Introduction
Lung cancer is a relatively common cancer and is a
major cause of cancer-related death worldwide (1,2). The
majority of lung cancers are non-small cell cancers (NSCLC),
consisting of adenocarcinomas and squamous cell carcinomas.
Treatment for NSCLC has improved dramatically in the past 15 years
thanks to molecular-targeted drugs (3–5). Recently,
new molecular-targeted drugs or immunotherapy drugs, such as immune
checkpoint inhibitors, have been developed, and treatment
strategies, particularly those for squamous cell carcinoma, have
been improved using personalized medicine (6,7). However,
the outcome of cancer treatment is still far from satisfactory, and
further examinations are required.
The epithelial-mesenchymal transition (EMT) is a
biological step during which epithelial cells lose their cell
polarity and cell-cell adhesion. This change is considered an
important step in both invasion and metastasis and the development
of treatment resistance in cancer. Cancer cells that have undergone
an EMT acquire the ability to infiltrate and migrate to other
organs (8,9). Recent studies have suggested that the
EMT is also involved in the acquisition of characteristics of
cancer-stem-like cells (10,11). Therefore, we aimed to analyze the
EMT-related mechanism of squamous cell carcinoma and to develop a
new treatment strategy for squamous cell carcinoma of the lung.
The Klotho gene, which is a 1014-amino acid
single-pass transmembrane protein expressed predominantly in renal
tubular epithelial cells, has been characterized as a systemic
anti-aging hormone and was originally identified in mice homozygous
for the mutated allele (kl−/−) (12–14). These
mice show a human-like aging-related syndrome and develop multiple
disorders such as hypogonadism, ectopic calcification,
osteoporosis, skin atrophy, and pulmonary emphysema (13). In contrast, transgenic mice
overexpressing Klotho show an extended life span that is 30% longer
in males and 20% longer in females. Previously, we reported an
association between the level of Klotho expression and overall
survival in patients with small cell lung cancer (SCLC) and large
cell neuroendocrine carcinoma (LCNEC). Expression of the Klotho
gene was an important postoperative prognosticator among patients
with SCLC and LCNEC (15,16). Recently, the Klotho gene was shown to
suppress the EMT and to inhibit transforming growth factor-β1
(TGF-β1) signaling, which is related to the invasion and metastasis
of cancer (17). We hypothesized that
the expression of Klotho regulates the EMT in squamous cell
carcinoma. The objective of the present study was to evaluate the
association between the expression of Klotho and the regulation of
EMT in squamous cell lung cancers. Accordingly, we investigated how
the expression of the Klotho gene regulates cell proliferation and
examined its relevance to the EMT in squamous lung cancer.
Materials and methods
Patient selection
For the analysis of non-invasive squamous cell
carcinoma, such as carcinoma in situ (cis), at Tokyo Medical
University Hospital (Usuda J moved to Nippon Medical School on
December 2012) between June 2009 and December 2011, we identified
10 patients with centrally located early lung cancers (CLELC) that
had been diagnosed during a bronchoscopy performed because of
abnormal sputum production and/or sputum cytological abnormalities
in a mass screening. These 10 patients, who received photodynamic
therapy using NPe6 (talaporfin sodium), were enrolled in the
present study. This retrospective study was conducted with the
approval of the Ethics Committee of Tokyo Medical University.
For the analysis of invasive squamous cell
carcinoma, among 161 patients who underwent surgical resection for
primary lung cancer between April 2007 and December 2008 at Nippon
Medical School Hospital, 30 patients with squamous cell carcinoma
were enrolled in the present study. Clinical information was
extracted from the medical records. The disease stage was based on
the TNM classification, 7th edition, using the International Union
Against Cancer (UICC) staging system. The all-histological types
were diagnosed by experienced pathologists at the Department of
Pathology, Nippon Medical School Hospital, according to the
histological typing of lung and pleural tumors in the WHO
International Histological Classification of Tumors, 4th edition.
This retrospective study was conducted with the approval of the
Ethics Committee of Nippon Medical School Hospital.
Immunohistochemical staining for
Klotho
We immunohistochemically examined the expression of
Klotho in patients with lung squamous cell carcinoma who had
undergone surgical resection or photodynamic therapy.
Immunohistochemical staining for Klotho was performed on 4-µM
formalin-fixed, paraffin-embedded tissue sections (15–17). The
slides were deparaffinized in xylene and dehydrated in a graded
ethanol series. Endogenous peroxidase was blocked with 0.3%
H2O2 in methanol for 30 min. All the slides
were heated to 95ºC by exposure to microwave irradiation for 20
min. The slides were then cooled for 1 h at room temperature and
washed in phosphate-buffered saline (PBS). Non-specific binding was
blocked by pre-incubation with 1% BSA for 30 min. After washing
with PBS, the slides were incubated with an anti-Klotho antibody
(KM2076; Kyowa Hakko Kirin, Tokyo, Japan) at 4ºC overnight. The
slides were then incubated with anti-Klotho antibody for 30 min at
room temperature. After washing in PBS, the slides were then
incubated with a peroxidase-conjugated secondary antibody. Negative
controls were prepared by omitting the primary antibody under the
same experimental conditions. Antibody staining was considered
positive when at least 10% of the tumor cells were stained, based
on the use of a 10% cutoff level in several previous studies
(15,16). All the slides were examined by two
observers who had no knowledge of the patients' clinical data.
