold PBS and
lysed by incubation in 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 10%
glycerol, 1% Triton X-100, 1 mM EDTA, 100 µg/ml
4-(2-aminoethyl)benzenesulfonyl fluoride, 10 µg/ml aprotinin, 1
µg/ml pepstatin A, 0.5 µg/ml leupeptin, 80 mM β-glycerophosphate,
25 mM sodium fluoride and 1 mM sodium orthovanadate for 30 min at
4°C. Cell lysates were clarified at 12,500 × g for 20 min at 4°C,
and the supernatants were subjected to western blot analysis as
described previously (22,23). Total protein was quantified with the
Quick Start™ Bradford 1X Dye Reagent (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) using bovine serum albumin
(BSA; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for the
standard. Protein extracts representing 40 mg total protein were
separated on 10% SDS-PAGE gel using the Bio-Rad Mini Protean 3
System (Bio-Rad Laboratories, Inc.) and electro-blotted onto
Protran® nitrocellulose membranes (Sigma-Aldrich; Merck
KGaA). Membranes were blocked in 5% BSA in PBS/0.025% Tween-20
(Sigma-Aldrich; Merck KGaA) for 1 h at room temperature. The
primary antibodies used were specific for p-ERK, ERK, p-Akt, Akt,
p-p70s6k, p-pRb(S780), p-pRb(S811) (Cell Signaling Technology,
Inc.) and Cdk4, Cdk2, cyclin D, cyclin E, β-actin (Santa Cruz
Biotechnology, Inc.). The primary antibodies were diluted
(dilution, 1:1,000) in 5% BSA in PBST, and incubated with the
membrane overnight at 4°C. The secondary antibodies were applied at
a 1:2,000 dilution in 5% BSA in PBST and incubated for 1 h at room
temperature, then processed for detection with the Supersignal West
Pico Chemiluminescent Substrate (Thermo Fisher Scientific, Inc.),
using the Amersham™ Imager 600 and Imaging Software (ver
0.4.4; GE Healthcare Life Sciences, Chalfont, UK). All western blot
analyses are representative of ≥3 independent experiments.
Migration assay
Cell migration was quantified via in vitro
wound-healing assay as described previously (24). Following plating of cells on 48-well
plates (4×104 cells/well) and allowing them to grow to
confluence, a single wound was created in the center of the cell
monolayer by gentle removal of the attached cells using a sterile
plastic pipette tip. Following serum starvation with EBM-2 for 2 h
at 37°C, cells were incubated for 16 h at 37°C in EGM-2 BulletKit
medium in the presence or absence of ETR (1–25 µg/ml). Cells were
fixed with methanol and then stained with 0.04% Giemsa solution
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Migration of the
cells into the wound was observed, and still images were captured
following incubation for 16 h. Images were captured using a Nikon
Digital Sight DS-U1 microscope (Nikon Corporation, Tokyo,
Japan).
Invasion assay
The upper side of the Transwell insert (6.5-mm
diameter insert, 8-µm pore size; Corning Incorporated, Corning, NY,
USA) was coated with 50 µl 1 mg/ml Matrigel® basement
membrane matrix (10.4 mg/ml; BD Biosciences) diluted in EBM-2.
Aliquots (100 µl) of HUVECs (5×104 cells/ml) resuspended
in EBM-2 were added to the upper compartment of the Matrigel-coated
Transwell and 600 µl EBM-2 was added to the lower compartment.
Following serum starvation with EBM-2 for 2 h, cells were incubated
for 15 h at 37°C in EGM-2 Bullet kit media in the presence or
absence of ETR (1–25 µg/ml). The inserts were fixed with 95–100%
methanol (Merck KGaA, Darmstadt, Germany; #106009.1011) and the
non-invasive cells were removed from the top of the membrane using
a cotton-tipped swab. Following staining with 0.04% Giemsa
solution, the number of invasive cells was determined from six
fields using ×200 objective magnification. Images were captured
using a Nikon Digital Sight DS-U1 microscope (Nikon
Corporation).
Tube formation assays
Matrigel basement membrane matrix (10.4 mg/ml; BD
Biosciences) was thawed overnight at 4°C, and each well of
pre-chilled 24-well plates was coated with 200 µl Matrigel and then
incubated at 37°C for 30 min. Following serum starvation with EBM-2
medium for 2 h, cells (4×104 cells/ml) were added to
Matrigel-coated plates and treated with ETR (1–25 µg/ml) for 6 h at
37°C. Tube formation was observed using an inverted microscope
(Eclipse TE2000-U; Nikon Corporation) and NIS-Elements F 3.0
software (Nikon Corporation).
