Introduction
Ovarian cancer (OC) is the most lethal of all types
of gynecological malignancy and the third leading cause of cancer
mortality among females worldwide (1). Due to the lack of specific biomarkers
for the diagnosis, more than half of patients are found to be in
the advanced stage of OC at the time of initial diagnosis (2,3). Among the
multiple types of ovarian cancer that exist, primary epithelial
ovarian cancer (PEOC) accounts for 85–90% in total (4). Another type, metastatic ovarian
carcinoma (MOC), can form from gastrointestinal tumors and breast
cancers with peritoneal dissemination and/or lymphatic metastasis
(5–7).
The clinical use of cancer antigen (CA)-125,
estrogen receptor (ER) and progesterone receptor (PR) for the
diagnosis of OC is currently not effective due to low sensitivity
and specificity (8,9). Therefore, the screening and
identification of a specific biomarker for OC diagnosis is
important. In a previous study, paired-box 8 (PAX8) has been
suggested to be a sensitive marker for tumors of the thyroid,
kidney and thymus, and tumors derived from the Müllerian pipe organ
(10–12). It was also reported that PAX8 is
widely expressed in various types of ovarian tumors, particularly
in serous tumors such as ovarian endometrioid carcinoma and clear
cell carcinoma (11). Through the
collating, summarizing and long-term follow-up inspection of
preliminary clinical data on ovarian cancer, the present study
mainly discusses the value of PAX8 in the differential diagnosis of
primary and metastatic ovarian carcinoma, and analyzes the
association between the expression of PAX8, clinicopathological
features of primary ovarian carcinoma and prognosis for patients
with ovarian cancer.
Materials and methods
Research subjects
The present study was approved by the hospital
Review Board and the Ethics Committee of the Medical Faculty at the
Shanghai Jiao Tong University (Shanghai, China). All patients
provided written informed consent of using their tumor tissues
prior to the beginning of the study. In total, 60 PEOC patients
enrolled in the Gynecology and Obstetrics Department of the
Shanghai Ninth People's Hospital (Shanghai, China), whom underwent
radical resection between January 2008 and December 2012, were
included in the present study. The age of patients ranged between
39 and 68 years, with a median age of 52. The group included 44
patients with ovarian serous carcinoma, 9 patients with mucinous
carcinoma, 2 patients with endometrial carcinoma and 5 patients
with clear cell carcinoma. The control group included 20 patients
with benign ovary tumors and 10 patients with MOC. In the MOC
subgroup, the MOC had metastasized in 5 patients from gastric
cancer, from colon cancer in 3 patients and from breast cancer in 2
patients. All clinicopathological profiles were evaluated in
accordance with the criteria of the National Comprehensive Cancer
Network Clinical Practice Guidelines in Oncology (Fort Washington,
PA, USA) (3). None of the patients
received preoperative radiotherapy or chemotherapy prior to
admission to hospital, confirmed by pathology following the
surgery, and all were treated with platinum-based chemotherapy in
combination with taxol subsequent to the surgery.
The last follow-up was on December 31, 2014, with an
average follow-up time of 20.58±5.71 months. During the follow-up
period, 45 patients succumbed to recurrence or metastasis. The
remaining 15 patients had censored data (among which 3 succumbed to
other diseases, 2 were lost to follow-up and 10 are still alive).
Surviving patients had a minimum follow-up period of 16 months,
with the longest being 36 months.
Immunohistochemical (IHC)
staining
Surgically-obtained tumor tissue specimens were
prepared in the form of 4 µm thick sections, prepared with
hematoxylin and eosin staining and IHC staining, respectively. The
avidin-biotin complex (ABC) method of IHC staining was adopted. The
sections were treated by normal dewaxing, graded ethanol hydration,
3% H2O2 closed endogenous peroxidase,
microwave heating antigen retrieval and PBS rinsing. The primary
antibody used was an anti-PAX8 rabbit anti-human monoclonal
antibody (dilution 1:200; #ab189249; Abcam, Cambridge, UK) and the
biotinylated secondary antibody was a goat anti-rabbit antibody
(dilution, 1:200; #ab7010; Abcam). ABC reagent was used to
visualize the staining (ABC kit; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) and samples were subsequently stained with
3,3′-diaminobenzidine. Next, the samples were re-stained with
hematoxylin and were resin-sealed. A PAX8 positive result was taken
to be the nucleus appearing yellowish-brown or brown. For the
negative control, PBS was used to replace the primary antibody.