Cell culture and transfection
The human squamous lung cancer cell line SQ5 and the
human adenocarcinoma cell line A549 were maintained in Dulbecco's
modified Eagle's medium (DMEM) supplemented with 10% fetal bovine
serum (FBS). We transfected a GFP-Klotho plasmid, which was kindly
provided by Dr Nabeshima (Foundation for Biomedical Research and
Innovation, Kobe, Japan), into SQ5 cells using Lipofectamin 3000
transfection reagent, according to the manufacturer's protocol
(Invitrogen, Carlsbad, CA, USA).
Flow cytometry
We transfected the GFP-Klotho plasmid or a GFP
vector into SQ5 cells; 24 h later, the cells were pelleted by
centrifugation, resuspended in PBS to a final density of
~2.9×106 cells/ml, and then filtered through a nylon
membrane to remove cell aggregates. We sorted the GFP-positive
cells using flow cytometry and a FACSCanto II (BD Biosciences, CA,
USA) with activation at 488 nm and fluorescence emission monitoring
at 508 nm (GFP). The data acquisition and analysis were performed
using FlowJo software (TreeStar, Ashland, OR, USA). A minimum of
10,000 events were collected for each analysis. We eliminated dead
cells and debris from the analysis using forward-scatter and
side-scatter parameters, and the remaining cells were then sorted
into GFP-positive and GFP-negative populations. The resulting cells
were resuspended in PBS. The protein expression levels were then
analyzed using an immunoblot analysis.
Immunoblot analysis
Cells were harvested by centrifugation and washed
twice with ice-cold PBS. The cell pellets were incubated in a lysis
buffer (50 mM Tris-HCl, pH 7.6, 120 mM NaCl, 1% Triton X-100, 0.2%
sodium-deoxycholate and protease inhibitor). The lysates were
cooled on ice for 10 min and then centrifuged at 14,000 × g
for 30 min. Each sample of 6.56 µg of total protein was separated
using SDS-PAGE on 7.5% gels and transferred to polyvinylidene
difluoride membranes.
The membranes, after blocking with 5% skimmed milk,
were incubated with the following antibodies: rat anti-human Klotho
monoclonal antibody (1:500 dilution; Kyowa Hakko Kirin, Tokyo,
Japan), rabbit anti-human E-cadherin monoclonal antibody (1:1,000
dilution), rabbit anti-human N-cadherin monoclonal antibody
(1:1,000 dilution), rabbit anti-human vimentin monoclonal antibody
(1:1000 dilution), or rabbit anti-human Snail monoclonal antibody
(1:500 dilution) (all from Cell Signaling Technology, Inc.,
Danvers, MA, USA) for 1 h. After rinsing with PBS containing 0.1%
(v/v) Triton X-100, the membranes were incubated with goat anti-rat
immunoglobulin-G (IgG)-conjugated horseradish peroxidase (HRP)
(1:10,000 dilution; Kirkegaard & Perry Laboratories,
Gaithersburg, MD, USA) for the anti-Klotho antibody or goat
anti-rabbit IgG conjugated HRP (1:2,000 dilution; Cell Signaling
Technology, Inc.) for the other antibodies. As a loading control,
blots were probed with β-actin (1:1,000 dilution; Cell Signaling
Technology, Inc.). The membranes were washed and developed using
western blotting enhanced chemiluminescence detection reagents
(Bio-Rad Laboratories, Richmond, CA, USA).
Statistics
The statistical analyses were performed using EZR
(Saitama Medical Center, Jichii Medical University, Saitama,
Japan), which is a graphical user interface for R (The R Foundation
for Statistical Computing, Vienna, Austria) (18). The study variables were compared
between the study groups using the Fisher's exact test for
categorical variables and the Student's t-test for
continuous variables. P-values of <0.05 were considered
significant.