Zymogram analysis
Activities of matrix metalloproteinases (MMPs) were
evaluated using zymography (25,26).
Aliquots of basic EBM 2 medium collected from HUVECs treated with
ETR (1–25 µg/ml) for 16 h at room temperature were diluted in
sample buffer (Bio-Rad Laboratories, Inc.; #161-0764) and applied
to 8% polyacrylamide gels supplemented with 1 mg/ml gelatin
(Sigma-Aldrich; Merck KGaA) as a substrate. Following
electrophoresis, the gels were incubated in 2.5% Triton X-100 for 1
h at room temperature in order to remove SDS and allow
re-naturalization of MMPs, and then further incubated in developing
buffer (Bio-Rad Laboratories, Inc.; #161-0766) supplemented with 50
mM Tris-HCl (pH 7.5), 10 mM CaCl2 and 150 mM NaCl for 16
h at 37°C. The gels were stained with 0.5% Coomassie Brilliant Blue
R-250 in 30% methanol-10% acetic acid for 3 h, followed by
de-staining with 30% methanol-10% acetic acid. Gelatinolytic
activities were detected as unstained bands against the background
of the Coomassie Brilliant Blue R-250 Blue-stained gelatin.
Statistical analysis
Statistical analysis was performed by a Student's
t-test using Microsoft Excel 2007 software (Microsoft Corporation,
Redmond, WA, USA). Results are presented as the mean ± standard
deviation. P<0.05 was considered to indicate a statistically
significant difference.
Results
ETR inhibits endothelial cell
proliferation by regulating the expression level of cell
cycle-associated proteins
To investigate the effects of ETR on the cellular
responses of human endothelial cells, the present study first
examined the ability of ETR to regulate cell proliferation in
HUVECs. ETR treatment suppressed cell proliferation in a
