Introduction
Breast cancer is the most frequent type of
malignancy in females observed worldwide (1). The incidence of breast cancer is
increasing. The prognosis of patients with late-stage breast cancer
is closely associated with metastasis, as once the tumor
metastasizes; there are few effective treatment options available
(2). Tumor initiation and development
is a complicated process and is associated with the loss of normal
regulatory pathways in cell proliferation, differentiation and
apoptosis (3,4).
Curcumin, a well-known chemopreventive agent
extracted from turmeric, has a history of 5,000 years (5,6). In recent
years, studies have indicated that curcumin is able to inhibit
proliferation and survival, induce apoptotic and non-apoptotic
(autophagocytosis and paraptosis) cell death, and reduce invasion
and migration in various types of malignant cancer cells (7–9). Through
regulation of the expression of genes associated with programmed
cell death, curcumin causes a high degree of apoptosis in human
breast cancer cells (10). However,
few studies have been performed that investigate the underlying
mechanisms of curcumin as a potential agent in breast cancer
therapy. Notably, curcumin is a potent inhibitor of nuclear
factor-κ-light-chain-enhancer of activated B cells (NF-κB),
indicating its potential role in inhibiting tumor pathogenesis in a
number of human malignancies and has a dose-dependent
pharmacological effect (11,12). The present study aimed to investigate
the anticancer effects of curcumin in MCF-7 cells and to
investigate the underlying mechanisms.
Materials and methods
Cells and reagents
Dulbecco's modified Eagle's medium (DMEM) and fetal
bovine serum (FBS) were obtained from HyClone™ (GE Healthcare,
Chicago, IL, USA). Curcumin (purity >98%) was supplied by
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Cell culture
Human breast cancer MCF-7 cell line was purchased
from Shanghai Institute of Cell Biology, Chinese Academy of
Sciences (Shanghai, China). The MCF-7 cells were cultured in DMEM
supplemented with 10% FBS, 100 U/ml penicillin and 100 U/ml
streptomycin (Sigma-Aldrich; Merck KGaA) in a humidified atmosphere
of 95% air with 5% CO2 at 37°C.
Cell viability and proliferation
assays
The viability and proliferation of MCF-7 cells were
quantified using a standard MTT reduction assay. To measure the
effects of curcumin, MCF-7 cells were seeded into 96-well plates at
a density of 2×103 cells/well and allowed to adhere
overnight. The density of viable cells was determined in a control
plate. Curcumin was prepared immediately prior to use and added to
test wells at a range of concentrations (2.5, 5, 10, 20 and 40 µM)
for 24, 48 and 72 h. MTT (10 µl MTT/100 µl DMEM; Sigma-Aldrich;
Merck KGaA) solution was added to the wells. Optical density of
each culture was read by using a microplate reader at a wavelength
of 570 nm.
Flow cytometry
To elucidate the mechanism underlying curcumin
treatment on MCF-7 cells, cell cycle analysis by flow cytometry was
performed. Approximately 1–5×106 MCF-7 cells were
treated with 20 µM curcumin at 24, 48 and 72 h after the cells were
collected, in accordance with the Annexin V-fluorescein
isothiocyanate (FITC) kit manufacturer's protocol (ab14085; Abcam,
Cambridge, MA, USA). Briefly, 195 µl cell suspension was added to 5
µl Annexin V-FITC solution, and the cells were incubated at room
temperature for 10 min. The cells were then washed with PBS and
suspended in 190 µl binding buffer from the kit. A total of 10 µl
propidium iodide was added, and cells undergoing early apoptosis
was detected by flow cytometry (BD FACSCalibur™; BD Biosciences,
Franklin Lakes, NJ, USA). The BD FACSCalibur was equipped with a
dual laser (488 and 635 nm) and CellQuest version 3.0 software (BD
Biosciences).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from 1–5×106
MCF-7 cells co-cultured with curcumin for various durations using 1
ml TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Waltham, MA, USA), according to the manufacturer's
protocol. The RNA sample was dissolved in RNase-free water and
quantified spectrophotometrically. PrimeScript™ RT Master mix was