According to the intensity and range of PAX8
expression, the present study divided PEOC with PAX8 expression
into 5 categories: No staining was assigned as negative (Fig. 1A and B); 1–24% staining as 1+
(Fig. 1C and D); 25–50% staining as
2+ (Fig. 1E and F); 51–75% staining
as 3+ (Fig. 1G and H); >75%
staining as 4+ (Fig. 1I and J).
 | Figure 1.Immunohistochemical staining of PAX8
in ovarian tumors. A PAX8 positive result was taken as the nucleus
appearing yellowish-brown or brown. (A) Lesions of metastatic
ovarian cancer from gastric adenocarcinoma, with PAX8 negative
staining (magnification, ×200). (B) Lesions of metastatic ovarian
cancer from gastric adenocarcinoma, with PAX8 negative staining
(magnification, ×400). (C) Lesions of ovarian teratoma, for which
PAX8 staining was 1+ (magnification, ×200). (D) Lesions of ovarian
teratoma, for which PAX8 staining was 1+ (magnification, ×400). (E)
Lesions of ovarian serous carcinoma, for which PAX8 staining was 2+
(magnification, ×200). (F) Lesions of ovarian serous carcinoma, for
which PAX8 staining was 2+ (magnification, ×400). (G) Lesions of
ovarian serous carcinoma, for which PAX8 staining was 3+
(magnification, ×200). (H) Lesions of ovarian serous carcinoma, for
which PAX8 staining was 3+ (magnification, ×400). (I) Lesions of
ovarian serous carcinoma, for which PAX8 staining was 4+
(magnification, ×200). (J) Lesions of ovarian serous carcinoma, for
which PAX8 staining was 4+ (magnification, ×400). PAX8, paired-box
8. |
The categorization criteria were set as follows:
Each slice was observed in 5 independent images, then 100 cells
were counted per image and the percentage of stained cells in each
slice was calculated.
The aforementioned results were obtained via a
double-blind method. Each section was examined by 2 pathologists
and the patients with consistent results were enrolled in the
present study.
Statistical analysis
SPSS 13.0 (SPSS, Inc., Chicago, IL, USA) was used
for statistical analysis. The association between PAX8 and
clinicopathological characteristics was tested using a
χ2 test for comparisons between groups, and a
Mann-Whitney U test and Kruskal-Wallis H test for comparisons
within the same population. For survival rate analysis, a
Kaplan-Meier test was used to compare the differences between
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
PAX8 expression in ovarian tumor
lesions
There were 44 patients with ovarian serous carcinoma
(73.3%), 9 patients with mucinous carcinoma (15%), 2 patients with
endometrioid carcinoma (3.3%) and 5 patients with clear cell
carcinoma (8.3%) within the total 60 patients with PEOC (Fig. 1).
The IHC staining of PAX8 in PEOC had an overall
positive rate of 92% (57/60). By contrast, all MOC samples showed
negative staining. Statistical analysis showed a significant
difference between the PEOC and MOC groups (P<0.001). However,
no significant difference was observed between the PEOC groups with
different pathological types (P=0.871).
The positive rate of PAX8 in 20 patients with
ovarian benign tumor was 85% (17/20), showing no significant
statistical difference in comparison with the PEOC group (P=0.761).
In addition, no significant difference with respect to the positive
expression of PAX8 was found among ovarian benign tumors with
different pathologies (P=0.533). All data shown in Table I.
 | Table I.Expression of PAX8 in ovarian tumor
lesions. |
Table I.