dose-dependent manner (Fig. 1A) and
did not alter cell viability (Fig.
1C), indicating that ETR inhibition of endothelial cell
proliferation was not mediated by induction of apoptosis or
cytotoxicity. Based on these results, the present study
subsequently analyzed the alterations in the expression level of
cell cycle-associated proteins, Cdks, cyclins and pRb in
ETR-treated HUVECs. Phosphorylation of pRb by Cdk/cyclin complexes
is essential for the transition from the G1 to the S
phase of the cell cycle (27). As
presented in Fig. 1B, ETR treatment
markedly reduced the expression levels of Cdk2 and cyclin E, which
induced inhibition of pRb phosphorylation in response to mitogenic
stimulation. These results demonstrated that ETR downregulated the
expression level of cell cycle-associated proteins, resulting in
inhibition of cell cycle progression and cell proliferation in
HUVECs.
 | Figure 1.Anti-proliferative effect of ETR on
mitogen-induced HUVECs is mediated by downregulation of cell
cycle-associated proteins. (A) Cell viability of quiescent HUVECs
incubated for 24 h in CM supplemented with growth factors with or
without ETR (1, 10 and 25 µg/ml). (B) Cell proliferation of cells
treated with ETR (1, 10 and 25 µg/ml). The results from triplicate
determinations (mean ± standard deviation) are presented as the
percentage of viable cells out of the total cell count. Statistical
significance is indicated (*P<0.05, compared with CM-treated
cells). (C) Cell lysate expression levels were determined by
western blotting with anti-Cdk4, anti-Cdk2, anti-cyclin D,
anti-cyclin E, anti-p-pRb or anti-β-actin antibodies. Results are
representative of ≥3 independent experiments. ETR, ethanolic