Results
Expression of Klotho in clinical
specimens
To elucidate the association between
cancer-invasiveness and the expression of Klotho, we first examined
the expression of Klotho in lung cancer patients with squamous cell
carcinoma in situ (cis) using an immunohistochemical
analysis. The clinicopathological characteristics of the patients
are listed in Table I. The median
patient age at the time of diagnosis was 68 years (range, 57–81
years). All the patients were men and were heavy smokers with a
smoking history of >30 pack-years. In all 10 patients with
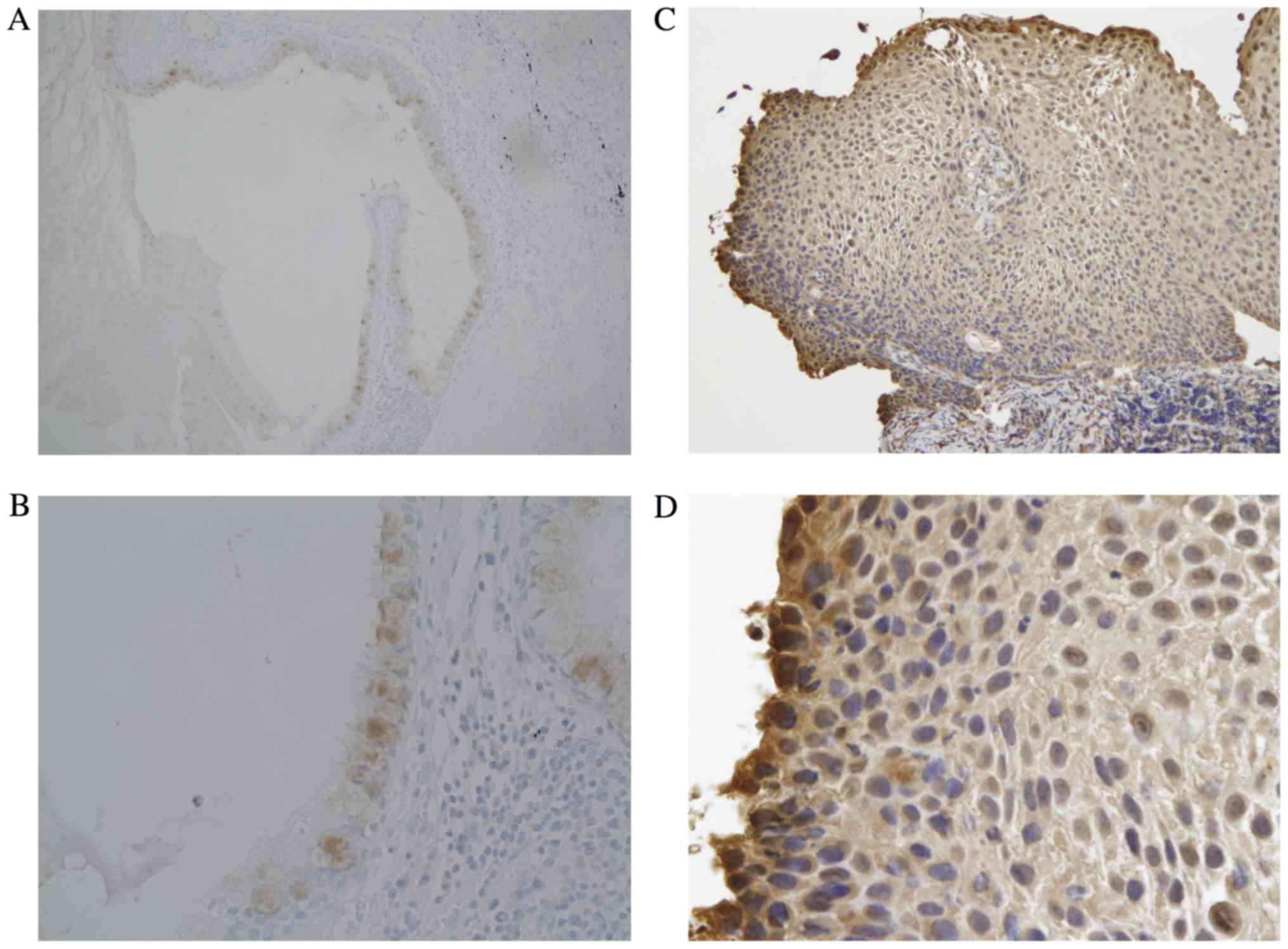
carcinoma in situ, we observed the expression of Klotho as
shown in Fig. 1C and D. The
expression of Klotho was observed in not only normal bronchial
epithelial cells, but also centrally located early lung cancers,
which were all carcinoma in situ and had been treated using
PDT. The criteria for a centrally located early lung cancer (CLELC)
were strictly defined in 1975. The tumor must be located only as
far as the segmental bronchi and must be a carcinoma in situ
or with only limited invasion into the bronchial wall. Fig. 1C and D shows the typical features of
CLELC. In these centrally located early lung cancers, cells that
were located closer to the basal membrane epithelial cells had
lower expression levels of Klotho. These results suggest that the
expression of Klotho may be associated with cancer
invasiveness.
 | Table I.Clinicopathologic Characteristics of
the patients with centrally located early lung cancer who underwent
PDT. |
Table I.