used to transcribe cDNA, according to the manufacturer's protocol
(Takara Biotechnology Co., Ltd., Dalian, China). The EzOmics SYBR
qPCR kit was purchased from Biomics USA Inc., (Palo Alto, CA, USA),
which included 5 µl cDNA template and 15 µl reaction mixture,
containing 10 µl 2X SYBR Green mix, 0.5 µM forward primer, 0.5 µM
reverse primer and RNase-free H2O. RT-qPCR analysis was
performed to detect the relative expression levels of B-cell
lymphoma 2 (Bcl-2), Bcl-2-associated X (Bax) and β-actin. β-actin
was used as an internal control for input RNA. The amplification
procedure was as follows: 40 cycles of 95°C for 30 sec, 95°C for 5
sec, 95°C for 30 sec and 72°C for 30 sec, followed by 95°C for 1
min, 95°C for 30 sec and 75°C for 30 sec. Primers were designed
using the Primer Express version 3.0 software package (Applied
Biosystems; Thermo Fisher Scientific, Inc.) for the expression
experiments. The primers (Sangon Biotech Co., Ltd., Shanghai,
China) used are as follows: Bcl-2 forward,
5′-GTTTGATTTCTCCTGGCTGTCTC-3′ and reverse,
5′-GAACCTTTTGCATATTTGTTTGG-3′; Bax forward,
5′-AAGCTGAGCGAGTGTCTCAAG-3′ and reverse,
5′-CAAAGTAGAAAAGGGCGACAAC-3′; β-actin forward,
5′-TGGCACCCAGCACAATGAA-3′ and reverse,
5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′. The mRNA expression of each gene
was normalized to that of β-actin. Data were analyzed using ABI
Prism 7900 Sequence Detection system version 2.3 software (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The relative mRNA
expression was calculated using the comparative Cq method
(2−ΔΔCq) (13).
Western blot analysis
The BCA Protein Assay kit (Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA) was used to semi-quantitatively assess
protein concentration. A total of 50 µg protein from each well was
loaded onto a 10% SDS-PAGE and, following this, electroblotted onto
a nitrocellulose membrane. The membranes were blocked in TBST (pH
7.4; 20 mM Tris HCl, 150 mM NaCl and 0.1% Tween-20) containing 5%
skimmed milk for 2 h at 4°C, and then incubated for 16 h at 4°C
with anti-NF-κB p65 (1:2,000; cat. no. SC-8008; Santa Cruz
Biotechnology, Inc.) and IκB (1:1,000; cat. no. SC-371; Santa Cruz
Biotechnology, Inc.) antibodies. The membrane was washed twice
using TBS and 0.05% Tween-20 (Biosharp Life Sciences, Hefei,
China), and subsequently incubated at room temperature for 2 h with
horseradish peroxidase-conjugated rabbit anti-mouse immunoglobulin
G (1:1,000; cat. no. SC-358922; Santa Cruz Biotechnology, Inc.). An
electrochemiluminescence kit (Beyotime Institute of Biotechnology,
Haimen, China) was used to visualize the immunoreactions according
to the manufacturer's instructions.
Statistical analysis
Statistical analyses were performed with one-way
analysis of variance followed by Bonferroni test or t-test when
appropriate using SPSS version 10.0 statistical software (SPSS,
Inc., Chicago, IL, USA). P<0.05 was used to indicate a
statistically significant difference. Data of continuous variables
are presented as the mean ± standard deviation.
Results
Inhibition of cell growth by curcumin
treatment
Following treatment of the MCF-7 cells for 24, 48
and 72 h with curcumin, the growth inhibitory activity of curcumin
was determined, and then the viability of the cells was measured by
MTT assay. Briefly, MCF-7 cells exposed to curcumin exhibited a
significant decrease in cell proliferation in a time- and
dose-dependent manner compared with those treated with control
(P<0.05; Fig. 1).
Effect of curcumin treatment on cell
cycle and apoptosis
Following treatment of MCF 7-cells with 20 µM
curcumin for 24, 48 and 72 h, the apoptotic rate was 20.52, 28.65
and 36.25%, respectively. Compared with the control group, this was
a significant difference at each time point (P<0.05; Table I). Cell cycle measurements revealed
that MCF-7 cells treated with curcumin significantly increased the
proportion of cells in the G0/G1 phase (P<0.01) and
significantly decreased the proportion of cells in the S phase
(P<0.01; Table I).
 | Table I.Effect of curcumin treatment on cell
cycle and apoptosis as determined by flow cytometry. |
Table I.