extract of Trigonostemon reidioides; HUVECs, human umbilical
vein endothelial cells; CM, complete medium; Cdk, cyclin-dependent
kinase; pRb, retinoblastoma protein. |
ETR inhibits endothelial cell
adhesion, migration, invasion and capillary structure
formation
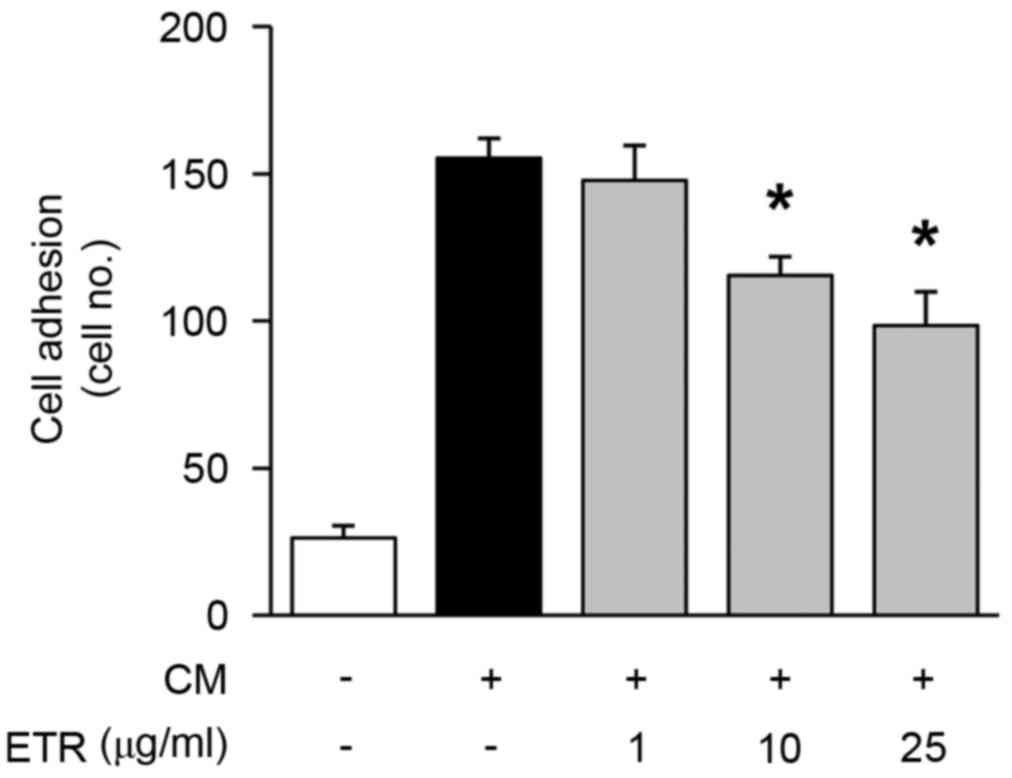
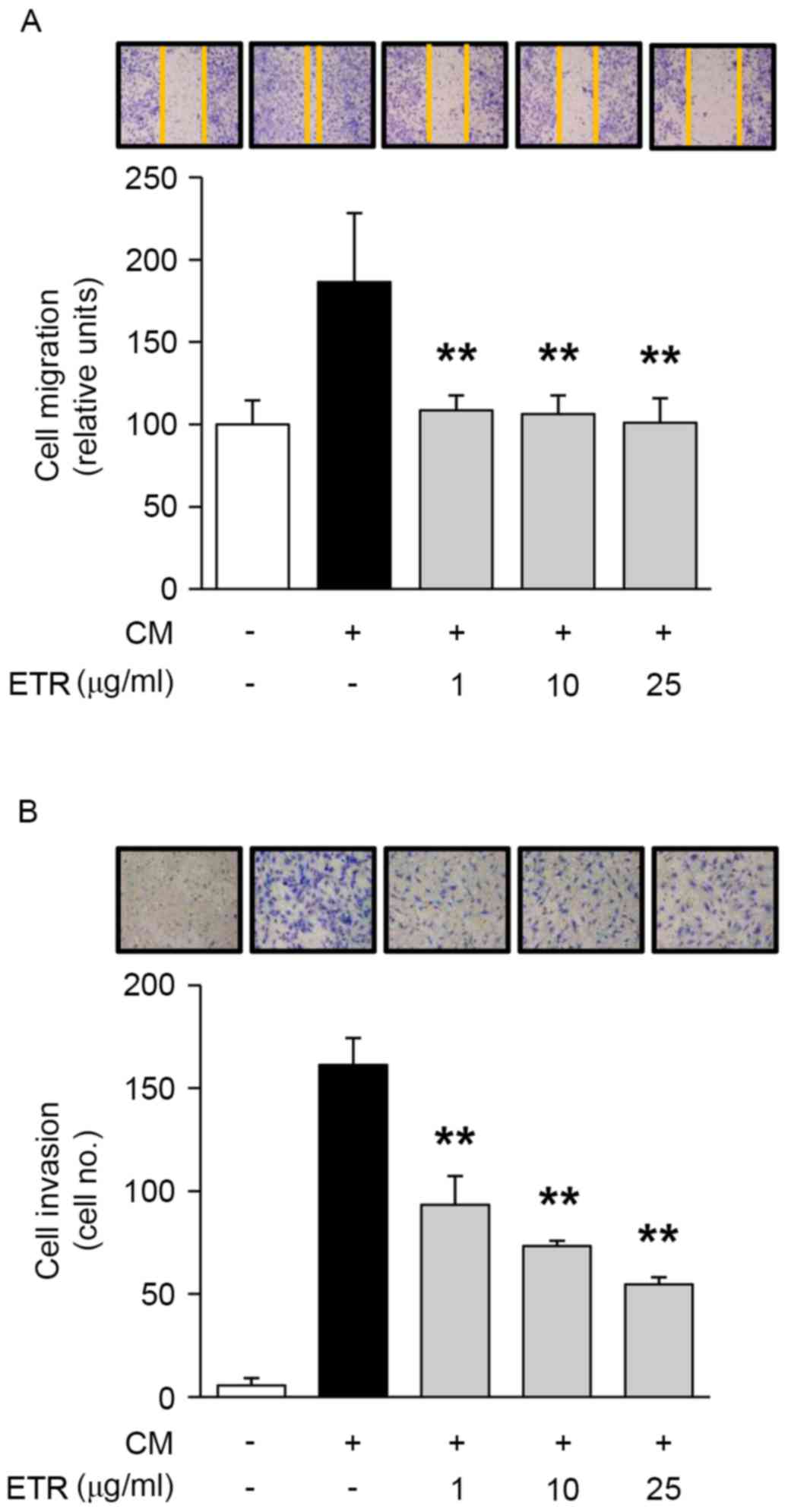
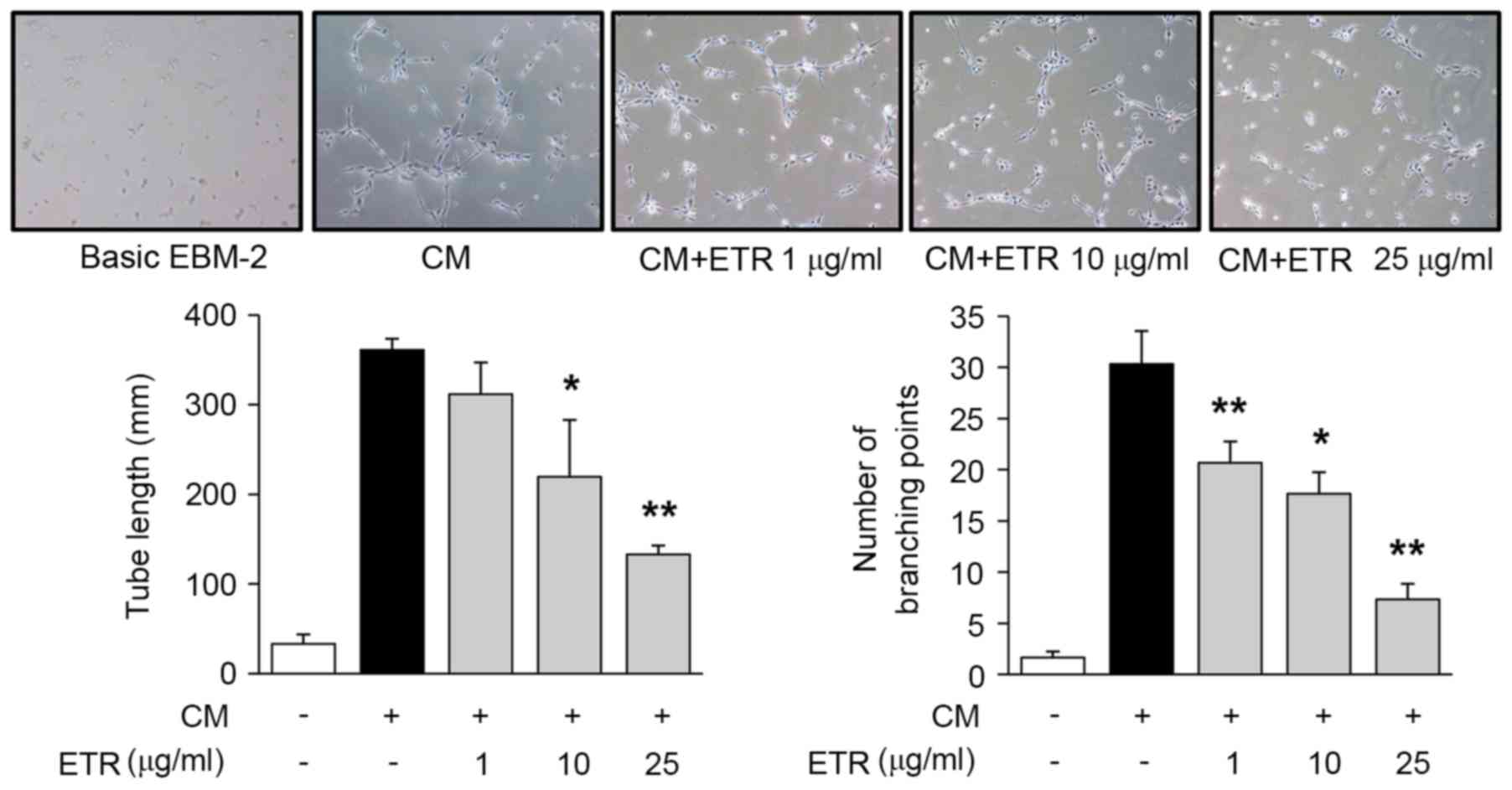
The effect of ETR on endothelial cell adhesion,
migration, invasion and tube formation was analyzed, which all
serve important roles in cancer and angiogenesis-associated
diseases (2). As presented in
Fig. 2, ETR treatment
dose-dependently reduced cell adhesion in HUVECs. In addition, ETR
significantly inhibited cell migration, cell invasion (Fig. 3A and B, respectively) and tube
formation in HUVECs (Fig. 4).
Collectively, these results suggested that the pharmacological
roles of ETR in regulating endothelial cell adhesion, migration,
invasion and tubular formation resulted in the regulation of
angiogenic responses in vitro.
Anti-angiogenic activities of ETR are
mediated by inhibition of mitogenic signaling pathways and
downregulation of MMP-2
In order to further investigate the molecular
mechanisms underlying the ETR-mediated regulation of
mitogen-induced endothelial cell proliferation, adhesion,
migration, invasion and tubular formation, the present study
examined the alterations in activation of mitogenic signaling
pathways, including ERK, PI3K/Akt and mammalian target of
rapamycin/p70S6K, which serve pivotal roles in cellular fate
(28). As presented in Fig. 5A, ETR treatment markedly inhibited
mitogen-induced phosphorylation/activation of Akt but not of ERK or
p70S6K in HUVECs when compared with that in unstimulated control