Expression of PAX8 in ovarian tumor
lesions.
| Tumor | Patient, n | Expression of PAX8, n
(%) |
|---|
| Primary epithelial
ovarian cancer | 60 | 57/60 (92.00) |
| Serous
carcinoma | 44 | 42/44 (95.45) |
| Mucinous
carcinoma | 9 | 8/9 (88.89) |
|
Endometrial carcinoma | 2 | 2/2 (100.00) |
| Clear
cell carcinoma | 5 | 5/5 (100.00) |
| Metastatic ovarian
carcinoma | 10 | 0/10 (0.00) |
| From
gastric cancer | 5 | 0/10 (0.00) |
| From
colon cancer | 3 | 0/10 (0.00) |
| From
breast cancer | 2 | 0/10 (0.00) |
| Benign ovary
tumor | 20 | 17/20 (85.00) |
|
Endometriosis | 7 | 6/7 (85.71) |
|
Teratoma | 5 | 3/5 (60.00) |
| Serous
cystadenoma | 8 | 8/8 (100.00) |
Association between PAX8 expression
and clinicopathological characteristics of PEOC
Based on the aforementioned categorization and
statistical analysis, the present study revealed that PAX8
expression was associated with the degree of cancer cell
differentiation and International Federation of Gynaecological
Oncologists (FIGO) stage (3). The
higher the PAX8 expression, the lower the cancer cell was
differentiated (P=0.033) and the more advanced the FIGO stage was
(P=0.003; Table II). There was no
significant difference demonstrated between the expression levels
of PAX8 in PEOC (P=0.574) of different pathological types (Table II).
 | Table II.Association between PAX8 expression
and clinicopathological characteristics of primary epithelial
ovarian cancer. |
Table II.
Association between PAX8 expression
and clinicopathological characteristics of primary epithelial
ovarian cancer.
|
|
| PAX8 |
|
|
|---|
|
|
|
|
|
|
|---|
| Clinical
characteristic | Patient, n | Negative | 1+ | 2+ | 3+ | 4+ | χ2 | P-value |
|---|
| Pathological
Characteristic |
|
|
|
|
|
| 0.317 | 0.574 |
| Serous
carcinoma | 44 | 2 | 4 | 7 | 13 | 18 |
|
|
|
Non-serous carcinoma | 16 | 1 | 1 | 2 | 8 | 4 |
|
|
| Differentiation
degree |
|
|
|
|
|
| 6.850 | 0.033 |
| High | 11 | 1 | 1 | 5 | 3 | 1 |
|
|
|
Moderate | 23 | 2 | 3 | 0 | 8 | 10 |
|
|
| Low | 26 | 0 | 1 | 4 | 10 | 11 |
|
|
| FIGO stage |
|
|
|
|
|
| 15.607 | 0.003 |
| I | 9 | 2 | 2 | 2 | 1 | 2 |
|
|
| II | 17 | 1 | 2 | 5 | 7 | 2 |
|
|
|
III–IV | 34 | 0 | 1 | 2 | 13 | 18 |
|
|
Association between PAX8 expression
and the prognosis of patients with PEOC
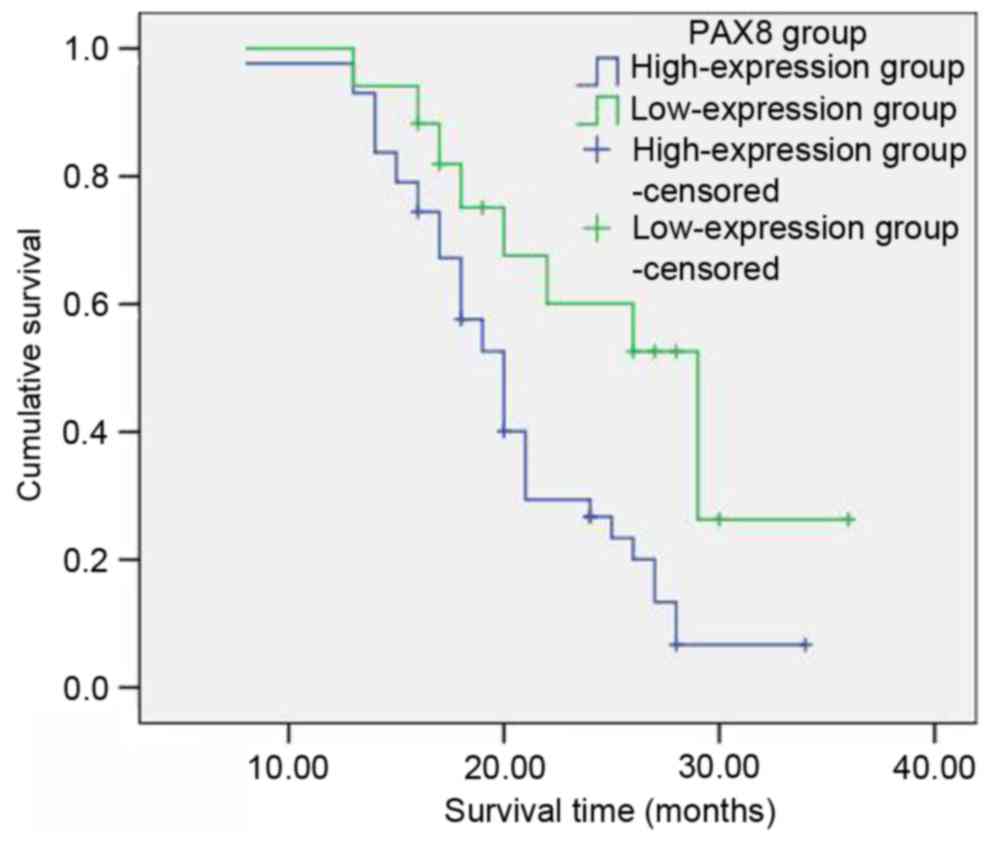
Among the group of 60 patients with primary ovarian
cancer, the 1-year survival rate was 98%, the 2-year survival rate
was 39% and the 3-year survival rate was 13%. According to the
aforementioned categorization based on the PAX8 staining intensity