Effect of curcumin treatment on cell
cycle and apoptosis as determined by flow cytometry.
|
| Cell cycle, % |
|
|---|
|
|
|
|
|---|
| Time, h | G1/S | G2/M | Apoptosis, % |
|---|
| 0 (control) | 35.68 | 65.29 | 4.52 |
| 24 | 36.27a | 56.71a | 20.52a |
| 48 | 52.46a | 36.19a | 28.65a |
| 72 | 65.84a | 15.63a | 36.25a |
Effect of curcumin treatment on the
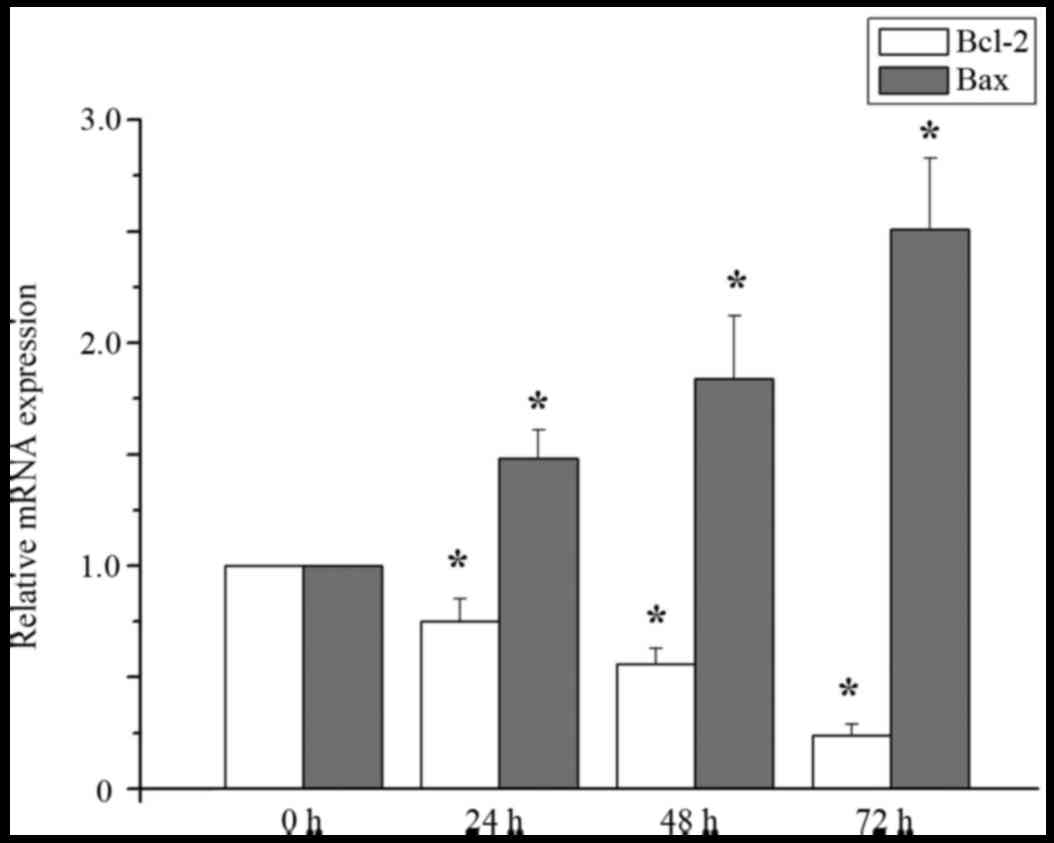
levels of Bcl-2 and Bax RNA expression
Compared with the control group, the expression
level of bcl-2 mRNA in MCF-7 cells treated with 20 µM curcumin was
significantly decreased at treatment at 24, 48 and 72 h, but the
level of bax mRNA was significantly increased at 24, 48, and 72 h
(P<0.05; Fig. 2).