cells. Activation of MMP-9 and MMP-2 has previously been reported
to promote endothelial cell migration, invasion and tube formation
(2,3,8). In order
to confirm the regulatory effects of ETR on endothelial cell
migration, invasion and tube formation, the present study
subsequently analyzed the changes in activation of MMP-9 and MMP-2.
As presented in Fig. 5B, ETR
treatment (25 µg/ml) inhibited mitogen-induced activation of MMP-2
in CM of HUVECs. Conversely, the activation of MMP-9 in HUVECs was
not altered by ETR treatment. Taken together, these results
demonstrated that the inhibitory effects of ETR on endothelial cell
proliferation, adhesion, migration, invasion and tube formation may
be mediated by inactivation of the PI3K/Akt signaling pathway and
subsequent downregulation of MMP-2.
 | Figure 5.ETR inhibits mitogen-induced Akt and
MMP-2 activities. Quiescent cells were treated with ETR (1, 10 and
25 µg/ml) for 15 min. (A) Cell lysates were analyzed by western
blotting with anti-p-ERK, anti-ERK, anti-p-Akt, anti-Akt, and
anti-p-p70S6K antibodies. (B) Gelatin zymogram analysis was
performed using basic EBM 2 medium from cell culture. Zymogram gel
loading was normalized to total protein concentration. Results are
representative of ≥3 independent experiments. ETR, ethanolic
extract of Trigonostemon reidioides; CM, complete medium;
MMP, matrix metalloproteinase; p, phosphorylated; ERK,
extracellular signal-regulated kinase; p70S6K, p70 ribosomal S6
kinase. |
Discussion
Previous studies have demonstrated that TR contained
bioactive compounds, including trigonostemone, a phenanthrenone,
and lotthanongine, a novel flavonoidal indole alkaloid (29,30).