(Table II), with respect to the low
PAX8 expression groups (negative, 1+ and 2+; 17 patients in total),
the survival rates subsequent to 1, 2 and 3 years were 100, 60.1
and 26.3%, respectively. With respect to the high PAX8 groups (3+
and 4+; 43 patients in total), the survival rates subsequent to 1,
2 and 3 years were 97.7, 26.7 and 6.7%, respectively. The
postoperative survival rate of patients with ovarian cancer in the
PAX8 low-expression groups was significantly longer than that of
the PAX8 high-expression groups. The difference was considered to
be statistically significant (χ2=6.820, P=0.009;
Table III; Fig. 2).
 | Table III.Influence of clinicopathological
characteristics on the prognosis of 60 patients with primary
epithelial ovarian cancer. |
Table III.
Influence of clinicopathological
characteristics on the prognosis of 60 patients with primary
epithelial ovarian cancer.
| Characteristic | Patient, n | 1-year survival
rate, % | 2-year survival
rate, % | 3-year survival
rate, % | χ2 | P-value |
|---|
| PAX8 |
|
|
|
|
|
|
|
−/1+/2+ | 17 | 100 | 60.1 | 26.3 | 6.820 | 0.009 |
|
3+/4+ | 43 | 97.7 | 26.7 | 6.7 |
|
|
| Pathological
characteristic |
|
|
|
|
|
|
| Serous
carcinoma | 44 | 97.7 | 29.7 | 0 | 2.920 | 0.087 |
|
Non-serous carcinoma | 16 | 100 | 50 | 23.4 |
|
|
| Differentiation
degree |
|
|
|
|
|
|
|
High | 11 | 100 | 42.4 | 0 | 1.566 | 0.457 |
|
Moderate | 23 | 100 | 44.3 | 25.9 |
|
|
|
Low | 26 | 96.2 | 24.1 | 6 |
|
|
| FIGO stage |
|
|
|
|
|
|
|
I–II | 26 | 100 | 56.4 | 33.8 | 10.428 | 0.001 |
|
III–IV | 34 | 97.1 | 21.7 | 0 |
|
|
Discussion
PAX8 belongs to the paired-box (PAX) gene family.
The PAX protein family (PAX l-9) has a highly conserved region
formed of 128 amino acids. The paired box gene family is able to
recognize the specific DNA binding site of the target genes to
regulate gene expression (13). PAX8
proteins are composed of 450 amino acids located in the 2q12-14
section of encoding genes with a molecular weight of 48 kb
(14). PAX8 was first reported to be
involved in thyroid gland development in mice (15,16).
During embryonic development, PAX8 performs a key role in the
development of organs such as the thyroid, kidney, part of the
central nervous system, inner ear, eye and Wolffian and Müllerian
ducts (17). Furthermore, PAX8
performs a role in maintaining the normal function of cells
subsequent to the birth of the fetus. According to several studies,
PAX8 can be a sensitive marker for thyroid, kidney, Müllerian duct
and thymus tumors (10–12).