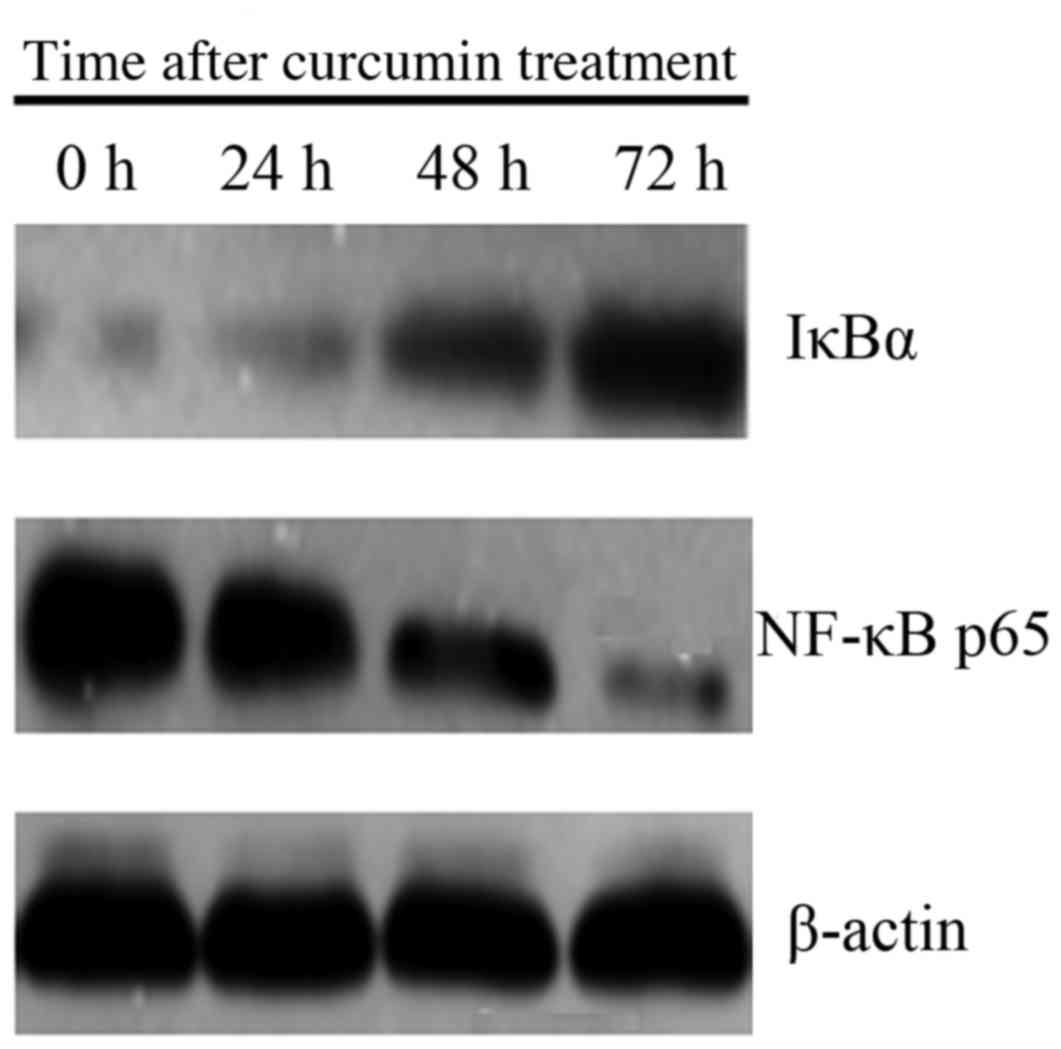
Effect of curcumin treatment on NF-κB
and IκBα protein expression
Level of NF-κB p65 protein expression was lower, and
expression of IκBα was higher in cells treated with 20 µM curcumin
at 24, 48 and 72 h, compared with 0 h (P<0.05; Fig. 3).
Discussion
The breast cancer MCF-7 cell line was selected to
assess the potential effects of curcumin. The results of the
present study demonstrated that curcumin was able to inhibit MCF-7
cell proliferation and promote apoptosis, possibly by regulating
the NF-κB signaling pathway.
It is well known that breast cancer is one of the
most common malignancies in females and the main cause of
cancer-associated mortality of females worldwide (14,15). In
breast cancer the complicated process is able to cause the loss of
normal regulatory pathways between cell proliferation,
differentiation and apoptosis (3).
The cytotoxic activity of curcumin in certain types of cancer cells
is well known, with many reports highlighting the
anti-proliferative effect of curcumin on cells. Curcumin inhibits
cell survival and proliferation and leads to apoptosis of lung
cancer cells by reducing the expression level of Axl receptor
tyrosine kinase and affecting Axl activation of non-small cell lung
cancer (16). Curcumin significantly
suppressed cell growth, decreased clonogenic potential, inhibited
migration and invasion and induced apoptosis and cell cycle arrest
in pancreatic cancer cells, liver cancer HepG2 cells and prostate
cancer DU-145 cells (17,18). It has been reported that a high
concentration of curcumin is able to induce apoptosis in human
leukemia cells (19). However it has
also been demonstrated that curcumin is able to inhibit apoptosis
in T lymphocytes (20).