Clinicopathologic Characteristics of
the patients with centrally located early lung cancer who underwent
PDT.
| Characteristics | N0. |
|---|
| Patients | 10 |
| Age (y) | 57–81 (68.6) |
| Gender | Male: 10 |
|
| Female: 0 |
| Size (mm) | 6–20 (10.8) |
| Endoscopic
findings | Flat type: 8 |
|
| Polypoid: 1 |
|
| Nodular: 1 |
| CR rate | 100% |
| Klotho
expression | Positive: 10 |
|
| Negative: 0 |
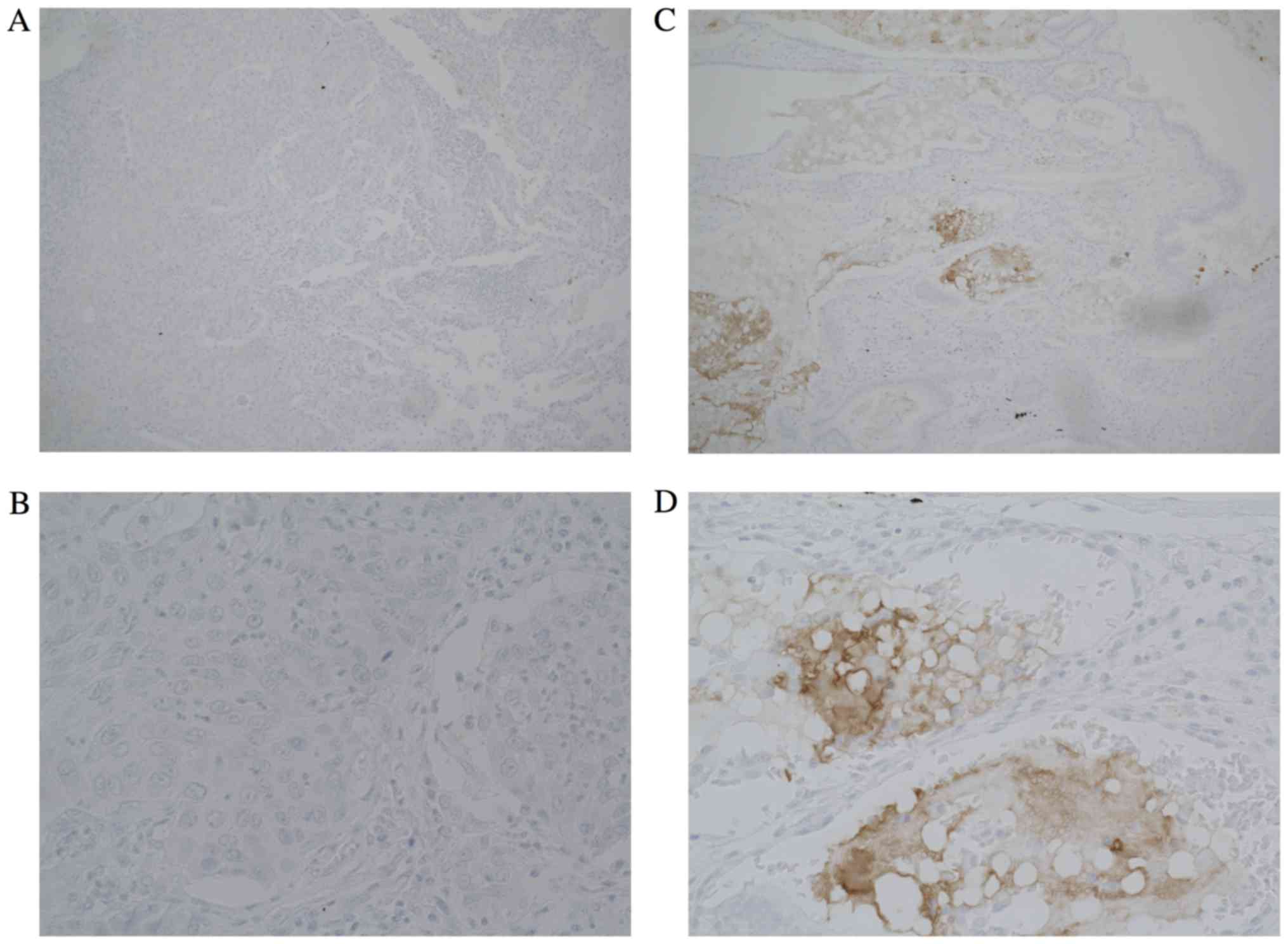
Next, to analyze invasive squamous cell carcinoma,
we performed immunohistochemical staining for specimens from
patients who had undergone surgical resection. The
clinicopathological characteristics of the patients with invasive
squamous cell carcinoma are listed in Table II. Though the sample number in Klotho
positive group is small, no significant differences in the
clinicopathological characteristics were observed between the
Klotho-positive group and the Klotho-negative group (Table III). However, in lung cancer
patients with invasive or advanced squamous cell carcinoma, who had
undergone a complete surgical resection, Klotho expression was
observed in only 4 patients (13%) (Fig.
2). Therefore, these results suggested that the expression of
Klotho may be associated with tumor development in squamous cell
lung cancer.
 | Table II.Clinicopathological characteristics of
30 patients with invasive squamous cell carcinoma who underwent
lung operation. |
Table II.