The female reproductive tract originates from the
fetal Müllerian system and PAX8 is involved in the regulation of
the formation of the tract (17).
Female mice lacking PAX8 expression cannot form the genital tract,
leading to sterility (18). For
humans, PAX8 is expressed in the tissues and organs, including in
secretory cells, derived from the Müllerian system. Additionally,
PAX8 expression is found in tumors derived from the epithelial
cells of the Müllerian system (12,19).
Previous studies have revealed that PAX8 is widely expressed in
various types of ovarian tumor, particularly in serous tumors such
as ovarian endometrioid carcinoma and clear cell carcinoma
(11,12). The majority of serous ovarian cancer
cells are considered to originate from the normal secretory cells
of the fallopian tube in which PAX8 is highly expressed. Mucinous
carcinoma of the Müllerian system is distinguished from metastatic
cancer of the digestive tract, and cytokeratin (CK)-7 and CK20 are
found to perform an important role in the differentiation process
(20). However, in some patients it
is difficult to judge the cancer source based on CK7/20 positivity
or negativity (20). In certain types
of mucinous carcinoma in the female reproductive tract, PAX8 may be
partially expressed (21). However,
PAX8 is not expressed in carcinoma of the stomach, colon, bile
duct, duodenal ampulla, hepatocellular or pancreatic acinar cell
carcinoma, and is rarely expressed in adenocarcinoma,
pancreas-pseudopapillary tumors of the pancreas or esophageal
adenocarcinoma (21). Where PAX8 is
expressed, metastatic tumors of the digestive tract may be
excluded, so the source of PAX8 may be considered to derive from
the Müllerian duct. Similarly, PAX8 can be used for the clinical
differential diagnosis of breast and ovarian cancer, ovarian sex
cord-stromal tumor and malignant mesothelioma (22,23).
In the present study, PAX8 was found to be highly
expressed in PEOC, but not in MOC, suggesting that PAX8 exhibits a
high enough sensitivity and specificity to be used in clinical
practice. In addition, the present study found that PAX8 expression
levels are associated with cancer cell differentiation degree and
FIGO stage. Survival rate analysis demonstrated that patients with
high levels of PAX8 expression had a poor prognosis. The results of
the present suggest that there may be a link between PAX8 and the
progression of ovarian cancer.
In conclusion, PAX8 IHC detection exhibits high
sensitivity and specificity in terms of differentiating between
PEOC and MOC. The level of PAX8 expression is associated with the
differentiation degree of ovarian tumor cells and FIGO staging,
which can help to determine prognosis. However, determining PAX8
expression cannot be used to discriminate between benign and
malignant ovarian tumors.
Acknowledgements
The authors would like to thank Dr Guang-ye Du for
the pathological examinations.
References
|
1
|
Sopik V, Lgbal J, Rosen B and Narod SA:
Why have ovarian cancer mortality rates declined? Part I.
incidence. Gynecol Oncol. 138:741–749. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Tiwari RC, Murray T, Ghafoor A,
Samuels A, Ward E, Feuer EJ and Thun MJ: American Cancer Society:
Cancer statistics, 2004. CA Cancer J Clin. 54:8–29. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Morgan RJ Jr, Alvarez RD, Armstrong DK,
Burger RA, Chen LM, Copeland L, Crispens MA, Gershenson DM, Gray
HJ, Hakam A, et al: National comprehensive cancer networks: Ovarian
cancer, version 2.2013. J Natl Compr Canc Netw. 11:1199–1209. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Matias-Guiu X and Davidson B: Prognostic
biomarkers in endometrial and ovarian carcinoma. Virchows Arch.
464:315–331. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Horn LC, Einenkel J, Handzel R and Höhn
AK: Morphology of secondary ovarian tumors and metastases.
Pathologe. 35:336–347. 2014.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yonemura Y, Canbay E, Endou Y, Ishibashi
H, Mizumoto A, Miura M, Li Y, Liu Y, Takeshita K, Ichinose M, et
al: Peritoneal cancer treatment. Expert Opin Pharmacother.