Previously, novel daphnane diterpenes, namely rediocides A-F
(1–6),
were isolated from TR and exhibited potent anti-flea activity
(31–33). These diterpenes are effective
antiviral (human immunodeficiency virus-1) agents, and have been
reported to have antileukemic, antimycobacterial and anticancer
activities (34–36). In addition, these compounds have may
exert anticancer effects via cytotoxicity against various cancer
cell lines, including liver, cervical, oral, colon, lung and
gastric cancer cell lines (21).
However, the effects and molecular mechanisms underlying TR on
angiogenesis have not been reported to date.
Dysregulation of the PI3K/Akt signaling pathway is
closely associated with angiogenesis-associated diseases, including
cancer (9,10). The PI3K/Akt signaling pathway serves
pivotal roles in the growth, migration and formation of blood
vessels in endothelial cells (11,12). Our
group has previously reported that the ethanolic extracts of
Ligularia fischeri and Broussonetia kazinoki
inhibited the proliferation, invasion and tube formation of
endothelial cells by inactivation of the mitogen- and
VEGF-A-stimulated signaling pathways, including the PI3K/Akt
signaling pathway (37,38). To the best of our knowledge, the
present study demonstrated for the first time that ETR inhibited
mitogen-induced endothelial cell proliferation, adhesion,
migration, invasion and tube formation. These anti-angiogenic
activities of ETR were mediated by the downregulation of
mitogen-induced Cdks/cyclins, and the inhibition of
phosphorylation/activation of pRb, Akt and MMP-2, but not of ERK,
p70S6K or MMP-9. These results confirmed the possibility of ETR as
a novel anti-angiogenic agent that selectively targets the Akt
signaling pathway.
In conclusion, the results of the present study
provided pharmacological roles and mechanisms of ETR in the
regulation of angiogenesis, and warranted further evaluation and
development of ETR for the prevention and treatment of diseases
associated with angiogenesis.
Acknowledgements
The present study was supported by the National
Institute of Biological Resources under the Ministry of Environment
of the Republic of Korea (grant no. 2014-04-202).
Glossary
Abbreviations
Abbreviations:
|
Cdks
|
cyclin-dependent kinases
|
|
EBM-2
|
endothelial basal medium-2
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
ETR
|
ethanolic extract of Trigonostemon
reidioides
|
|
HUVECs
|
human umbilical vein endothelial
cells
|
|
MMPs
|
matrix metalloproteinases
|
|
mTOR
|
mammalian target of rapamycin
|
|
PI3K
|
phosphatidylinositol 3-kinase
|
|
pRb
|
retinoblastoma protein
|
|
p70S6K
|
p70 ribosomal S6 kinase
|
|
VEGF-A
|
vascular endothelial growth
factor-A
|
References
|
1
|
Cristofanilli M, Charnsangavej C and
Hortobagyi GN: Angiogenesis modulation in cancer research: Novel
clinical approaches. Nat Rev Drug Discov. 1:415–426. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Folkman J: Angiogenesis: An organizing
principle for drug discovery? Nat Rev Drug Discov. 6:273–286. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Carmeliet P and Jain RK: Principles and
mechanisms of vessel normalization for cancer and other angiogenic
diseases. Nat Rev Drug Discov. 10:417–427. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cook KM and Figg WD: Angiogenesis
inhibitors: Current strategies and future prospects. CA Cancer J
Clin. 60:222–243. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jain RK, Duda DG, Clark JW and Loeffler
JS: Lessons from phase III clinical trials on anti-VEGF therapy for
cancer. Nat Clin Prac Oncol. 3:24–40. 2006. View Article : Google Scholar
|
|
6
|
Ng EW, Shima DT, Calias P, Cunningham ET
Jr, Guyer DR and Adamis AP: Pegaptanib, a targeted anti-VEGF
aptamer for ocular vascular disease. Nat Rev Drug Discov.
5:123–132. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brown DM and Regillo CD: Anti-VEGF agents
in the treatment of neovascular age-related macular degeneration:
Applying clinical trial results to the treatment of everyday
patients. Am J Ophthalmol. 144:627–637. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ellis LM and Hicklin DJ: VEGF-targeted
therapy: Mechanisms of anti-tumour activity. Nat Rev Cancer.