Uncontrolled cell proliferation and inhibition of
cell death is central to cancer development. Tumor cells exhibit
resistance to apoptosis in order to survive and metastasize. The
common features of cancer development, including genetic
modification and changes in apoptotic pathways in the pathogenesis,
can provide an insight into therapeutic targets. Treatment
strategies that are designed to destroy the cancer cells by
activating apoptosis signaling pathways are required, ideally
therapies that are able to induce tumor cell-selective apoptosis,
and no harmful effects on normal cells (21). NF-κB is a critical regulator of
fundamental cell functions, including cell proliferation and
survival (22). The mammalian NF-κB
family consists of five transcription factors: p65 (RelA), RelB,
c-Rel, p105/p50 (NF-κB1) and p100/p52 (NF-κB2) (23). RelA, RelB and c-Rel are synthesized as
final proteins, and all of the members can form homo- and
heterodimers and shuttle from the cytoplasm to the nucleus in
response to cell stimulation (23).
NF-κB transcription factors are characterized by the presence of a
highly-conserved Rel homology domain (RHD), which is responsible
for dimerization, DNA binding and interaction with the inhibitor of
κB (IκB) proteins (24). The IκB
proteins, including IκBα, IκBβ, IκBε, IκBγ, Bcl-3 and the precursor
Rel proteins p100 and p105, are characterized by the presence of
multiple ankyrin repeats, which are protein-protein interaction
domains that interact with NF-κB via the RHD (24). Therefore, the present study
investigated the expression of NF-κB p65 and IκBa in NF-κB pathway.
The present study aimed to investigate the anticancer effects of
curcumin in breast cancer MCF-7 cells and to further investigate
the molecular mechanisms in human breast cancer cells. The results
of the present study revealed that proliferation of MCF-7 cells
treated with curcumin was markedly decreased in a concentration-
and time-dependent manner. The inhibitory effect of curcumin was
the greatest at a concentration of 20 µM. mRNA expression of Bax in
cells treated with curcumin was increased and Bcl-2 expression was
decreased compared with the non-treated cells. Protein expression
of NF-κB p65 was decreased and IκB was increased.
In conclusion, curcumin was able to inhibit
proliferation, and the potential mechanism may be associated with
the regulation of the NF-κB signaling pathway by curcumin.
Acknowledgements
The authors thank the Central Research Laboratory,
The Second Hospital of Shandong University (Shandong, China) for
technical assistance and support. The present study was supported
by grants from the National Natural Science Foundation of China
(grant no. 81400072), the Natural Science Foundation of Shandong
Province (grant nos. ZR2016HM67, 2013HQ047 and ZR2013HM103),
Science and Technology Development Project of Shandong (grant nos.
2016GSF201044 and 2016GSF201203) and the Medical and Health Science
Technology Development Plan Project of Shandong Province (grant no.
2015WS0307).
References
|
1
|
Pu Z, Yuan X, Zhang X, Chen Q and Xie H:
Meta-analysis on the association between CYP2D6*10 gene
polymorphism and disease free survival of breast cancer patients
receiving tamoxifen treatment in Asia. Bangladesh J Pharmacol.
9:652–662. 2014. View Article : Google Scholar
|
|
2
|
Li Z and Kang Y: Emerging therapeutic
targets in metastatic progression: A focus on breast cancer.
Pharmacol Ther. 161:79–96. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chung SS and Vadgama JV: Curcumin and
epigallocatechin gallate inhibit the cancer stem cell phenotype via
down-regulation of STAT3-NFκB signaling. Anticancer Res. 35:39–46.
2015.PubMed/NCBI
|
|
4
|
Ramachandran C and You W: Differential
sensitivity of human mammary epithelial and breast carcinoma cell
lines to curcumin. Breast Cancer Res Treat. 54:269–278. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li YB, Gao JL, Zhong ZF, Hoi PM, Lee SM
and Wang YT: Bisdemethoxycurcumin suppresses MCF-7 cells
proliferation by inducing ROS accumulation and modulating
senescence-related pathways. Pharmacol Rep. 65:700–709. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ramachandran C, Rodriguez S, Ramachandran
R, Nair Raveendran PK, Fonseca H, Khatib Z, Escalon E and Melnick
SJ: Expression profiles of apoptotic genes induced by curcumin in
human breast cancer and mammary epithelial cell lines. Anticancer
Res. 25:3293–3302. 2005.PubMed/NCBI
|
|
7
|
Basnet P and Skalko-Basnet N: Curcumin: An
anti-inflammatory molecule from a curry spice on the path to cancer
treatment. Molecules. 16:4567–4598. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen Y, Shu W, Chen W, Wu Q, Liu H and Cui
G: Curcumin, both histone deacetylase and p300/CBP-specific
inhibitor, represses the activity of nuclear factor kappa B and
Notch 1 in Raji cells. Basic Clin Pharmacol Toxicol. 101:427–433.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goel A, Kunnumakkara AB and Aggarwal BB:
Curcumin as ‘Curecumin’: from kitchen to clinic. Biochem Pharmacol.