Clinicopathological characteristics of
30 patients with invasive squamous cell carcinoma who underwent
lung operation.
| Characteristics | No. |
|---|
| Total | 30 |
| Age
(years) | 44–82 (71.7) |
| Gender |
|
| Male | 25 |
|
Female | 3 |
| Surgical
procedure |
|
|
Segmentectomy | 2 |
|
Lobectomy | 25 |
|
Pneumonectomy | 3 |
| Location |
|
|
central | 3 |
|
intermidiate | 10 |
|
peripheral | 17 |
| Pathological
Stage |
|
| IA | 10 |
| IB | 4 |
| IIA | 2 |
| IIB | 9 |
| IIIA | 5 |
| Diameter (mm) | 15–90 (37.3) |
|
Lymphangio-invasion |
|
| (−) | 5 |
| (+) | 25 |
| Lymph node meta |
|
| (−) | 21 |
| (+) | 9 |
| Pleural invasion |
|
| (−) | 20 |
| (+) | 10 |
| Pulmonary
metastasis |
|
| (−) | 29 |
| (+) | 1 |
| Recurrence |
|
| Yes | 9 |
| No | 21 |
| Alive |
|
| Yes | 18 |
| No | 12 |
| Klotho |
|
| Yes | 4 |
| No | 26 |
 | Table III.Clinicopathologic characteristics of
patients who underwent surgical resection, for Klotho positive and
negative groups. |
Table III.
Clinicopathologic characteristics of
patients who underwent surgical resection, for Klotho positive and
negative groups.
| Characteristics | Klotho (−) | Klotho (+) | P-value |
|---|
| No. of
patients | 26 | 4 |
|
| Age (years) | 72.6 | 65.8 | 0.099 |
| Gender |
|
|
|
|
Male | 23 | 4 | 1 |
|
Female | 3 | 0 |
|
| Surgical
procedure |
|
|
|
|
Segmentectomy | 2 | 0 | 1 |
|
Lobectomy | 21 | 4 |
|
|
Pneumonectomy 3 | 0 |
|
|
| Location |
|
|
|
|
Central | 3 | 0 | 0.752 |
|
Intermidiate | 8 | 2 |
|
|
Peripheral | 15 | 2 |
|
| Pathological
Stage |
|
|
|
| IA | 9 | 1 | 0.926 |
| IB | 3 | 1 |
|
|
IIA | 2 | 0 |
|
|
IIB | 8 | 1 |
|
|
IIIA | 4 | 1 |
|
| Diameter (mm) | 37.0 | 39.2 | 0.83 |
|
Lymphangio-invasion |
|
|
|
|
(−) | 4 | 1 | 0.538 |
|
(+) | 22 | 3 |
|
| Lymph node
meta |
|
|
|
|
(−) | 18 | 3 | 1 |
|
(+) | 8 | 1 |
|
| Pleural
invasion |
|
|
|
|
(−) | 17 | 3 | 1 |
|
(+) | 9 | 1 |
|
| Pulmonary
metastasis |
|
|
|
|
(−) | 25 | 4 | 1 |
|
(+) | 1 | 0 |
|
| Recurrence |
|
|
|
| No | 18 | 3 | 1 |
|
Yes | 8 | 1 |
|
| Alive |
|
|
|
|
Yes | 16 | 2 | 1 |
| No | 10 | 2 |
|
Klotho regulated the
epithelial-mesenchymal transition in vitro
We hypothesized that Klotho expression regulates
EMT-related proteins, which are associated with invasion and
metastasis. We next studied EMT markers such as E-cadherin,
N-cadherin, vimentin and Snail in lung squamous cancer cells. To
improve the efficiency of transfection, the SQ5 cells were
transfected with a GFP-Klotho expression vector and then sorted
into GFP-positive or GFP-negative populations using flow cytometry.
Overall, 14.8% of the cells were sorted as GFP-positive cells
(Fig. 3A, B).