8:579–591. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Coffer PJ, Jin J and Woodgett JR: Protein
kinase B (c-Akt): A multifunctional mediator of
phosphatidylinositol 3-kinase activation. Biochem J. 335:1–13.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kandel ES and Hay N: The regulation and
activities of the multifunctional serine/threonine kinase Akt/PKB.
Exp Cell Res. 253:210–229. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ackah E, Yu J, Zoellner S, Iwakiri Y,
Skurk C, Shibata R, Ouchi N, Easton RM, Galasso G, Birnbaum MJ, et
al: Akt1/protein kinase B is critical for ischemic and
VEGF-mediated angiogenesis. J Clin Invest. 115:2119–2127. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang XM, Wang YS, Zhang J, Li Y, Xu JF,
Zhu J, Zhao W, Chu DK and Wiedemann P: Role of PI3K/Akt and MEK/ERK
in mediating hypoxia-induced expression of HIF-1alpha and VEGF in
laser-induced rat choroidal neovascularization. Invest Ophthalmol
Vis Sci. 50:1873–1879. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
You JJ, Yang CH, Yang CM and Chen MS:
Cyr61 induces the expression of monocyte chemoattractant protein-1
via the integrin ανβ3, FAK, PI3K/Akt and NF-κB pathways in retinal
vascular endothelial cells. Cell Signal. 26:133–140. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Di Y, Zhang Y, Nie Q and Chen X:
CCN1/Cyr61-PI3K/AKT signaling promotes retinal neovascularization
in oxygen-induced retinopathy. Int J Mol Med. 36:1507–1518. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Clark AS, West K, Streicher S and Dennis
PA: Constitutive and inducible Akt activity promotes resistance to
chemotherapy, trastuzumab, or tamoxifen in breast cancer cells. Mol
Cancer Ther. 1:707–717. 2002.PubMed/NCBI
|
|
16
|
Knuefermann C, Lu Y, Liu B, Jin W, Liang
K, Wu L, Schmidt M, Mills GB, Mendelsohn J and Fan Z:
HER2/PI-3K/Akt activation leads to a multidrug resistance in human
breast adenocarcinoma cells. Oncogene. 22:3205–3212. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Katso R, Okkenhaug K, Ahmadi K, White S,
Timms J and Waterfield MD: Cellular function of phosphoinositide
3-kinases: Implications for development, homeostasis, and cancer.
Annu Rev Cell Dev Biol. 17:615–675. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tempeam A, Thasana N, Pavaro C, Chuakul W,
Siripong P and Ruchirawat S: A new cytotoxic daphnane diterpenoid,
rediocide G, from Trigonostemon reidioides. Chem Pharm Bull
(Tokyo). 53:1321–1323. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Biodiversity of Cambodia, Cardamom
protected forest and Seima biodiversity conservation area. NIBR.
1642012.
|
|
20
|
Chuakul W, Saralump P and Prathanturarug
S: Medicinal Plants in Thailand. 2. Amarin Printing and Publishing
Public Co., Ltd.; Bangkok: 1997
|
|
21
|
Tempeam A, Thasana N, Thavornkitcharat A,
Pavaro C and Ruchirawat S: In vitro cytotoxicity of some Thai
medicinal plants and daphnane diterpenoid from Trigonostemon
redioides. Mahidol U J Pharm Sci. 29:25–31. 2002.
|
|
22
|
Seo DW, Kim SH, Eom SH, Yoon HJ, Cho YR,
Kim PH, Kim YK, Han JW, Diaz T, Wei BY and Stetler-Stevenson WG:
TIMP-2 disrupts FGF-2-induced downstream signaling pathways.
Microvasc Res. 76:145–151. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Seo DW, Li H, Qu CK, Oh J, Kim YS, Diaz T,
Wei B, Han JW and Stetler-Stevenson WG: Shp-1 mediates the
antiproliferative activity of tissue inhibitor of
metalloproteinase-2 in human microvascular endothelial cells. J
Biol Chem. 281:3711–3721. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cho YR, Kim SH, Ko HY, Kim MD, Choi SW and
Seo DW: Sepiapterin inhibits cell proliferation and migration of
ovarian cancer cells via down-regulation of p70S6K-dependent
VEGFR-2 expression. Oncol Rep. 26:861–867. 2011.PubMed/NCBI
|
|
25
|
Cho YR, Choi SW and Seo DW: The in
vitro antitumor activity of Siegesbeckia glabrescens against
ovarian cancer through suppression of receptor tyrosine kinase
expression and the signaling pathways. Oncol Rep. 30:221–226. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee HN, Joo JH, Oh JS, Choi SW and Seo DW:
Regulatory effects of Siegesbeckia glabrescens on non-small cell
lung cancer cell proliferation and invasion. Am J Chin Med.