75:787–809. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Y, Yu J, Cui R, Lin J and Ding X:
Curcumin in treating breast cancer: A review. J Lab Autom.
21:723–731. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hatcher H, Planalp R, Cho J, Torti FM and
Torti SV: Curcumin: From ancient medicine to current clinical
trials. Cell Mol Life Sci. 65:1631–1652. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin YG, Kunnumakkara AB, Nair A, Merritt
WM, Han LY, Armaiz-Pena GN, Kamat AA, Spannuth WA, Gershenson DM,
Lutgendorf SK, et al: Curcumin inhibits tumor growth and
angiogenesis in ovarian carcinoma by targeting the nuclear
factor-kappaB pathway. Clin Cancer Res. 13:3423–3430. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rengarajan T, Nandakumar N, Rajendran P,
Haribabu L, Nishigaki I and Balasubramanian MP: D-pinitol promotes
apoptosis in MCF-7 cells via induction of p53 and Bax and
inhibition of Bcl-2 and NF-κB. Asian Pac J Cancer Prev.
15:1757–1762. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim KC, Baek SH and Lee C:
Curcumin-induced downregulation of Axl receptor tyrosine kinase
inhibits cell proliferation and circumvents chemoresistance in
non-small lung cancer cells. Int J Oncol. 47:2296–2303. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou X, Su J, Feng S, Wang L, Yin X, Yan J
and Wang Z: Antitumor activity of curcumin is involved in
down-regulation of YAP/TAZ expression in pancreatic cancer cells.
Oncotarget. 7:79076–79088. 2016.PubMed/NCBI
|
|
18
|
Sha J, Li J, Wang W, Pan L, Cheng J, Li L,
Zhao H and Lin W: Curcumin induces G0/G1 arrest and apoptosis in
hormone independent prostate cancer DU-145 cells by down regulating
Notch signaling. Biomed Pharmacother. 84:177–184. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Iqbal B, Ghildiyal A, Sahabjada, Singh S,
Arshad M, Mahdi AA and Tiwari S: Antiproliferative and apoptotic
effect of curcumin and TRAIL (TNF Related Apoptosis inducing
Ligand) in chronic myeloid leukaemic cells. J Clin Diagn Res.
10:XC01–XC05. 2016.PubMed/NCBI
|
|
20
|
Somasundaram S, Edmund NA, Moore DT, Small
GW, Shi YY and Orlowski RZ: Dietary curcumin inhibits
chemotherapy-induced apoptosis in models of human breast cancer.
Cancer Res. 62:3868–3875. 2002.PubMed/NCBI
|
|
21
|
Tor YS, Yazan LS, Foo JB, Armania N, Cheah
YK, Abdullah R, Imam MU, Ismail N and Ismail M: Induction of
apoptosis through oxidative stress-related pathways in MCF-7, human
breast cancer cells, by ethyl acetate extract of Dillenia
suffruticosa. BMC Complement Altern Med. 14:552014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Oida K, Matsuda A, Jung K, Xia Y, Jang H,
Amagai Y, Ahn G, Nishikawa S, Ishizaka S, Jensen-Jarolim E, et al:
Nuclear factor-ĸB plays a critical role in both intrinsic and
acquired resistance against endocrine therapy in human breast
cancer cells. Sci Rep. 4:40572014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Colomer C, Marruecos L, Vert A, Bigas A
and Espinosa L: NF-κB members left home: NF-κB-independent roles in
cancer. biomedicines. 5:pii: E26. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ghosh S, May MJ and Kopp EB: NF-kappa B
and Rel proteins: Evolutionarily conserved mediators of immune
responses. Annu Rev Immunol. 16:225–260. 1998. View Article : Google Scholar : PubMed/NCBI
|