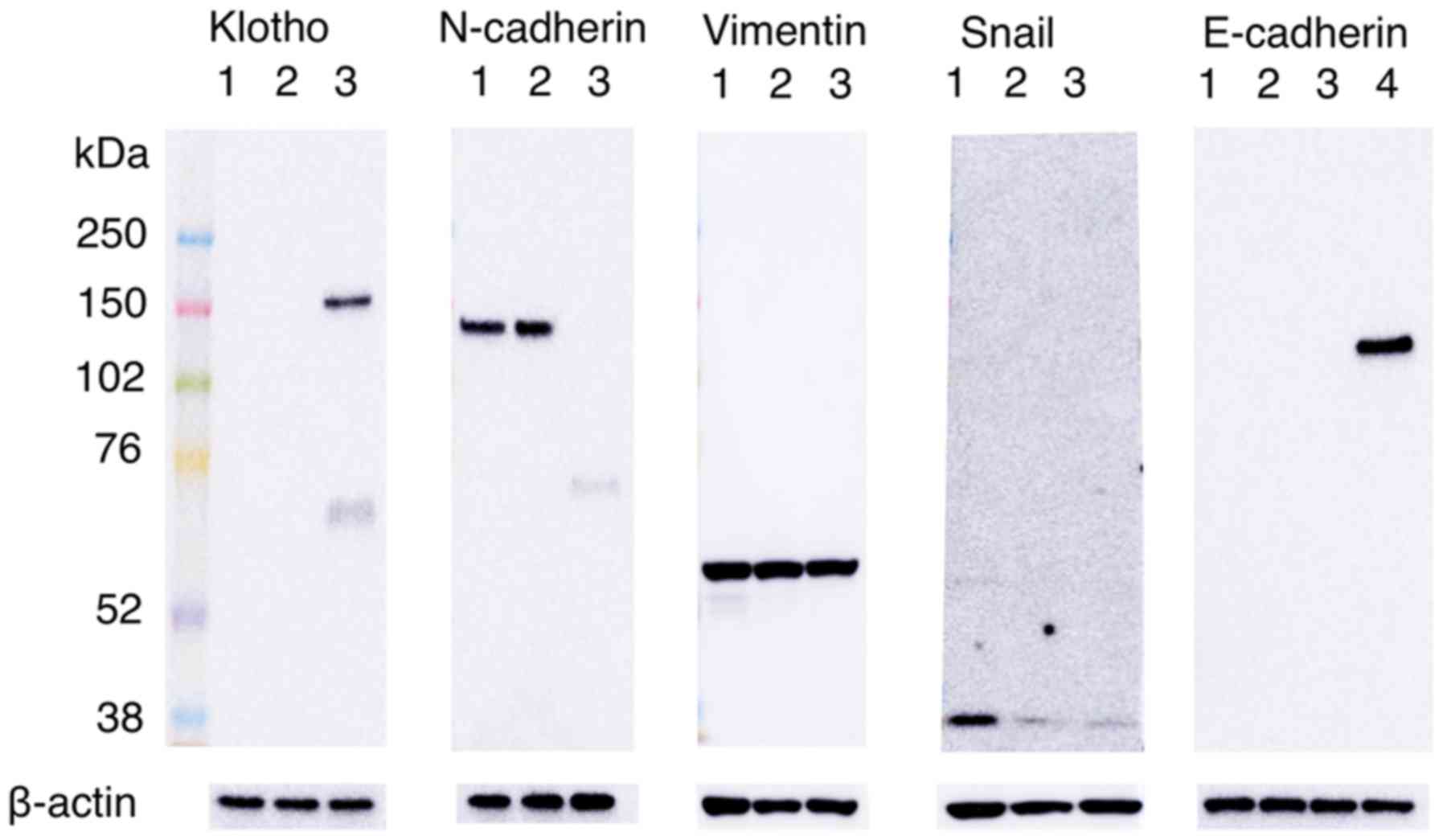
To determine whether Klotho expression affects the
protein expressions of EMT markers, an immunoblot assay was
performed (Fig. 4). The GFP-positive
sorted cells showed an overexpression of Klotho. Moreover, in SQ5
cells transiently overexpressing GFP-Klotho, the expression of
N-cadherin, which is a mesenchymal marker, was completely
inhibited, compared with wild-type SQ5 cells or SQ5 cells
transfected with only the GFP vector. Namely, the overexpression of
Klotho resulted in a loss of N-cadherin expression in the SQ5
cells. The overexpression of Klotho did not affect the regulations
of other mesenchymal markers, such as vimentin or Snail, or the
epithelial marker E-cadherin. Thus, the present in vitro
study confirmed that Klotho expression inhibits N-cadherin
expression, which is an EMT marker, in lung squamous cancer
cells.
Discussion
Several insights have emerged as a result of our
immunohistochemical analyses. Klotho expression was observed not
only in normal bronchial epithelial cells, but also in centrally
located early lung cancers in all the patients. In patients with
invasive squamous cell carcinoma, however, Klotho expression was
only seen in 4 cases (13%). Several reports have identified an
association between Klotho expression and cancerous changes. Some
reports have shown a reduction in the expression of Klotho in
invasive breast cancers or cervical cancers, compared with normal
lesions (19,20). Moreover, the expression of Klotho has
been observed in carcinoma in situ, but not in invasive
carcinoma (20,21). As shown in Figs. 1 and 2,
the expression of Klotho was associated with the inhibition of
cancer invasiveness, similar to the results of previous reports.
These results suggest that Klotho expression is associated with
invasion and that a loss of Klotho expression can promote cancer
progression in squamous cell lung carcinoma.
In this study, we hypothesized that the expression
of Klotho can inhibit the progression of lung cancer and regulate
the EMT. To elucidate the association between the expression of
Klotho and the expression of EMT markers, we examined the protein
levels using a western blot analysis and a human squamous lung
cancer cell line, SQ5, transfected with GFP-Klotho plasmid DNA, as
shown in Fig. 4. The Klotho blot
showed two bands in SQ5 cells transfected with the GFP-Klotho
plasmid DNA (lane 3). This is caused by two forms: membrane Klotho
and secreted Klotho. The extracellular domain is able to ectodomain
shedding. Klotho protein is clipped on the cell surface by
membrane-anchored proteases and the entire extra cellular domain is
released into systemic circulation, functioning as a hormone
(14,17). We observed that the overexpression of
Klotho almost completely suppressed the expression of N-cadherin.