15:623–636. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Young RH: From krukenberg to today: The
ever present problems posed by metastatic tumors in the ovary: Part
I. Historical perspective, general principles, mutinous tumors
including the krukenberg tumor. Adv Anat Pathol. 13:205–227. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhu W and Michael CW: WT1, monoclonal CEA.
TTF1, and CA125 antibodies in the differential diagnosis of lung,
breast, and ovarian adenocarcinomas in serous effusions. Diagn
Cytopathol. 35:370–375. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee BH, Hecht JL, Pinkus JL and Pinkus GS:
WT1, estrogen receptor, and progesterone receptor as markers for
breast or ovarian primary sites in metastatic adenocarcinoma to
body fluids. Am J Clin Pathol. 117:745–750. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ozcan A, Steven SS, Hamilton C, Anjana K,
Coffey D, Krishnan B and Truong LD: PAX 8 expression in
non-neoplastic tissues, primary tumors, and metastatic tumors: A
cornprehensive immunohistochemical study. Mod Pathol. 24:751–764.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nonaka D, Chiriboga L and Soslow RA:
Expression of pax8 as a useful marker in distinguishing ovarian
carcinomas from mammary carcinomas. Am J Surg Pathol. 32:1566–1571.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tong GX, Devaraj K, Hamele-Bena D, Yu WM,
Turk A, Chen X, Wright JD and Greenebaum E: PAX8: A marker for
carcinoma of Müllerian origin in serous effusions. Diagn
Cytopathol. 39:567–574. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mansouri A, Goudreau G and Gruss P: PAX
genes and their role in organogenesis. Cancer Res. 59 7
Suppl:S1707–S1710. 1999.
|
|
14
|
Lang D, Powell SK, Plummer RS, Young KP
and Ruggeri BA: PAX genes: Roles in development, pathophysiology,
and cancer. Biochem Pharmacol. 73:1–14. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Plachov D, Chowdhury K, Walther C, Simon
D, Guenet JL and Gruss P: Pax8, a murine paired box gene expressed
in the developing excretory system and thyroid gland. Development.
110:643–651. 1990.PubMed/NCBI
|
|
16
|
Poleev A, Fickenscher H, Mundlos S,
Winterpacht A, Zabel B, Fidler A, Gruss P and Plachov D: PAX8, a
human paired box gene: Isolation and expression in developing
thyroid, kidney and Wilms' tumors. Development. 116:611–623.
1992.PubMed/NCBI
|
|
17
|
Bouchard M, de Caprona D, Busslinger M, Xu
P and Fritzsch B: Pax2 and Pax8 cooperate in mouse inner ear
morphogenesis and innervation. BMC Dev Biol. 10:892010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mittag J, Winterhager E, Bauer K and
Grümmer R: Congenital hypothyroid female pax8-deficient mice are
infertile despite thyroid hormone replacement therapy.
Endocrinology. 148:719–725. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Levanon K, Ng V, Piao HY, Zhang Y, Chang
MC, Roh MH, Kindelberger DW, Hirsch MS, Crum CP, Marto JA and
Drapkin R: Primary ex vivo cultures of human fallopian tube
epithelium as a model for serous ovarian carcinogenesis. Oncogene.
29:1103–1113. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vang R, Gown AM, Barry TS, Wheeler DT,
Yemelyanova A, Seidman JD and Ronnett BM: Cytokeratins 7 and 20 in
primary and secondary mucinous tumors of the ovary: Analysis of
coordinate immunohistochemical expression profiles and staining
distribution in 179 cases. Am J Surg Pathol. 30:1130–1139. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ordóñez NG: Value of PAX 8 immunostaining
in tumor diagnosis: A review and update. Adv Anat Pathol.
19:140–151. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ordóñez NG: Value of PAX8, PAX2,
claudin-4, and h-caldesmon immunostaining in distinguishing
peritoneal epithelioid mesotheliomas from serous carcinomas. Mod
Pathol. 26:553–562. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Laury AR, Hornick JL, Perets R, Krane JF,
Corson J, Drapkin R and Hirsch MS: PAX8 reliably distinguishes
ovarian serous tumors from malignant mesothelioma. Am J Surg
Pathol. 34:627–635. 2010.PubMed/NCBI
|