42:453–463. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Harbour JW, Luo RX, Santi AD, Postigo AA
and Dean DC: Cdk phosphorylation triggers sequential intramolecular
interactions that progressively block Rb functions as cells move
through G1. Cell. 98:859–869. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lemmon MA and Schlessinger J: Cell
signaling by receptor tyrosine kinases. Cell. 141:1117–1134. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kokpol U, Thebpatiphat S, Boonyaratavej S,
Chedchuskulcai V, Ni CZ, Clardy J, Chaichantipyuth C, Chittawong V
and Miles DH: Structure of trigonostemone, a new phenanthrenone
from the Thai plant Trigonostemon reidioides. J Nat Prod.
53:1148–1151. 1990. View Article : Google Scholar
|
|
30
|
Kanchanapoom T, Kasai R, Chumsri P,
Kraisintu K and Yamasaki K: Lotthanongine, an unprecedented
flavonoidal indole alkaloid from the roots of Thai medicinal plant,
Trigonostemon reidioides. Tetrahedron Lett. 43:2941–2943. 2002.
View Article : Google Scholar
|
|
31
|
Jayasuriya H, Zink DL, Singh SB, Borris
RP, Nanakorn W, Beck HT, Balick MJ, Goetz MA, Slayton L, Gregory L,
et al: Structure and stereochemistry of rediocide A, a highly
modified daphnane from Trigonostemon reidioides exhibiting potent
insecticidal activity. J Am Chem Soc. 122:4998–4999. 2000.
View Article : Google Scholar
|
|
32
|
Jayasuriya H, Zink DL, Borris RP, Nanakorn
W, Beck HT, Balick MJ, Goetz MA, Gregory L, Shoop WL and Singh SB:
Rediocides B-E, potent insecticides from Trigonostemon reidioides.
J Nat Prod. 67:228–231. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Soonthornchareonnon N, Sakayarojkul M,
Isaka M, Mahakittikun V, Chuakul W and Wongsinkongman P: Acaricidal
daphnane diterpenoids from Trigonostemon reidioides (KURZ) CRAIB
roots. Chem Pharm Bull (Tokyo). 53:241–243. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
He W, Cik M, Appendino G, Puyvelde LV,
Leysen JE and De Kimpe N: Daphnane-type diterpene orthoesters and
their biological activities. Mini Rev Med Chem. 2:185–200. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pettit GR, Ducki S, Tan R, Gardella RS,
McMahon JB, Boyd MR, Pettit GR III, Blumberg PM, Lewin NE, Doubek
DL, et al: Isolation and structure of pedilstatin from a republic
of maldives Pedilanthus sp. J Nat Prod. 65:1262–1265. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chumkaew P, Karalai C, Ponglimanont C and
Chantrapromma K: Antimycobacterial activity of phorbol esters from
the fruits of Sapium indicum. J Nat Prod. 66:540–543. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim JH, Kim HJ, Kim JK, Ahn EK, Ko HJ, Cho
YR, Lee SJ, Bae GU, Kim YK, Park JW, et al: Ligularia fischeri
inhibits endothelial cell proliferation, invasion and tube
formation through the inactivation of mitogenic signaling pathways
and regulation of vascular endothelial cadherin distribution and
matrix metalloproteinase expression. Oncol Rep. 34:221–226. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cho YR, Kim JH, Kim JK, Ahn EK, Ko HJ, In
JK, Lee SJ, Bae GU, Kim YK, Oh JS, et al: Broussonetia
kazinoki modulates the expression of VEGFR-2 and MMP-2 through
the inhibition of ERK, Akt and p70S6K-dependent signaling pathways:
Its implication in endothelial cell proliferation, migration and
tubular formation. Oncol Rep. 32:1531–1536. 2014. View Article : Google Scholar : PubMed/NCBI
|