These results indicate that Klotho inhibited the expression of
N-cadherin, which is a mesenchymal marker, and Klotho may inhibit
the progression of cancer cells. The N-cadherin blot showed smaller
band in GFP-Klotho transfected SQ5 cells. This appears to be caused
by the protein destruction of N-cadherin or the suppression of the
N-cadherin expression at the mRNA level, followed by overexpression
of Klotho. The E-cadherin blot did not show any band in three SQ5
samples. This result indicates that E-cadherin is not expressed
originally and E-cadherin expression is not caused by Klotho
transfection in SQ5 cells. The Snail blot showed that both the
GFP-vector control and the GFP-Klotho samples have a reduced level
of Snail. This may suggest that the transfection may be causing
this reduction.
The regulation of N-cadherin expression by Klotho is
reportedly related to several signaling pathways. The Wnt signaling
pathway inhibits glycogen synthase kinase-3β, which stabilizes β
catenin, promoting N-cadherin expression. Klotho can inhibit the
activation of the Wnt signaling pathway (22). Lee reported that Klotho functioned as
a Wnt antagonist in a cervical cancer cell line and that the
expression of Klotho in SiHa cells resulted in a decrease in
N-cadherin (23). In addition, the
IGF-1 signaling pathway has been shown to have a strong influence
on the EMT process (14). Klotho
binds to a cell-surface receptor to inhibit the activation of
insulin and IGF-1 and repress the intercellular signals of insulin
and IGF-1. Moreover, Klotho has been reported to inhibit
TGF-β1-induced EMT responses, resulting in an increase in
N-cadherin and a decrease in E-cadherin in A549 cells (17). From these studies, we can speculate
that Klotho may play a critical role as a suppressor of the
expression of N-cadherin, thereby inhibiting the Wnt, IGF-1, and
TGF-β1 signaling pathways simultaneously.
To our knowledge, this is the first study that the
Klotho gene was a suppressor of the EMT in lung squamous cell
carcinoma. However, the limitations of this study include that we
used only SQ5 cell line, that we did not clarified whether the
overexpression of Klotho can inhibit the invasiveness of cancer
cells, and that we did not evaluate expression of E-cadherin
markers in immunohistochemical analysis. Therefore, additional
studies are needed to make a more definitive conclusion regarding
the ability of Klotho to inhibit cellular infiltration, cell
proliferation, and cell motility in more squamous lung cancer cell
lines. We would like to investigate expression of EMT markers in
immunohistochemical analysis in the future.
In this immunohistochemical analysis of specimens
from patients with lung squamous cell carcinoma who had undergone
surgical resection, Klotho expression did not have a significantly
favorable effect on patient outcome (data not shown). There was,
however, a trend for an improvement in disease-free survival
consistent with Klotho expression. We previously reported that the
expression of Klotho was an important prognosticator for lung large
cell neuroendocrine carcinoma and lung small cell carcinoma
(15,16). Further studies in more patients with
lung squamous cell carcinoma are needed.
Because the EMT has been shown to affect not only
carcinoma invasion or metastasis but also the acquisition of
cancer-stem cell traits or resistance to anticancer drugs or
radiation therapy, the EMT may play an important role as a
treatment target for cancer. Therefore, we suggest that Klotho may
regulate the EMT and could be useful as a new and effective
anti-cancer drug in lung squamous cell carcinoma. In the future, we
hope to develop innovative treatment strategies for squamous cell
lung carcinoma that include not only postoperative adjuvant
chemotherapy, but also new drug discovery.
Acknowledgements
This study was supported in part by Grant-in-Aid for
Scientific Research (C) from Japan Society for the Promotion of
Science (JSPS), KAKENHI 16K10693 (J.U.) and supported by the
Research on Development of New Medical Devices from Japan Agency
for Medical Research and development (AMED), 16hk0102025h0002
(J.U.). We would like to thank Professor Yo-ichi Nabeshima,
Laboratory of Molecular Life Science, Institute of Biomedical
Research and Innovation Foudation for Biomedical Research and
Innovation, for giving Klotho-GFP plasmid DNA.
References
|
1
|
American Cancer Society: Cancer Facts
& Figures 2016. American Cancer Society; Atlanta: 2016
|
|
2
|
International Agency for Research on
Cancer: GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and
Prevalence Worldwide in 2012. http://globocan.iarc.fr/Default.aspxAccessed on.
August 22–2016.
|
|
3
|
Maemondo M, Inoue A, Kobayashi K, Sugawara
S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I,
et al: Gefitinib or chemotherapy for non-small-cell lung cancer
with mutated EGFR. N Engl J Med. 362:2380–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mitsudomi T, Morita S, Yatabe Y, Negoro S,
Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, et
al: Gefitinib versus cisplatin plus docetaxel in patients with
non-small-cell lung cancer harbouring mutations of the epidermal
growth factor receptor (WJTOG3405): An open label, randomised phase
3 trial. Lancet Oncol. 11:121–128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shaw AT, Kim DW, Nakagawa K, Seto T, Crinó
L, Ahn MJ, De Pas T, Besse B, Solomon BJ, Blackhall F, et al:
Crizotinib versus chemotherapy in advanced ALK-positive lung
cancer. N Engl J Med. 368:2385–2394. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Soria JC, Felip E, Cobo M, Lu S, Syrigos
K, Lee KH, Göker E, Georgoulias V, Li W, Isla D, et al: Afatinib
versus erlotinib as second-line treatment of patients with advanced
squamous cell carcinoma of the lung (LUX-Lung 8): An open-label
randomised controlled phase 3 trial. Lancet Oncol. 16:897–907.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rizvi NA, Mazières J, Planchard D,
Stinchcombe TE, Dy GK, Antonia SJ, Horn L, Lena H, Minenza E,
Mennecier B, et al: Activity and safety of nivolumab, an anti-PD-1
immune checkpoint inhibitor, for patients with advanced, refractory
squamous non-small-cell lung cancer (CheckMate 063): A phase 2,
single-arm trial. Lancet Oncol. 16:257–265. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Singh A, Greninger P, Rhodes D, Koopman L,
Violette S, Bardeesy N and Settleman J: A gene expression signature
associated with ‘K-Ras addiction’ reveals regulators of EMT and
tumor cell survival. Cancer Cell. 15:489–500. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mani SA, Guo W, Liao M-J, Eaton EN,
Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et
al: The epithelial-mesenchymal transition generates cells with
properties of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wellner U, Schubert J, Burk UC,
Schmalhofer O, Zhu F, Sonntag A, Waldvogel B, Vannier C, Darling D,
zur Hausen A, et al: The EMT-activator ZEB1 promotes tumorigenicity
by repressing stemness-inhibiting microRNAs. Nat Cell Biol.
11:1487–1495. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi
H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, et
al: Mutation of the mouse klotho gene leads to a syndrome
resembling aging. Nature. 390:45–51. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Matsumura Y, Aizawa H, Shiraki-lida T,
Nagai R, Kuro-o M and Nabeshima Y: Identification of the human
klotho gene and its two transcripts encoding membrane and secreted
Klotho protein. Biochem Biophys Res Commun. 242:626–630. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kurosu H, Yamamoto M, Clark JD, Pastor JV,
Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M,
Kawaguchi H, et al: Suppression of aging in mice by the hormone
Klotho. Science. 309:1829–1833. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Usuda J, Ichinose S, Ishizumi T, Ohtani K,
Inoue T, Saji H, Kakihana M, Kajiwara N, Uchida O, Nomura M, et al:
Klotho predicts good clinical outcome in patients with
limited-disease small cell lung cancer who received surgery. Lung
Cancer. 74:332–337. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Usuda J, Ichinose S, Ishizumi T, Ohtani K,
Inoue T, Saji H, Kakihana M, Kajiwara N, Uchida O, Nomura M, et al:
Klotho is a novel biomarker for good survival in resected large
cell neuroendocrine carcinoma of the lung. Lung Cancer. 72:355–359.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Doi S, Zou Y, Togao O, Pastor JV, John GB,
Wang L, Shiizaki K, Gotschall R, Schiavi S, Yorioka N, et al:
Klotho inhibits transforming growth factor-beta1 (TGF-beta1)
signaling and suppresses renal fibrosis and cancer metastasis in
mice. J Biol Chem. 286:8655–8665. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Aviel-Ronen S, Rubinek T, Zadok O, Vituri
A, Avivi C, Wolf I and Barshack I: Klotho expression in cervical
cancer: Differential expression in adenocarcinoma and squamous cell
carcinoma. J Clin Pathol. 69:53–57. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wolf I, Levanon-Cohen S, Bose S, Ligumsky
H, Sredni B, Kanety H, Kuro-o M, Karlan B, Kaufman B, Koeffler HP
and Rubinek T: Klotho: A tumor suppressor and a modulator of the
IGF-1 and FGF pathways in human breast cancer. Oncogene.
27:7094–7105. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chang B, Kim J, Jeong D, Jeong Y, Jeon S,
Jung SI, Yang Y, Kim KI, Lim JS, Kim C and Lee MS: Klotho inhibits
the capacity of cell migration and invasion in cervical cancer.
Oncol Rep. 28:1022–1028. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen B, Ma X, Liu S, Zhao W and Wu J:
Inhibition of lung cancer cells growth, motility and induction of
apoptosis by Klotho, a novel secreted Wnt antagonist, in a
dose-dependent manner. Cancer Biol Ther. 13:1221–1228. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee J, Jeong DJ, Kim J, Lee S, Park JH,
Chang B, Jung SI, Yi L, Han Y, Yang Y, et al: The anti-aging gene
KLOTHO is a novel target for epigenetic silencing in human cervical
carcinoma. Mol Cancer. 9:1092010. View Article : Google Scholar : PubMed/NCBI
|